The minimal open preperitoneal (MOPP) approach for treating groin hernias: technique, indications, and results
Highlight box
Key findings
• Minimal open preperitoneal (MOPP) technique is a newcomer in the group of open preperitoneal techniques after trans inguinal preperitoneal and trans rectus preperitoneal techniques. We have presented a very detailed description of the technique, step by step. The MOPP technique has demonstrated good results in terms of chronic pain and recurrence rate. The technique it neither requires disposable equipment nor gas, it is environmentally friendly.
What is known and what is new?
• The two classic groups of primary groin hernia repair techniques Lichtenstein and endoscopic have often been compared ultimately indicating better results for the endoscopic group with regard to chronic pain. More recently the comparison between open preperitoneal techniques and the two classic groups shows a superiority of this third group versus Lichtenstein with at least as good a result as the endoscopic techniques.
• This manuscript adds to the last publications a proposition of a new preperitoneal technique as a basic intervention for primary groin hernias.
What is the implication, and what should change now?
• The major implication is to be able to offer MOPP for surgeons who are unwilling or unable to use endoscopic equipment. Or as a substitute technique for endoscopic surgeons who do not wish to perform their usual technique for some select patients while also avoiding the Lichtenstein technique or the no mesh repair technique. But it will be necessary to confirm the good results presented in this study by those which will have to be presented by new users.
Introduction
Background
The idea of utilizing a large preperitoneal prosthesis for the treatment of groin hernias was extensively advocated over 60 years ago by Nyhus (1). Notwithstanding, the principle of the preperitoneal “hammock prosthesis” as a treatment for groin hernias can be traced back to the thesis of French surgeon D. Corti in 1949, building on the work of E. Acquaviva (2-4). Wantz (5,6) shared a similar concept, introduced a trans-rectus procedure aiming to treat complex hernias (such as recurrent hernias) using local anesthesia in an ambulatory setting. Regrettably, due to technical challenges, and even by his own admission, this objective was not attained. The Wantz procedure eventually necessitated general anesthesia and traditional hospitalization. Inspired by Wantz and Stoppa (7), Franz Ugahary, the pioneer of the minimal open preperitoneal (MOPP) approach, realized this goal. He merged the concept of fortifying the visceral sac with a large prosthesis (as proposed by Stoppa) with his innovative minimal invasive surgery utilizing a small grid iron incision (8,9). However, many found the Ugahary technique challenging to replicate and teach. The technique did not gain widespread adoption, partly due to the absence of a prosthesis specifically designed for the minimal incision. Over the past two decades, with the rise of endoscopic surgery, inguinal hernia treatments have largely been divided into two primary techniques: the classic anterior Liechtenstein-type approach and the posterior endoscopic approach. However, a new prosthesis developed by Pelissier, featuring a rigid peripheral ring, made it easier to place the mesh in the preperitoneal space through an anterior approach. This innovation led to the birth of the transinguinal preperitoneal (TIPP) technique (10,11), the second minimal invasive open preperitoneal method following the Ugahary technique. The third variant is denoted by the trans-rectus preperitoneal (TREPP) technique (12). It is only in recent years that studies have emerged comparing this open preperitoneal method to the earlier two (13-19). The open preperitoneal approach has proven superior to the Lichtenstein techniques in terms of reducing chronic pain and has displayed results on par with endoscopic methods.
Objectives
The objectives of this article are two-fold:
- To provide a revised description of the MOPP technique. While the origins, technical principles, and preliminary outcomes of the MOPP method have been previously published (20,21), this article clarifies several fundamental steps of the procedure based on cadaveric dissection and the clinical experience from several thousand surgeries. The innovation hinges on the identification of the transversalis fascia (TF) during two pivotal stages: The TF covers the deep inguinal orifice. Recognizing it at this juncture initiates the entry into the preperitoneal space, paving the way for preperitoneal dissection. The TF also constitutes the internal spermatic fascia. Identifying and severing it systematically commences the parietalization of the cord elements in medial hernias. Rationalizing these two essential steps for placing a large prosthesis in the preperitoneal space makes the technique more easily reproducible and teachable.
- This article, for the first time, presents results from a substantial number of hernia repairs [1,401] using the MOPP technique. The data are sourced from the French “club hernie” database (22). The aim is to highlight the technique’s impressive outcomes in terms of minimal short and long-term complications, its low recurrence rate, minimal chronic discomfort, and the virtual absence of severe chronic pain when addressing primary groin hernias. I present this article in accordance with the STROBE reporting checklist (available at https://ales.amegroups.com/article/view/10.21037/ales-23-37/rc).
Methods
Prospective data
Patients included in the study: all primary, inguinal, femoral hernias and bilateral hernias in both men (including scrotal hernias) and women. Patients excluded from the study: strangulating hernias, unscheduled surgeries, and according to the HerniaSurge Group International guidelines (23), placing a mesh in the preperitoneal space is contraindicated if the posterior route has been previously used. Therefore, previous radical prostatectomy, pelvic irradiation, or the performance of a vascular bypass with dissection of the preperitoneal space are contraindications to the MOPP technique. As the MOPP technique is a preperitoneal route through the anterior wall, recurrent hernias are mostly contraindicated. When a strictly posterior approach is preferable, I employ the Ugahary technique [similar to endoscopic totally extraperitoneal (TEP) technique]. Our patients are informed of the necessity for long-term follow-up and data collection. The data have been integrated into the database of the “hernia club” following a rigorous methodology and a binding charter (22).
Between September 2011 and 29 April 2019, a total of 1,616 groin hernias were operated on in a single center by a single operator. A total of 1,500 were primary hernias, and 1,401 hernias (out of 1,146 patients) were operated on using the MOPP technique. During this period, 171 primary or recurrent hernias were operated on using other techniques: Ugahary (N=91), Lichtenstein and anterior mesh (N=64), posterior mesh (N=5), and no mesh (N=4).
Clinical control is performed by the operating surgeon at discharge, the tenth postoperative day, the thirtieth postoperative day and, in the case of any symptoms at one month, an additional visit is scheduled between the third and sixth months post-surgery and 1 year after the procedure. During face-to-face interviews, pain was assessed using the Visual Analog Scale (VAS): 0 to 10 for D0, D1, D8, D30, D90–180 if pain persisted at D30, and 1 year later. An additional follow-up at 2 years involves a phone interview following a validated phone questionnaire conducted by an independent clinical research assistant (CRA) shielded as to the surgical procedure deployed. During the phone interview at 1 and/or 2-year follow-up, since VAS was not applicable (not face-to-face), we utilized a four-level verbal rating scale (VRS)—no pain, mild pain, moderate pain, severe pain. Post-operative VAS and VRS scores were compared to the preoperative scores. If any event is reported over the phone, the patient is strongly advised to schedule a clinical visit.
Collected parameters
Data extracted from the registry included perioperative data such as patient age and gender, body mass index, comorbidities, hernia and operative characteristics, operating time, and length of stay. Post-operative outcomes occurring within the first 30 days post-surgery were noted. Complications were graded based on the Dindo-Clavien classification. Late outcomes and follow-up: at each follow-up stage, quality of life (QOL) and patient self-assessment of the surgery (PROM) were documented.
Pain assessment
Chronic postoperative pain is defined as pain lasting more than 3 months. To mitigate bias in data collection and results, it’s noteworthy that all eligible patients operated on during the study period were included without exception.
Statistical analysis
Categorical variables were presented as frequencies and percentages. We do not have a comparative analysis in this study.
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). As this study is a monocenter, monoperator observational retrospective study, embedded in a registry which complies with the General Data Protection Regulation (GDPR) and the French “Commission Nationale de l’Informatique et des Libertés” (National Commission for Information Technology and Liberties) CNIL (MR0003) requirements, IRB approval is not required. Informed consent was taken from all the participants.
Surgical technique
Preliminary considerations
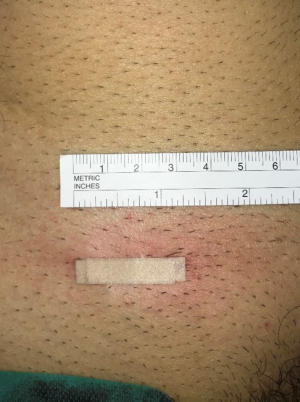
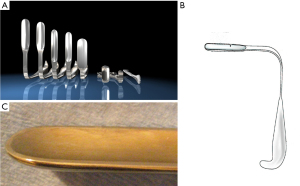
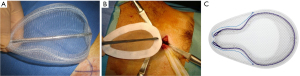
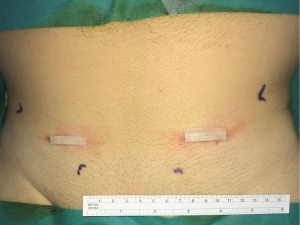
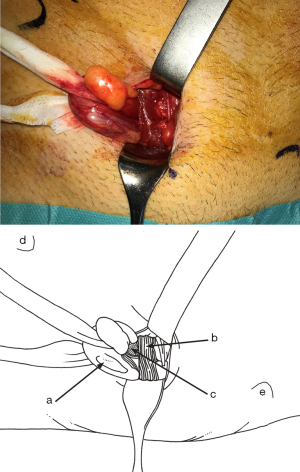
Minimal open and minimally invasive technique: The MOPP technique proposed here in is a synthesis between the TIPP technique, from which it uses the principles (10,11), and the Ugahary technique (8,9), from which it uses the same dissection technique of the planes with specific retractors of different sizes (Figure 1). This combination, based on our experience, typically limits the incision to 3 or 4 cm, hence the term “Minimal Open” (Figure 2). The operation can be performed under ilioinguinal block, transversus abdominis plane (TAP) block, or spinal anesthesia. In our practice, we now use general anesthesia without endotracheal intubation or curarization; local anesthesia is also applied, illustrating the “minimal invasive” principle. The “Minimal Open” and “minimal invasive” principles rationalize our preference for this technique when treating older and more fragile patients, even those with the largest primary hernias. The main principle of MOPP is to unroll in the preperitoneal space a large prosthesis far beyond the limits of the Fruchaud musculo-pectineal hole (24). It is a suture-less and tension-free technique. The prosthesis is held against the abdominal wall by the underlying pressure. Whenever the general conditions allow, the procedure is scheduled as outpatient (more than 90% in our practice).
Prosthesis-instrumentation
The prosthesis is chosen based on the need to unroll it in the preperitoneal space through a small incision. Some wide polypropylene mesh prostheses are specifically designed for this technique, with peripheral reinforcement, facilitating the proper deployment of the prosthesis (Figure 3). The quality of the prosthesis supports the minimally invasive approach. This technique requires very long but narrow retractors (Figure 1), allowing for extensive and deep dissection. A long dressing forceps with an atraumatic end is used to introduce the prosthesis behind the pubic bone, in contact with the bladder, without risking trauma.
Surgical technique step by step
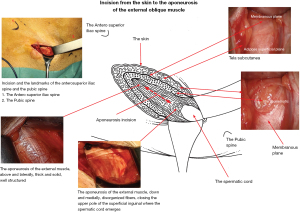
We use the new Fascial nomenclature (25) to describe the planes between the skin and the external oblique fascia in the inguinal area: the tela subcutanea (Figure 4). The superficial adipose plane is the new name for Camper’s fascia. The membranous plane has been renamed from Scarpa’s fascia. The fat between the membranous plane and the external oblique aponeurosis is the pre-aponeurotic fat.
The skin incision
The skin incision is deliberately minimized. With experience, it can range between 25 and 40 mm. It is located immediately in front of the deep inguinal ring. Several landmarks can be drawn on the patient’s skin (Figure 2). It is simpler to connect the superior anterior iliac spine to the pubic tubercle and mark the incision transversely at the junction of the internal and middle third (Figure 5).
The incision of the subcutaneous tissue (tela subcutanea)
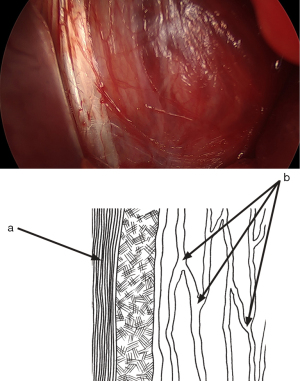
Refer to the diagram illustrating the surgical anatomy from the skin to the external oblique muscle aponeurosis (Figure 4), per the new anatomical nomenclature of the fascia (25). After dissecting the superficial adipose plane, previously known as Camper’s fascia, we observe a membranous plane, formerly called Scarpa’s fascia, which is sometimes a very thin membrane that can be hard to discern (Figure 6). This structure varies between individuals. The membranous plane is incised transversely (Figure 7). It will be sutured with a thin absorbable thread at the end of the procedure. Beneath the membranous plane, an adipose plane must be dissected to visualize the external oblique muscle aponeurosis.
External oblique muscle aponeurosis incision
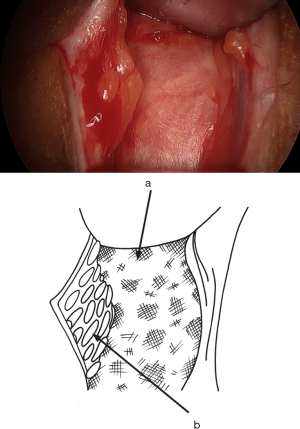
Cranially and laterally, the fibers of the aponeurosis typically form a thick, sturdy ligament. Caudally and medially, they often appear disorganized, closing the upper pole of the superficial inguinal ring (Figure 4). The incision of the aponeurosis is oblique, downwards and inwards, centered over this area where the fibers are disorganized and situated above and outside the superficial inguinal orifice. It’s not necessary to open the orifice (Figure 4). At this point, the ilioinguinal nerve can be identified. Care must be taken not to cut it during the aponeurosis incision (and similarly when closing it). With the incision, the inguinal canal is now wide open, but the cord is not immediately visible as it’s covered by cremaster fibers (Figure 8). The cremaster fibers are situated between the external spermatic fascia, which originates from the external oblique, and the internal spermatic fascia, derived from the TF (26). While the appearance of the cremaster muscle varies significantly among patients—sometimes thick with organized fibers or sometimes consisting of a few scattered fibers—the fibers are always preserved and pushed medially.
Dissection of the spermatic cord, the funicular pedicle, and the genital branch of the genito-femoral nerve
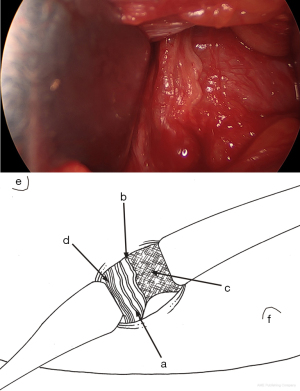
From the aponeurosis of the external oblique muscle laterally, the cord is moved medially to reveal the funicular pedicle (blue line) laterally and posteriorly, which contains the genital branch of the genitofemoral nerve that must be carefully preserved (Figure 9). The funicular pedicle is separated from the spermatic cord, and the posterior part of the cord is separated from the inguinal canal.
The cremaster muscle
The fibers of the cremaster muscle, which more or less densely cover the anterior surface of the cord, are pushed inwards, revealing the spermatic cord also covered proximally by the internal spermatic fascia, an extension of the TF. Typically, at this stage, the external spermatic fascia is not distinguished (26). As Fruchaud noted, it’s possible to perform an intra-fibrocremasteric cleavage of the cord and free the cord elements from the fibrocremasteric sheath surrounding them. Fruchaud divided the main lateral fascicle of the cremaster laterally and the deep accessory fascicle medially (27). For this technique, I begin near the external oblique aponeurosis and gradually push all cremasteric fibers inwards as a block. They remain intact medially, and the spermatic cord is entirely dissected, only covered by the internal spermatic fascia (Figure 10).
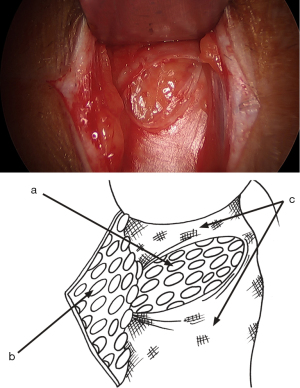
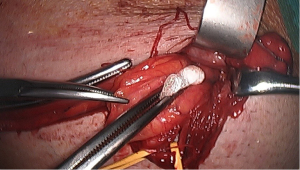
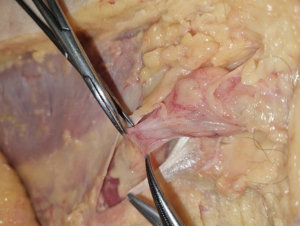
Lateral sac, lipoma
At this stage, a lateral hernia sac is looked for. Identifying a large, old sac is easy, but sometimes a small sac is found in the cord’s most proximal part. The lateral sac is separated from the cord. Similarly, a lipoma of the cord is also dissected (Figure 11). It’s either repressed or resected, as retaining such a lipoma may cause postoperative pain and give the impression of a recurrence. At this stage, one can begin the parietalization of the cord by pushing back the hernia sac, or if there’s no lateral sac, by pushing the peritoneum’s line of reflection after sectioning the internal spermatic fascia, as we’ll examine in more detail during the parietalization step.
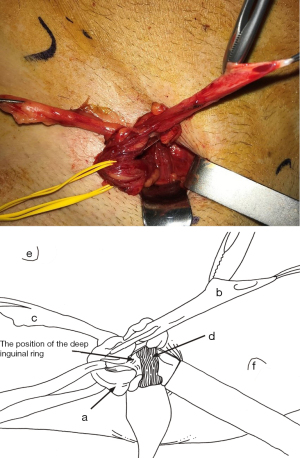
The deep inguinal ring
In the MOPP technique, the preperitoneal route always passes through the deep inguinal ring, regardless of the hernia type: lateral, medial, or femoral. Thus, it’s crucial to detail the methods of penetrating the deep inguinal orifice before releasing the preperitoneal space.
An essential point is that the TF covers the deep inguinal orifice. The approach is to push it inwards, starting from the internal edge of the spermatic cord or the internal edge of the lateral sac. Gradually, the yellow fat of the preperitoneal space becomes visible (Figure 12). Repeating this same pushback motion inside the TF, more yellow fat is seen, and the inferior epigastric pedicle (artery and veins) emerges (Figure 13). These vessels constitute a crucial landmark before entering the preperitoneal space. Preparing for this step, it’s beneficial to study the TF through cadaver dissection (Figure 14). The dissection continues behind the vessels, first with a nut (Figure 15). Then the vessels are pressed against the anterior abdominal wall, protected throughout the procedure with a retractor.
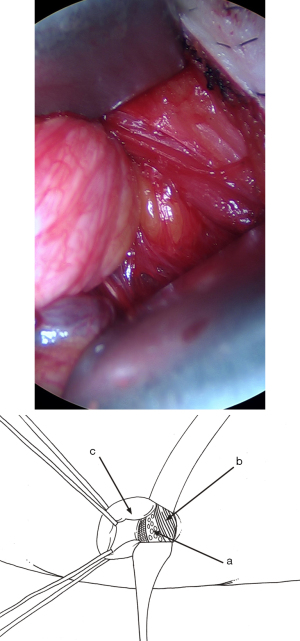
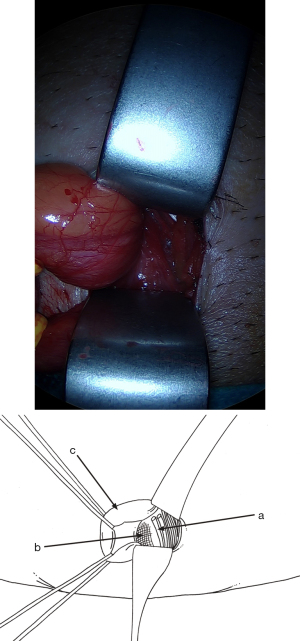

Cleavage of the preperitoneal space
As soon as the dissection passes medially beyond the deep side of the inferior epigastric pedicle, the conditions are met for extensive release of the preperitoneal space, where the lower and internal part of the mesh will be positioned. Using retractors specifically designed for the procedure, and increasing the sizes of retractors as per Ugahary’s dissection principles (Figure 1), the dissection extends into the avascular plane, medially and laterally along the inferior epigastric vessels towards the iliac vessels. Quickly and easily, the Bogros and Retzius spaces are dissected, revealing the pubis, Cooper’s ligament, and the retropubic space, with the bladder pushed back (Figure 16). During this stage, a medial sac may be repressed, and a femoral sac, if present, is always sought and repressed if necessary. Sometimes, this is just a small lipoma incarcerated in the femoral ring. The obturator region is also systematically checked. The dissection is atraumatic for the pelvic vascular structures. The space’s dissection continues upwards and medially. Above the incision, the peritoneal and fascial planes might adhere more or less to the superficial aponeurotic plane. Occasionally, dissection with scissors may be necessary to ensure proper deployment of the prosthesis at this level. Above and laterally, dissection is easier up to the psoas muscle.
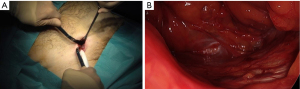
Parietalization of the spermatic cord (visualization of the internal spermatic fascia)
This is a critical step for the proper deployment of the prosthesis. Complete parietalization is essential and Its realization is delicate. As Wantz mentioned, the elements of the spermatic cord must be separated from the peritoneum over approximately 10 cm from the deep inguinal ring (5,6), preserving the spermatic fascia as indicated by Stoppa (28), thus achieving the cord’s parietalization (Figure 17). If there’s an external oblique sac already separated from the spermatic cord, the dissection will merely prolong the separation of the spermatic cord from the visceral sac upwards. Without a lateral sac, finding the correct plane might be more challenging. In such situations, it’s crucial and straightforward to individualize the internal spermatic fascia. Cutting the internal spermatic fascia just before the spermatic cord penetrates the deep inguinal ring will open the plane between the spermatic cord and the visceral sac, without risking opening the peritoneum. Preparing for this step with cadaver dissection can be beneficial (Figure 18). At this level, the vas deferens might closely adhere to the peritoneum. It is the sectioning of the internal spermatic fascia that facilitates the separation of the vas deferens from the peritoneum. After starting the parietalization this way, it then becomes easy to entirely separate the elements of the spermatic cord from the visceral sac, with a low risk of opening the visceral sac. The dissection can begin with a nut. The separation is then performed with specific retractors/dissectors according to Ugahary’s principles. A deeper skin retractor can replace the original one, facilitating the dissection’s progress. Applying a compress can also help push the visceral sac upwards. During this step, the dissection should respect the spermatic fascia if possible. The dissection is complete when the so-called “parietalization triangle” becomes visible (Figure 17). Its base is the line of reflection of the repressed peritoneum, and its peak is the reunification of the spermatic vessels with the vas deferens at the formed spermatic cord’s level. The internal edge is limited by the deferent, which plunges backward and inward. The outer edge consists of the spermatic vessels. According to Stoppa, careful preservation of this “spermatic sheath” during the cord’s mobilization is recommended to avoid perivascular sclerosis due to contact with the large mesh. This might be potentially useful if a reoperation is needed for vascular surgery, organ transplant, or lymph node dissection (28). This extensive dissection then allows the wide and easy deployment of the prosthesis’s upper and external parts. This might explain our low recurrence rate.
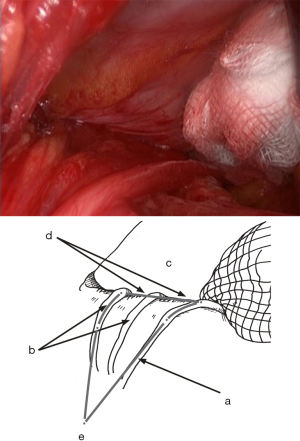
Placing the prosthesis
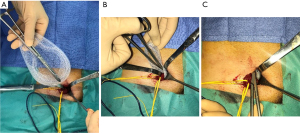
We utilize a mesh with peripheral reinforcement, available in two sizes: medium (3.4×5.6 inches) and large (4.0×6.2 inches) (Figure 3). The dissected preperitoneal space is kept open with three retractors. One retractor lifts the anterior abdominal wall, protecting the epigastric vessels, while the other two long and narrow retractors push back the visceral bag and the bladder. To prepare the prosthesis’s introduction, we use an atraumatic clamp (similar to dressing forceps) that gauges the distance between the retro-pubic region and the incision (Figure 19A). The prosthesis is grasped with the atraumatic forceps at its lower and median edge’s middle part and introduced through the incision, parallel to the inguinal ligament, up to the retro-pubic area with consideration to the measurement made earlier (Figure 19B). The same forceps grasps the prosthesis’s upper and lateral part and introduces it into the preperitoneal dissection area’s upper and lateral parts (Figure 19C). The prosthesis’s lower end is placed behind the pubis, and its upper end is placed near the psoas muscle. The prosthesis is thus partially deployed in the dissection space. Its deployment is completed using retractors, a finger or forceps. The prosthesis’s correct position can be controlled and improved using a spatula instrument, possibly removing a fold from the prosthesis, optimizing the proper deployment of its periphery (Figure 20). When the prosthesis’s positioning is satisfactory, the spermatic cord is reintroduced under the external oblique muscle fascia. The prosthesis is not fixed, however, it can be a discussion point for specific cases (similar to giant hernias with weakened tissues due to corticosteroid therapy, radiotherapy, etc.). With the prosthesis in place, the surgeon notices the deep inguinal ring partially closes spontaneously, “like a sphincter.” It’s usually unnecessary to suture the musculo-fascial plane. Exceptionally, in the case of a large hernia, suturing the TF with a slowly absorbable suture might be beneficial to close the deep inguinal orifice around the spermatic cord. In a massive medial hernia context, the excess TF can also be sutured to avoid a seroma. When closing the external oblique aponeurosis, care is taken to avoid the ilio-inguinal nerve. The subcutaneous membranous plane is closed with an absorbable 4-0 (1.5) thread non-interrupted suture. Two reversing stitches with the same thread close the superficial adipose plane, and adhesive strips are applied to the skin (Figure 21). An adhesive bandage protects the adhesive strips.
Results
Missing data, loss of follow-up
For pre, per and immediate postoperative data, the loss was negligible. Given our follow-up policy and patient education, the rate of loss to follow-up remains low. However, after five failed contact attempts at different times on different days (by the CRA), patients were considered lost to follow-up. Those who declined participation in the clinical control or telephone interview were recorded separately as potential negative outcomes. Results pertain to primary hernias operated on during scheduled surgeries using the MOPP technique and with a minimal one-year outcome from September 2011 to 29 April 2019, after excluding previously mentioned contraindications.
Demographics, pain status at baseline and per operative data are detailed in Table 1
Table 1
| Technique minimal open preperitoneal | Data, n (%) or mean ± SD |
|---|---|
| Cases | 1,401 (100.0) |
| Males/females | 1,258 (89.80)/ 143 (10.20) |
| Age (years) | 68.33±14.22 |
| BMI (kg/m2) | 24.54±2.8 |
| American Society of Anesthesiologists classification | |
| Missing data | 0 |
| ASA 1 | 579 (41.33) |
| ASA 2–3 | 822 (58.67) |
| Emergency surgery | 0 |
| Preoperative pain status (baseline) | |
| Preoperative Visual Analog Scale missing data | 445 (31.76) a |
| VAS, N=956 | 2.772±2.65 |
| VAS median for patient VAS >0 | 3.922±2.65 |
| VAS 0 | 373 (39.0) |
| VAS 1–3 | 292 (30.54) |
| VAS 4–6 | 184 (19.24) |
| VAS 7–10 | 107 (11.19) |
| Verbal rating scale | |
| Missing data | 0 |
| No pain (asymptomatic) | 359 (25.63) |
| Any pain or discomfort | 1,042 (74.37) |
| Mild pain (discomfort) | 423 (40.59) |
| Moderate pain | 356 (34.16) |
| Severe pain | 259 (24.85) |
| ‘Uncommon’ pain | 4 (0.38) |
| Groin hernia location | |
| Missing data | 4 (0.28) |
| Lateral | 893 (63.9) |
| Medial | 517 (37.0) |
| Lateral + medial | 44 (3.15) |
| Femoral total | 53 (3.80) |
| Femoral only | 32 (2.29) |
| Femoral + lateral | 19 (1.36) |
| Femoral + medial | 2 (0.14) |
| Mesh size | |
| Missing data | 1 (0.07) |
| ASPIDE M.O.P.P. (SURGIMESH®1)-U80914p medium (9 cm × 14 cm) | 506 (36.14) |
| ASPIDE M.O.P.P. (SURGIMESH®1)-U81116p large (11 cm × 16 cm) | 396 (28.28) |
| BARD-Polysoft 0130030 medium (7.5 cm × 14 cm) | 5 (0.35) |
| BARD-Polysoft 0130040 large (9.5 cm × 16 cm) | 37 (2.64) |
| BARD-Onflex 0115410 medium (8.6 cm × 14.2 cm) | 48 (3.42) |
| BARD-Onflex 0115411 large (10.2 cm × 15.7 cm) | 408 (29.14) |
| Total medium | 559 (39.90) |
| Total large | 841 (60.10) |
| Mesh fixation | |
| Missing data | 1 (0.07) |
| No fixation | 1,400 (99.93) |
| Operating time (minutes) | 40.17±14.1 |
a, preoperative VAS was not searched for previous patients. VAS, Visual Analog Scale.
Total patients: 1,401 (no missing data)—male: 1,258 (89.80%), female: 143 (10.20%). Average age: 68.33 years (±14.22), average BMI: 24.54 kg/m2 (±2.8), ASA I: 579 (41.33%), ASA II & III: 822 (58.67%). VAS: 60.97% of hernias registered a VAS score between 1 to 10, while 39% of hernias were asymptomatic. VRS: 25.63% were asymptomatic, 74.37% reported some pain or discomfort. Groin hernia location (missing data: 4)—lateral: 893 (63.9%), medial: 517 (37%), femoral (total): 53 (3.80%), or combined.
About 60% used large mesh (4.0” × 6.2”), 40% used medium mesh (3.4” × 5.6”). Mesh fixation was not employed. Average operative time: 40.17±14.1 minutes.
Hospital stays, postoperative course, and early outcomes (up to day 30) are detailed in Table 2
Table 2
| MOPP technique | Data, n (%) or mean ± SD |
|---|---|
| Postoperative course | |
| Studied cases | 1,401 (100.0) |
| Postoperative pain VAS 0–10 | |
| Missing data, D1; D8 | 445 (31.76); 31 (2.21) |
| Median at D1; D8 | 2.772±2.65; 1.543±1.75 |
| Postoperative complications (< D30), N=77 | |
| Missing data | 5 (0.35) |
| General | 20 (1.43)a |
| Surgical site occurrence | 57 (4.08)b |
| Deep infection (peri-prosthetic) | 0 |
| Death | 0 |
| Reoperation | 0 |
| Including mesh removal | 0 |
| Dindo-Clavien classification | |
| I–II | 77 (5.51) |
| IIIb | 0 |
| IV | 0 |
| V | 0 |
| Hospital stays | |
| Missing data | 0 |
| Outpatients | 1,316 (93.93) |
| Inpatients | 85 (6.06) |
| D-case not proposed | 62 (4.42) |
| D-case proposed but failed | 23 (1.64) |
| Hospital stays for inpatients (days) | 1.57±2.04c |
| Follow up at D30 | |
| Missing data | 173 (12.34) |
| Studied cases | 1,228 (87.65) |
| Median VAS 0–10 at D30 | 0.587±1.2 |
| VAS | |
| Median VAS for patient VAS >0 | 2.30±1.58 |
| VAS 0 | 915 (74.51) |
| VAS 1–3 | 260 (21.17) |
| VAS 4–6 | 43 (3.50) |
| VAS 7–10 | 10 (0.81) |
| Symptom at 1 month, N=1,250 | |
| Missing data | 151 (10.78) |
| No pain (asymptomatic) | 919 (73.52) |
| Any pain or discomfort | 331 (26.48) |
| Mild pain (discomfort) | 301 (24.08) |
| Moderate pain | 21 (1.68) |
| Severe pain | 9 (0.72) |
| Symptom at 3 months | |
| Patients seen again at 3 months | 181 (12.92) |
| Average VAS | 0.862±1.73 |
| Average VAS for patients symptomatic | 2.754±1.84 |
| Patients still symptomatic | 56 (4.0) |
| More symptoms than pre-operative condition | 20 (1.43) |
| Less symptoms than pre-operative condition | 36 (2.57) |
| Chronic pain | 20 (1.43) |
a, injection site phlebitis (n=1), phlebitis (n=2), hypoesthesia (n=8), urinary retention (n=5), other minor (n=4); b, subcutaneous seromas or hematomas healing spontaneously (n=50), infected superficial infection (n=2), not infected deep hematomas (n=5); c, one patient presented a decompensation of a Parkinson’s disease, hospitalization in a medical service during 16 days. D, day; MOPP, minimal open preperitoneal; VAS, Visual Analog Scale.
Out of 1,401 patients (no missing data): 93.93% were outpatients; No significant post-operative complications with only Dindo-Clavien grades I and II noted; no reoperations; a very low level of post-operative pain at day 30 with a VAS score of [0–3] in 95.68% of cases; severe pain was noted in 0.72% of cases.
At 3 months (chronic pain): Table 2. Of the 331 patients with a VAS score greater than 0 at 1 month, 181 were seen at 3 months. Their average VAS score was 0.862±1.73. The average VAS score of the 56 patients still symptomatic at 3 months was 2.754±1.84. Among these, 20 reported symptoms more severe than their pre-operative condition, while 36 reported milder symptoms. Thus, the rate of identified chronic pain in this study is 1.43% (20 out of 1,401).
Follow-up and late outcomes are detailed in Table 3
Table 3
| Minimal open preperitoneal | Data, n (%) or mean ± SD |
|---|---|
| Lost to follow-up | 162 (11.56) |
| Mean follow-up (days) | 1,236±603 |
| Follow-up ≥1 year | 1,239 (88.43) |
| Phone questionnaire completed | 983 (79.33) |
| Clinical visit | 256 (20.66) |
| Identified recurrence | 1 (0.08) |
| Late complications | |
| Testicular atrophy | 0 |
| Late superficial infection operated | 1 (0.08) |
| Late sepsis or chronic sinus operated twice (no recurrence) | 1 (0.08) |
| In another center: abscessed sigmoid diverticulosis, prosthesis removal | 1 (0.08) |
| In another center: meshoma, prothesis removal, recurrence reoperated | 1 (0.08) |
| Bowel obstruction or erosion | 0 |
| Total reintervention for four patients | 5 (0.36) |
| Total of recurrences | 2 (0.14) |
Of the 1,239 hernias reviewed with a minimum of 365 days of follow-up, 162 (11.56%) were lost to follow-up after 365 days. The review process was conducted via phone with self-palpation during coughing (79.33%) or at a medical office (20.66%). Results: out of the 1,239 hernias, 5 reinterventions were needed for 4 patients (0.36%), with a total recurrence rate of 2 patients (0.14%). No cases of testicular atrophy or intraperitoneal complications were noted.
Symptomatic patients, PROM are detailed in Table 4
Table 4
| MOPP technique (minimal outcome: 24 months) | Data, n (%) |
|---|---|
| Q1. Since your operation does your abdominal wall seem: | |
| Missing data | 194 (13.84) |
| Solid | 1,203 (99.67) |
| Not solid | 4 (0.33) |
| Q2. Do you have a new hernia or bulge in the operated groin? | |
| Missing data | 193 (13.77) |
| No | 1,200 (99.34) |
| Yes | 8 (0.66) |
| Q3. Do you currently feel any pain or local discomfort? | |
| Missing data | 196 (13.99) |
| No (asymptomatic) | 1,121 (93.02) |
| Yes | 84 (6.97) |
| Mild pain or discomfort | 58 (4.81) |
| Moderate pain | 26 (2.158) |
| Severe pain | 0 |
| Q4. These symptoms | N=84 |
| Missing data | 0 |
| Do not interfere with your daily life | 75 (6.224) |
| Allow to pursue the ongoing activity | 6 (0.497) |
| Cause a temporary interruption of your activity | 2 (0.165) |
| Prevent certain activities (impairment) | 1 (0.082) |
| Q5. These symptoms are | |
| Missing data | 1 (1.19) |
| Less bothersome than the hernia | 77 (6.4) |
| More bothersome than the hernia | 6a (0.50) |
| Yes (please specify) | 0 |
| Q6. How do you assess the result of your hernia operation | |
| Missing data | 205 (14.63) |
| Excellent or good | 1,185 (99.08) |
| Medium | 10 (0.836) |
| Bad | 1b (0.08) |
a, in these cases the result is judged by the patient: excellent [2], good [3], bad [1]; b, superficial infection operated with a good result, having caused temporary pain prohibiting certain activities but completely resolving. MOPP, minimal open preperitoneal.
Using the PROMs procedure, patients answered questions as detailed in Table 4. Out of 1,401 patients, 196 had missing data (13.99%). The results demonstrate the patients’ overall positive perception: 99.67% felt the wall was solid, 0.66% noticed a bulge, and 6.97% reported some pain or local discomfort with none experiencing severe pain. These symptoms were less bothersome than the hernia for 84 cases and more bothersome for 6 cases. Overall, 99% of the patients rated their experience as excellent or good.
Discussion
Regarding the technique, modifications to the TIPP technique have enabled the use of a larger prosthesis in most cases, measuring 10.2 cm × 15.7 cm, through a small incision. These technical adjustments, based on identifying the fascia transversalis at two levels as previously described, have standardized critical steps of the procedure, making them more straightforward. This standardization facilitates teaching, as evidenced by the positive outcomes presented. However, the reproducibility of this technique remains to be proven. While we might be optimistic based on the outcomes of analogous techniques like TIPP and TREPP as supported by cited studies, we cannot overlook the challenges posed by the learning curve. Merola et al. (29) suggest that a surgical residency’s learning curve starts around 40 hernia repairs using the Lichtenstein method. This concept is extensively discussed in “International guidelines for groin hernia” by The HerniaSurge Group (23), where they mention: a learning curve of 300 cases at the Shouldice Institute for the “Shouldice” technique. A longer learning curve for laparoendoscopic hernia repair, especially TEP, than for the open Lichtenstein method, ranging between 50 and 100 procedures. TAPP has a shorter learning curve than TEP. From my experience, the learning curve mainly results in extended procedure durations, not necessarily inferior initial results. Given the remarkably low rates of complications, chronic pain, and recurrences, this assumption seems plausible, but it does warrant specific investigation. For novice surgeons, during the initial learning phase, I recommend starting with an incision similar in size to the one used for the Lichtenstein technique (approximately 6 cm). This approach simplifies the dissection of anatomical planes, including the cord’s parietalization. Over time, they can then progressively decrease the incision size and operation duration. Currently, I cannot specify the number of procedures required to surpass the learning curve, but observations of surgeons undergoing training should be able to provide an accurate gauge over time. While this article meticulously defines the MOPP technique and its outcomes, it is currently not feasible to compare it directly with other methods due to the lack of comparative studies. However, drawing parallels to other preperitoneal techniques suggests that MOPP’s outcomes might align with other open or endoscopic methods such as TREPP, TEP, and TAPP. Hurel and colleagues (15) support this assumption in their conclusion from a recent propensity score matching analysis comparing 1-year postoperative chronic pain using Lichtenstein, TIPP, TAPP, and TEP techniques. Their findings highlight Lichtenstein’s clear disadvantage and an indistinguishable difference between TIPP, TAPP, and TEP.
To further explore the potential advantages of the open preperitoneal approach, including MOPP, consider these studies: Reinhorn and colleagues (13) emphasize the potential benefits of open posterior mesh placement (TREPP) over endoscopic repair in terms of short-term QOL and seroma formation, with equivalent hernia recurrence rates. Agarwal et al. (14) show the advantages of TREPP/OPP over Lichtenstein regarding patient-reported QoL, sustained for a year, and reduced opioid consumption 30 days post-surgery. Zwols (16) and Koning (18) also highlight the superiority of preperitoneal techniques over the Lichtenstein method. Conversely, Posthuma and colleagues (17) argue that TEP offers better outcomes than TIPP, resulting in reduced postoperative pain and wound complications, even though recurrence rates and reoperations are comparable between the two. But no significant differences in QOL, reoperations, recurrence rate, and readmission within 30 days were observed. To validate the hypothesis that MOPP should yield results analogous to other open preperitoneal (TREPP) or endoscopic (TEP, TAPP) methods, I plan to conduct a study comparing MOPP to TIPP using a propensity test on a vast patient pool. Similarly, another study will compare MOPP with the TREPP technique.
Limitations concerning the technique
The author has specifically devoted his activity to being a groin hernia surgeon using the preperitoneal route for 30 years. This makes it easier for him to approach this particular type of intervention compared to surgeons who do not have experience with the open preperitoneal route. However, the author has focused his entire surgical approach on making the MOPP technique reproducible. This approach faces the reactions of surgeons in training or those already experienced who come to learn the technique in the operating room. If the technique, as practiced by the author, uses specific instrumentation, it can be replaced by basic instruments used in general surgery. Regarding the MOPP technique, the primary weak point is identical to that of the TIPP technique. The MOPP approach is not a purely posterior approach, unlike the Ugahary technique, the TREPP technique, or endoscopic techniques. Therefore, there is an anterior dissection through the inguinal canal before entering the preperitoneal space, which by definition is completely dissected. The anterior and posterior spaces are heavily remodeled during the same procedure. But this theoretically significant disadvantage is counterbalanced by the fact that recurrences are very rare. Reoperations after MOPP recurrence are difficult and time-consuming. For the patients in this study, the two recurrences were treated using the Lichtenstein technique, without complications, and with very good results after more than a year of follow-up. In the period following this study, two new recurrences were also reoperated on under challenging anterior dissection conditions (by Lichtenstein), but also with good results. For recurrent hernias previously operated on via an anterior approach (no mesh or Lichtenstein), I use the Ugahary technique because it is a purely posterior approach, like TREPP and the endoscopic technique TEP. I had introduced the Ugahary technique in France in 2005 and used it for all primary groin hernias with good results. it was, however, challenging to teach the technique and it didn’t seem reproducible enough. This was most likely because of the preperitoneal approach, which was not known to most surgeons at that time and because of the absence of specifically dedicated prostheses. This is the justification for the creation of the MOPP technique.
Limitations in relation to the prosthetic material
As shown in this study, it is preferable to use a specific prosthesis that is easy to introduce and unroll through the small incision. This prosthesis must not be too flexible and should have a peripheral reinforcement or even a hem to facilitate its unrolling and control its positioning. The prostheses used by the endoscopic route are generally very difficult to unroll anteriorly or require specific technical resources. One of the goals of the technique is to make a small incision (minimal access); which can be a difficulty during learning. It is then possible for beginners to make an incision as in the Lichtenstein technique and to reduce it with experience.
Limitations regarding the data and the results
This is a monocentric study with a single operator particularly invested in groin hernia surgery. The results could differ in the population of general surgeons. Nevertheless, these data have strong internal validity because they were prospectively collected, and all consecutive patients were registered with a high follow-up rate.
Teaching and development of the technique
Several parameters precisely developed in our study make it possible to propose the diffusion of this technique. Firstly, notions of surgical anatomy concerning the TF can help, and cadaveric dissections can be beneficial. Secondly, the use of prosthetic material dedicated to the procedure. Thirdly, we have benefited for several years from feedback from experienced and young colleagues who have participated in workshops with us. Thus, after a constant evolution of the technique during the first years after its conception, it is now mature. The technique can be offered to experienced or novice surgeons within the framework of adequate teaching.
Conclusions
The MOPP technique offers minimal access and is minimally invasive. It is a variant of the TIPP technique, utilizing simple yet specifically dedicated instrumentation (Figure 1). The primary principles involve unrolling a large prosthesis in the preperitoneal space. This suture-less and tension-free technique requires a small (3–4 cm) incision near the deep inguinal ring (Figure 2). A thorough understanding of the surgical anatomy, particularly the TF, facilitates and optimizes the key steps of the MOPP technique. All primary groin hernias in adults can be addressed with this method. Fragile or elderly patients fall within the standard indications, with procedures typically performed as outpatient. Both the recurrence rate and the level of chronic pain observed are very low. The objective of this work is to introduce a new open preperitoneal technique that is more straightforward to teach and, consequently, more reproducible. This assertion remains to be validated by other practitioners.
Acknowledgments
The author wishes to express gratitude to the Club-Hernie members and the independent clinical research assistant for their rigorous scientific approach and dedication to the registry.
Funding: None.
Footnote
Reporting Checklist: The author has completed the STROBE reporting checklist. Available at https://ales.amegroups.com/article/view/10.21037/ales-23-37/rc
Data Sharing Statement: Available at https://ales.amegroups.com/article/view/10.21037/ales-23-37/dss
Peer Review File: Available at https://ales.amegroups.com/article/view/10.21037/ales-23-37/prf
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://ales.amegroups.com/article/view/10.21037/ales-23-37/coif). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). As this study is a monocenter, monoperator observational retrospective study, embedded in a registry which complies with the General Data Protection Regulation (GDPR) and the French “Commission Nationale de l’Informatique et des Libertés” (National Commission for Information Technology and Liberties) CNIL (MR0003) requirements, IRB approval is not required. Informed consent was taken from all the participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nyhus LM, Condon RE, Harkins HN. Clinical experiences with preperitoneal hernial repair for all types of hernia of the groin, with particular reference to the importance of transversalis fascia analogues. Am J Surg. 1960;100:234-244. [Crossref] [PubMed]
- Corti D. Nylon et plastie Thèse Marseille. 1949.
- Acquaviva DE, Bourret P, Corti D. Considérations sur l’emploi des plaques de nylon dites crinoplaques comme matériel de plastie pariétale. Cong fr de chirurgie 1949; Paris. Masson: 453.
- Soler M, Giuly JA, Bendavid R. Don Eugène Acquaviva (1897-1976): The real founder of modern parietology using a prosthetic mesh! Hernia 2021;25:1733-5. [Crossref] [PubMed]
- Wantz GE, Fischer E. Unilateral giant prosthetic reinforcement of visceral bag: Preperitoneal hernioplasties with Dacron. In Bendavid R, Ed, Abdominal wall hernias. Principles and management. New York, Springer-Verlag, 2001 pp 396-400.
- Wantz GE. Giant prosthetic reinforcement of the visceral sac. Surg Gynecol Obstet 1989;169:408-17. [PubMed]
- Stoppa R, Petit J, Abourachid H. Original procedure method of groin hernia repair interposition without fixation of Dacron tulle prosthesis by subperitoneal median approach. Chirurgie 1973;99:119-23. [PubMed]
- Ugahary F, Simmermacher RKJ. Groin hernia repair via a grid-iron incision: an alternative technique for preperitoneal mesh incision. Hernia 1998;2:123-51. [Crossref]
- Soler M, Ugahary F. e-memories of the National Academy of Surgery, 2004;3: 28-33. Available online: https://e-memoire.academie-chirurgie.fr/ememoires/005_2004_3_3_28x33.pdf
- Pelissier EP. Inguinal hernia: preperitoneal placement of a memory-patch ring by anterior approach. Preliminary experience. Hernia 2006;10:248-52. [Crossref] [PubMed]
- Gillion JF, Pelissier E. Transinguinal Preperitoneal (TIPP) Inguinal Hernia Repair Using a Totally Extraperitoneal, Parietalized, Memory-Ring Patch. In: Campanelli G, (eds) The Art of Hernia Surgery. Springer, Cham., 2018
- Akkersdijk WL, Andeweg CS, Bokkerink WJ, et al. Teaching the transrectus sheath preperitoneal mesh repair: TREPP in 9 steps. Int J Surg 2016;30:150-4. [Crossref] [PubMed]
- Reinhorn M, Fullington N, Agarwal D, et al. Posterior mesh inguinal hernia repairs: a propensity score matched analysis of laparoscopic and robotic versus open approaches. Hernia 2023;27:93-104. [Crossref] [PubMed]
- Agarwal D, Bharani T, Fullington N, et al. Improved patient-reported outcomes after open preperitoneal inguinal hernia repair compared to anterior Lichtenstein repair: 10-year ACHQC analysis. Hernia 2023;27:1139-54. [Crossref] [PubMed]
- Hurel R, Bouazzi L, Barbe C, et al. Lichtenstein versus TIPP versus TAPP versus TEP for primary inguinal hernia, a matched propensity score study on the French Club Hernie Registry. Hernia 2023;27:1165-77. [Crossref] [PubMed]
- Zwols TLR, Slagter N, Veeger NJGM, et al. Transrectus sheath pre-peritoneal (TREPP) procedure versus totally extraperitoneal (TEP) procedure and Lichtenstein technique: a propensity-score-matched analysis in Dutch high-volume regional hospitals. Hernia 2021;25:1265-70. [Crossref] [PubMed]
- Posthuma JJ, Sandkuyl R, Sloothaak DA, et al. Transinguinal preperitoneal (TIPP) vs endoscopic total extraperitoneal (TEP) procedure in unilateral inguinal hernia repair: a randomized controlled trial. Hernia 2023;27:119-25. [Crossref] [PubMed]
- Koning GG, Keus F, Koeslag L, et al. Randomized clinical trial of chronic pain after the transinguinal preperitoneal technique compared with Lichtenstein’s method for inguinal hernia repair. Br J Surg 2012;99:1365-73. [Crossref] [PubMed]
- Romain B, Gillion JF, Ortega-Deballon P, et al. Patient’s satisfaction at 2 years after groin hernia repair: any difference according to the technique? Hernia 2018;22:801-12. [Crossref] [PubMed]
- Soler M. The minimal open preperitoneal (MOPP) approach to treat the groin hernias, with the history of the preperitoneal approach. Ann Laparosc Endosc Surg 2017;2:133. [Crossref]
- Soler M. (2018) Minimal Open Preperitoneal (MOPP) Technique. in: Campanelli G. editor. The Art of Hernia Surgery A Step-by-Step Guide. Milan: Springer, 2018: 319-326 eBook ISBN 978-3-319-72626-7
- Club Hernie | Groupement Chirurgical. Available online: https://www.club-hernie.com/fr/
- International guidelines for groin hernia management. Hernia 2018;22:1-165. [Crossref] [PubMed]
- Fruchaud H, Translated by Bendavid R. Bendavid R (ed). The surgical anatomy of hernias of the groin, Toronto: 2006 p 131.
- Schleip R, Hedley G, Yucesoy CA. Fascial nomenclature: Update on related consensus process. Clin Anat 2019;32:929-33. [Crossref] [PubMed]
- Loriau J. Anatomy of the Inguinal Region. In: Campanelli G. (ed) The Art of Hernia Surgery a step-by-step guide. Springer, 2018 pp. 159-173.
- Fruchaud H, Translated by Bendavid R. 2006. Bendavid R (ed). The surgical anatomy of hernias of the groin, Toronto: 2006 p 54-55.
- Stoppa R, Diarra B, Mertl P. The retroparietal spermatic sheath - An anatomical structure of surgical interest. Hernia 1997;1:55-9. [Crossref]
- Merola G, Cavallaro G, Iorio O, et al. Learning curve in open inguinal hernia repair: a quality improvement multicentre study about Lichtenstein technique. Hernia 2020;24:651-9. [Crossref] [PubMed]
Cite this article as: Soler M. The minimal open preperitoneal (MOPP) approach for treating groin hernias: technique, indications, and results. Ann Laparosc Endosc Surg 2024;9:3.