Outcomes of laparoscopic vs. open gastrectomy for Siewert type II/III adenocarcinoma of the esophagogastric junction: a systematic review and meta-analysis
Highlight box
Key findings
• This study found that laparoscopic gastrectomy (LG) for Siewert type II/III adenocarcinoma of the esophagogastric junction (AEG) was more conducive to postoperative rehabilitation and improved overall survival (OS) and disease-free survival (DFS).
What is known and what is new?
• Compared with open gastrectomy (OG), LG is associated with less blood loss, fewer postoperative complications, more lymph nodes dissected, and longer operation time.
• This study systematically analyzed the postoperative survival of these patients by calculating OS, DFS, and hazard ratio, and found that LG showed a significant advantage in OS and DFS compared with OG.
What is the implication, and what should change now?
• Total gastrectomy is a reliable method for the treatment of AEG patients, and has certain advantages in postoperative recovery and long-term prognosis. However, more high-quality studies are needed to verify these findings, especially the difference between the two surgical modalities in the patients after neoadjuvant therapy.
Introduction
Gastric cancer (GC) is one of the most common malignant tumors, and it is the third leading cause of cancer death in the world (1). From a global perspective, East Asian countries have a high incidence of GC, among which China accounts for half of the new cases of GC in the world every year (2,3). It is worth noting that the incidence of adenocarcinoma of esophagogastric junction (AEG) is gradually increasing, which poses a serious threat to the physical and mental health of people (4). Due to the special location of AEG, the treatment and surgical methods applied to AEG are more complicated than those applied at other GC sites, and no consensus has so far been reached.
Although there is much debate about the best treatment for patients with AEG, surgery remains the cornerstone of different strategies (5). Overall, Siewert type I is treated as esophageal cancer, while Siewert II/III type AEG tends to be treated as proximal GC (6). Since Kitano et al. reported the first laparoscopic radical gastrectomy of distal GC in 1994 (7), this technique has been widely used in the treatment of GC, because it not only provides good short-term outcomes but also has a comparable tumor prognosis (8). However, current studies on laparoscopic gastrectomy (LG) vs. open gastrectomy (OG) mainly focus on the treatment of distal GC. The KLASS-01 study from Korea demonstrated that laparoscopic distal gastrectomy has a lower incidence of postoperative complications, especially wound complications, than open distal gastrectomy for stage I GC (9). The CLASS-01 randomized clinical trial demonstrated that in patients with locally advanced GC indicated by preoperative clinical stage, laparoscopic distal gastrectomy was comparable to open distal gastrectomy with disease-free survival (DFS) (10). These findings proved that laparoscopic surgery was safe and reliable, and provided a strong reference for us to further explore the application of laparoscopic technology in patients with AEG.
The surgical treatment of AEG is more complicated than that of distal GC, and there are differences in the selection of surgical methods, the length of esophageal dissection, and the scope of gastrectomy, especially lymph node (LN) dissection and other aspects. Chen et al. (11) analyzed the incidence of LN metastasis in each station of Siewert II/III type AEG and found that the LN metastasis rate was 10% in No. 110 and 1–3% in No. 111, which also confirmed the importance of lower mediastinal lymphadenectomy. Laparoscopy has the advantage of being performed in a narrow space, which allows for finer dissection of the mediastinal LNs. Theoretically, the number of dissected LNs increases, thus affecting the survival rate of patients (12).
The intraoperative outcomes, number of dissected LNs, postoperative outcomes, overall survival (OS), and DFS were analyzed in the two groups, and the advantages and disadvantages of the two surgical methods were comprehensively compared. In addition, we also compared OS and DFS in detail, which was lacking in previous meta-analyses.
Although these studies have reported differences between the two procedures, reliable evidence is still lacking due to the small sample size of the studies. The purpose of the study was to investigate the clinical efficacy and postoperative survival differences between LG and OG in patients with Siewert type II/III AEG. On this basis, evidence-based medical evidence is provided for the selection of clinical treatment. We present this article in accordance with the PRISMA reporting checklist (available at https://ales.amegroups.com/article/view/10.21037/ales-23-39/rc).
Methods
Literature search
PubMed, Embase, Web of Science, Cochrane Central Register of Controlled Trials, and Wanfang Database were searched from database inception to April 2022. The searches were performed based on the PICOS (population, intervention, comparison, outcomes, and study design) criteria. The search strategy included ((“Esophagogastric Junction”) OR (((((Junction, Esophagogastric) OR (Gastroesophageal Junction)) OR (Gastroesophageal Junctions)) OR (Junction, Gastroesophageal)) OR (Junction, Gastroesophageal))) AND ((open) OR (laparoscopic))) AND (gastrectomy). Figure 1 shows the study selection process in our meta-analysis.
Inclusion criteria
All randomized controlled trials (RCTs), prospective and retrospective studies that met the following criteria were included: (I) studies focusing only on patients with Siewert type II/III AEG; (II) a comparison between LG and OG for patients with AEG; and (III) at least one of the intraoperative outcomes, number of dissected LNs, postoperative outcomes, OS, and DFS should be reported.
Exclusion criteria
Studies were excluded if they were any of the following: (I) studies without sufficient data on the outcomes mentioned, such as case reports, literature reviews, and conference abstracts; (II) studies without available full text; (III) repeated studies based on the same author or center; and (IV) studies without sufficient data on patients with Siewert type II/III AEG.
Data extraction
Data from the included studies were extracted and summarized independently by two researchers. If there was no consensus, the study was reviewed by a third researcher. The following data were extracted from each study: (I) basic study information, including author, date, country, center of study, and sample size; (II) surgery-related information, including specific surgical methods (laparoscopic or open, proximal or distal gastrectomy), Siewert type, blood loss, operation time, number of dissected (LNs), and number of LN metastases; (III) perioperative outcomes, including postoperative hospital stay, recovery time of gastrointestinal function, time to first ambulation, time to first liquid diet, postoperative complications, and mortality in 30 days; and (IV) long-term outcomes, including OS, DFS, hazard ratio (HR), and Kaplan-Meier survival curves.
Quality assessment
Two researchers independently evaluated the articles, and in case of disagreement, a third researcher was invited for evaluation. The quality of all studies was evaluated by the Newcastle-Ottawa Scale (13). If the study had a score of 6 or higher, it was defined as a high-quality study. Based on this evaluation, all the articles in this meta-analysis were graded as high-quality articles (Table 1).
Table 1
| Study | Selection | Comparability | Outcomes | Quality scores | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ① | ② | ③ | ④ | ⑤ | ⑥ | ⑦ | ⑧ | ⑨ | ||||
| Hong, 2013 | * | * | * | * | * | * | 6 | |||||
| Huang, 2017 | * | * | * | * | * | * | * | * | 8 | |||
| Shi, 2018 | * | * | * | * | * | * | * | * | 8 | |||
| Zhang, 2018 | * | * | * | * | * | * | * | 7 | ||||
| Jia, 2018 | * | * | * | * | * | * | 6 | |||||
| Wang, 2019 | * | * | * | * | * | * | 6 | |||||
| Zhao, 2019 | * | * | * | * | * | * | * | * | 8 | |||
| Lee, 2019 | * | * | * | * | * | * | * | * | 8 | |||
| Sugita, 2021 | * | * | * | * | * | * | * | * | 8 | |||
| Zhang, 2021 | * | * | * | * | * | * | 6 | |||||
| Lin, 2022 | * | * | * | * | * | * | * | * | * | 9 | ||
| Song, 2022 | * | * | * | * | * | * | * | * | * | 9 | ||
*, one point. ①, representativeness of exposed cohort; ②, selection of nonexposed cohort; ③, ascertainment of exposure; ④, outcome of interest was not present at start of study; ⑤, study controls for age, sex, and marital status; ⑥, study controls for any additional factors; ⑦, assessment of outcomes; ⑧, follow-up long enough for outcomes to occur; ⑨, adequacy of follow-up. NOS, Newcastle-Ottawa Scale.
Statistical analysis
Review Manager 5.3 software was used to analyze the data extracted in the study. Continuous variables were effectively evaluated by weight mean difference (WMD) and odds ratio (OR) with 95% confidence interval (CI). Similarly, dichotomous variables were evaluated using ORs and 95% CIs. OS, DFS, and HR were used to assess the long-term prognosis of patients. For studies that only provided a Kaplan-Meier curve, we used Engauge Digitizer 12.1 and Get Data Graph Digitizer 2.25 to extract data and reconstruct the curve. The HR and 95% CI between the LG and OG groups were calculated using the Cox proportional hazard regression model. Then, we used Review Manager 5.3 for further analysis. I squared was used to measure the statistical heterogeneity between studies, and I squared values over 50% were considered high heterogeneity. Values of P<0.05 were considered indicative of statistical significance. Generally, a fixed-effects model was performed, and a random-effects model was used unless there was substantial interstudy heterogeneity. We also created funnel plots to identify potential bias.
Results
Study selection and characteristics
After reviewing 1,020 studies, we included 12 articles (14-25) (Table 2) about retrospective studies in this meta-analysis. A total of 301 articles were found to be duplicates and were then removed. After reviewing the titles, abstracts, and types of articles, we found that only 29 articles met the preliminary requirements. Finally, 12 studies involving 2,959 AEG patients were included in this meta-analysis by reviewing the full text.
Table 2
| Study | Country | Year | Group | Samples | Age (years) | Gender (M/F) | BMI (kg/m2) | Siewert type (II/III) | Extent of resection PG/TG | Extent of LN dissection | Tumor stage (I/II/III/IV) |
Tumor size (cm) | ASA (1/2/3) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hong, 2013 | China | 2008–2012 | LG | 100 | 53.23±11.03 | 71/29 | 24.13±2.31 | 100/0 | 0/100 | D2 | 6/53/41/0 | NA | 6/92/2 |
| OG | 104 | 54.45±10.44 | 76/28 | 24.35±11.24 | 104/0 | 0/104 | 5/54/45/0 | NA | 5/96/3 | ||||
| Huang, 2017 | China | 2007–2014 | LG | 171 | 62.4±8.9 | 152/19 | 22.2±2.9 | 87/84 | 0/171 | D2 | 27/47/97/0 | 52.4±21.5 | 103/45/23 |
| OG | 171 | 61.4±10.0 | 152/19 | 21.9±3.0 | 87/84 | 0/171 | 29/42/100/0 | 53.2±23.3 | 103/45/23 | ||||
| Shi, 2018 | China | 2013–2015 | LG | 132 | 60.08±8.37 | 114/18 | 23.13±3.09 | NA | 20/112 | D2 | 28/50/54/0 | NA | NA |
| OG | 264 | 60.54±9.06 | 228/36 | 23.29±3.27 | NA | 40/223 | 40/103/121/0 | NA | NA | ||||
| Zhang, 2018 | China | 2010–2011 | LG | 36 | 62.22±9.93 | 20/16 | NA | 17/19 | 31/5 | D2 | 0/15/21/0 | NA | 21/12/3 |
| OG | 41 | 61.1±9.33 | 24/17 | NA | 19/22 | 34/7 | 0/16/25/0 | NA | 27/10/4 | ||||
| Jia, 2018 | China | 2015–2017 | LG | 48 | 65.8±8.6 | 38/10 | NA | 32/16 | 48/0 | D2 | 16/26/6/0 | NA | NA |
| OG | 68 | 66.4±6.8 | 52/16 | NA | 46/22 | 68/0 | 18/38/12/0 | NA | NA | ||||
| Wang, 2019 | China | 2009–2014 | LG | 32 | 61.9±8.7 | 21/11 | NA | 17/15 | 0/32 | D2 | 11/17/4/0 | 2.73±0.67 | NA |
| OG | 43 | 61.9±6.0 | 23/20 | NA | 27/16 | 0/43 | 17/21/5/0 | 2.94±1.12 | NA | ||||
| Lee, 2019 | Korea | 2003–2015 | LG | 37 | NA | 26/11 | 22.0 (2.8) | 18/19 | 0/37 | D1+/D2 | 5/16/16/0 | NA | 22/44/5 |
| OG | 71 | NA | 46/25 | 22.0 (2.0) | 33/38 | 0/71 | 4/22/45/0 | NA | 18/18/1 | ||||
| Zhao, 2019 | China | 2007–2017 | LG | 468 | 60.42±10.40 | 330/138 | 22.51±2.61 | 150/318 | 0/468 | D2 | 0/37/301/130 | NA | 40/121/307 |
| OG | 217 | 58.99±10.58 | 175/42 | 22.71±2.61 | 45/172 | 0/217 | 0/7/132/78 | NA | 22/26/169 | ||||
| Sugita, 2021 | Japan | 2008–2018 | LG | 50 | 68 [40–86] | 38/12 | 22.7 [15.8–27.0] | 50/0 | 40/10 | D2+ | 26/17/7/0 | 35 [10–80] | 21/28/1 |
| OG | 29 | 65 [41–74] | 26/3 | 22.6 [19.1–27.0] | 29/0 | 6/23 | 8/7/14/0 | 50 [25–80] | 9/17/3 | ||||
| Zhang, 2021 | China | 2010–2019 | LG | 52 | 63.2±8.6 | 44/8 | 22.1±1.3 | NA | 52/0 | D1+ | 40/11/1/0 | NA | NA |
| OG | 61 | 61.2±7.2 | 54/7 | 22.6±1.2 | NA | 61/0 | 37/23/1/0 | NA | NA | ||||
| Lin, 2022 | China | 2004–2015 | LG | 93 | 58.25±9.20 | 75/18 | 21.68±2.05 | 47/46 | 37/56 | D2 | NA | NA | NA |
| OG | 93 | 59.16±10.27 | 70/23 | 21.57±2.92 | 45/48 | 37/56 | NA | NA | NA | ||||
| Song, 2022 | China | 2014–2019 | LG | 382 | 64 [58–69] | 338/44 | 24.45 [22.10–26.70] | 382/0 | 0/382 | D2 | 107/120/155/0 | 3.49±1.60 | 201/164/17 |
| OG | 196 | 63 [59–69] | 174/22 | 24.40 [22.50–27.25] | 196/0 | 0/196 | 49/70/77/0 | 3.69±1.62 | 100/82/14 |
Continuous variables are presented as the mean ± SD, median [range], or n (%). M/F, male/female; BMI, body mass index; PG/TG, proximal gastrectomy/total gastrectomy; LN, lymph node; ASA, American Society of Anesthesiologists; LG, laparoscopic gastrectomy; D2, day 2; NA, not available; OG, open gastrectomy; SD, standard deviation.
Of these, 1,601 patients underwent LG and 1,358 patients underwent OG. The patients included in these studies were mainly from China, with a small number from Japan and South Korea. The detailed screening process is shown in Figure 1.
Intraoperative outcomes
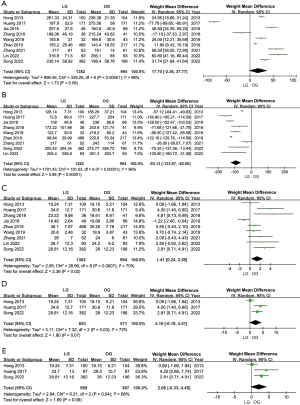
All selected studies recorded the operation time and estimated blood loss, but three articles (16,21,22) were not included in the data analysis because standard deviations (SDs) were not provided. Meta-analysis showed that there was no significant difference in operation time, while the amount of intraoperative blood loss in the LG group was significantly less than that in the OG group (Figure 2A,2B).
Number of dissected LNs
The mean and SD of the number of dissected LNs were clearly recorded in nine articles. Meta-analysis showed that LG was superior to OG in LN dissection (Figure 2C). Due to the difference in the scope of LN dissection between total gastrectomy (TG) and proximal gastrectomy, we classified the included studies again according to the scope of, surgery and then compared the difference between LG and OG. Analysis of the results of three studies showed that more LNs could still be removed during TG under LG (Figure 2D). However, only two (18,23) articles recorded the number of LNs dissected during proximal gastrectomy, and they showed no difference between LG and OG (Figure S1A). Siewert type II AEG tumors are located 1 cm above the esophagogastric junction (EGJ) to 2 cm below, and the surgical space is extremely narrow, so LN dissection is more difficult. By extracting and comparing the data of the Siewert II type separately, we found that LG had a certain trend over OG (WMD =2.06; 95% CI: −0.33, 4.45; P=0.09) (Figure 2E). In addition, two studies (15,25) included the number of positive LNs dissected, but there was no difference (Figure S1B). It is worth noting that a study by Sugita (22) explored laparoscopic mediastinal LN dissection. The results showed that LG vs. OG increased the number of lower mediastinal LNs detected for Siewert type II AEG (1 vs. 0, P=0.002).
Perioperative outcomes
Postoperative hospital stay was recorded in eight articles (14,15,17-20,23,24). This meta-analysis revealed that patients in the LG group had significantly shorter postoperative hospital stays than those in the OG group (WMD =−1.96 days; 95% CI: −2.11, −1.81; P<0.001), with little heterogeneity (I2=49%; P=0.06) (Figure 3A). Seven articles (14,18-20,23-25) reported the recovery time of gastrointestinal function. From the analysis results, the LG group was also superior the OG group, but the heterogeneity was high (I2=98%; P<0.00001), so we adopted the random-effects model (WMD =−0.97; 95% CI: −1.62, −0.31; P=0.004) (Figure 3B). In five articles (15,20,23-25), the time of the first liquid diet was recorded, and there was no obvious difference between LG and OG (WMD =−0.59 days; 95% CI: −1.30, 0.12; P=0.10) (Figure 3C). In five articles (14,15,20,24,25), the time to first ambulation was recorded, and patients in the LG group tended to start moving earlier than patients in the OG group (WMD =−0.84; 95% CI: −1.56, −0.13; P=0.02) (I2=99%; P<0.00001) (Figure 3D). Information on postoperative complications was recorded in all included articles. There was no significant difference among the studies (I2=0%; P=0.88). According to the meta-analysis, the overall incidence of postoperative complications in the LG group was lower than that in the OG group (OR =0.71; 95% CI: 0.58, 0.88; P=0.002) (Figure 3E). Considering the variety of postoperative complications, we conducted further subgroup analysis.
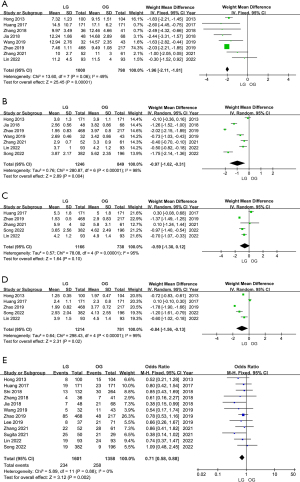
In terms of the incidence of postoperative pneumonia (OR =0.48; 95% CI: 0.28, 0.81; P=0.006) and wound infection (OR =0.62; 95% CI: 0.36, 1.06; P=0.08), LG had certain advantages compared with OG, and the differences among various studies were not significant (Figure S2A,S2B). However, there was no significant difference between LG and OG in the complications of anastomotic site bleeding (Figure S2C), anastomotic stricture (Figure S2D), anastomotic leakage (Figure S3A), abdominal abscess (Figure S3B), intestinal obstruction (Figure S3C), and pancreatic fistula (Figure S3D). In addition, four articles (14,15,20,24) included data mortality within 30 days of surgery and showed no difference between LG and OG (OR =0.47; 95% CI: 0.12, 1.92; P=0.30) (Figure S3E).
Long-term outcomes
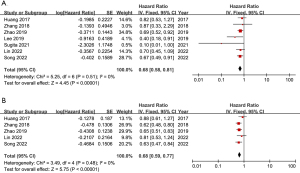
Seven papers included Kaplan-Meier curves for 5-year survival, but some did not provide HR. Therefore, we used Engauge Digitizer 12.1 and Get Data Graph Digitizer 2.25 to extract data and estimate HR. The final meta-analysis showed that the OS in the LG group was significantly better (HR =0.68; 95% CI: 0.58, 0.81; P<0.00001) than that in the OG group, and there was little heterogeneity (I2=0%; P=0.51) (Figure 4A). In addition, there were four articles in which the Kaplan-Meier curves of DFS for 5 years were described. We found that DFS (HR =0.68; 95% CI: 0.59, 0.77; P<0.00001) in the LG group was still better than that in the OG group with statistical significance and low heterogeneity (I2=0%; P=0.48) (Figure 4B).
Sensitivity analysis
Sensitivity analyses identified overall results with significant sources of heterogeneity by removing individual studies from the data and analyzing their effects on patients. These exclusions did not alter the results obtained.
Publication bias
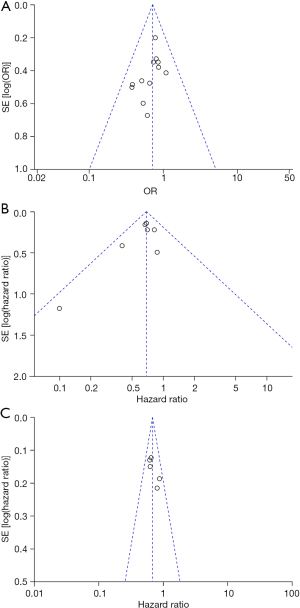
The funnel plots based on postoperative complications, OS and DFS is shown in Figure 5. There was no extensive evidence of publication bias as none of the studies exceeded the 95% CI range.
Discussion
In recent years, increasing attention has been given to the AEG. Compared with the downward trend of the global incidence of GC, the global incidence of AEG is on the rise, and the prognosis is poor (4,26,27). Due to the particularity of the tumor site and biological behavior, the early diagnosis, typing, and treatment of AEG are still controversial at present. The multidisciplinary collaborative treatment model is considered to be the trend and hope in treatment development, while the complete resection of tumors and standardized LN dissection are still the basis of the treatment (5). Although LG has been in use for more than 20 years, its development has been limited because successful reconstruction of the digestive tract is difficult to achieve laparoscopically. However, with technological progress and economic development, the application of laparoscopy in the field of GC has been greatly promoted. The application of laparoscopic radical gastrectomy has gradually expanded from distal gastrectomy to TG. Additionally, RCTs in Korea (KLASS-03) (28), Japan (JCOG1401) (29), and China (CLASS-02) (30) in recent years have demonstrated the safety and feasibility of laparoscopic TG. These studies provided a certain reference for further exploring the application effect of laparoscopy in patients with AEG.
Siewert typing is currently the most widely recognized AEG typing method, and has good guiding importance for tumor staging and the selection of treatment strategies (31). Siewert type II is considered to be a true AEG as, the tumor center is within the area extending 1 to 2 cm around the EGJ, with Siewert type I being 1 to 5 cm above the EGJ and Siewert type III being 2 to 5 cm below the EGJ. The surgical approach for Siewert type II has been controversial due to the proximity of the tumor to the EGJ. In the fifth edition of the Japanese Guidelines for Gastric Cancer (32), the abdominal transhiatal approach (TH) is recommended for AEG with esophageal infiltration <3 cm, while a combined thoracoabdominal approach is recommended for AEG with esophageal infiltration >3 cm, considering the curability. At present, the international view of type III AEG tends to be consistent, and the TH approach is recommended for surgical treatment. The scope of Siewert type II/III AEG resection focused on the choice of TG or proximal gastrectomy. A meta-analysis (33) of Siewert type II/III AEG patients after TG or proximal gastrectomy showed no significant difference in 5-year survival and postoperative complications between the two resection scopes. However, for advanced AEG with a large tumor volume, low location and high risk of peripheral LN metastasis, clinical experts still recommend TG. It was precisely because of the special anatomical location of AEG and the great trauma of traditional surgery that an increasing number of experts have begun to try minimally invasive surgery, especially with the rapid development of laparoscopic technology, which made minimally invasive treatment of AEG possible.
For Siewert II/III AEG, it has been controversial whether LG as a minimally invasive procedure is more advantageous than OG. Although RCTs are ideal cornerstones for meta-analysis, due to factors such as intraoperative variability, learning curve effect, and patient-informed consent, there are currently no high-quality RCTs to compare the advantages and disadvantages of these two surgical methods. However, we collected and collated high-quality multicenter retrospective studies in recent years to preliminarily evaluate the effectiveness and feasibility of LG in patients with AEG. In this study, we compared intraoperative outcomes, number of LNs dissected, perioperative outcomes, and long-term outcomes. From the literature available at present, our study is the most comprehensive and includes the largest number of studies.
In terms of intraoperative conditions, both LG and OG have their advantages. We found that there was significantly less surgical blood loss in the LG group but slightly more operation time than in the OG group. From a technical point of view, laparoscopy has the advantages of visual magnification and a high-definition surgical field of view, to better expose and locate blood vessels and nerves, which also provides a powerful weapon for surgeons to perform fine operation and reduce unnecessary intraoperative injuries. In addition, abdominal incisions of LG tend to be smaller than those of OG, which is one reason for reduced intraoperative blood loss. We also analyzed the reasons for the operation time of LG being slightly longer than that of OG. The main reasons are the higher technical requirements of laparoscopy and the learning curve, which are related to the experience of surgeons, their familiarity with surgical instruments and the cooperation of assistants (34). Although laparoscopy requires a certain amount of time to train, especially in the area of digestive tract reconstruction and LN dissection, our analysis found that experienced teams, such as Professor Huang’s team (15), could operate in less time than required in the OG group. Further advances in surgical techniques, especially those related to stapling devices and new instruments, may further reduce the operation time.
LN metastasis in AEG is associated with poor prognosis, and standardized LN dissection is an important part of surgery (35). LN metastasis in Siewert III type is mainly abdominal, but the rule of LN metastasis in Siewert II type is still under investigation, especially in the aspect of mediastinal LN dissection. Lower mediastinal LN dissection may improve survival in patients with esophagus-predominant AEG (36). Therefore, lower mediastinal LN dissection should be performed for any GC invading the esophagus as recommended by the Japanese guidelines for the treatment of GC (32). Japanese scholar Kurokawa et al. (37) analyzed mediastinal LN metastasis in Siewert type II AEG patients in a multicenter study, and the results suggested that the lower mediastinal LN metastasis rate was approximately 18%, and the longer the invasion distance of the esophagus, the greater the chance of mediastinal metastasis. Theoretically, laparoscopy has the advantages of an enlarged field of vision and a variable angle of view, which is conducive to LN dissection in a narrow space. The results of our meta-analysis were also consistent with this view. In general, the number of LNs dissected in the LG group was significantly higher than that in the OG group. In a separate analysis of Siewert type II lesions, we found that patients in the LG group still exhibited a higher number of LNs dissected. Different tumor sites, different pathological types, different stages, and different surgical techniques must have different LN dissection ranges, which may also be related to the surgical habits of the surgical team. Since our research data came from published research results, and they did not reflect detailed data such as the a of LN and LN dissection scope, we only carried out comparative analysis on the number of LNs dissected (Figure 2C). To make the results less biased, we performed an independent analysis of TG patients and proximal gastrectomy patients and found no statistical difference between the open and endoscopic group (Figure 2D and Figure S1A). In the study we included, only Sugita’s cohort (22) in Japan demonstrated that LG vs. OG increased the number of lower mediastinal LNs detected for Siewert type II AEG (1 vs. 0, P=0.002). However, data from only one cohort cannot be used for further meta-analysis, which is also one of the limitations of this study. Although there is now evidence that laparoscopy can remove more LNs than OG, more clinical trials are needed for lower mediastinal LN dissection. Indeed, the CLASS10 prospective study in China is designed to explore the answer to this question, and I believe that all of this will be verified in the near future.
Postoperative recovery of patients is an important index to evaluate these two surgical methods. Through analysis, the postoperative hospital stay and postoperative recovery time of gastrointestinal function in the LG group were significantly less than those in the OG group, which was mainly related to the low surgical trauma of laparoscopic surgery. Similarly, laparoscopic surgery is associated with a reduced incidence of overall postoperative complications. In the subgroup analysis, pneumonia and wound infection rates were lower in the LG group than in the OG group, but there were no differences in anastomotic leakage, anastomotic stenosis, pancreatic fistula, intestinal obstruction, and abdominal abscess. Because the abdominal incision of laparoscopic surgery is smaller than that of open surgery, the postoperative pain response of patients subjected to LG is smaller, so these patients will cough and expectorate actively, and the time to get out of bed will be shorter, which further reduces the incidence of crash pneumonia and wound infection. In addition, because laparoscopic digestive tract reconstruction has always been a difficult problem and the technical requirements of the surgeons are extremely high, sometimes doctors choose to perform anastomosis at the incision where the specimen is taken, so there is no difference in anastomotic complications between the two groups.
The survival of patients after surgery is the focus of our comparison, and it is also a favorable reference to evaluate the feasibility of surgical methods. Compared with the OG group, AEG patients in the LG group achieved better OS and DFS. The prognosis of patients is related to tumor-node-metastasis (TNM) stage, tumor size and age of patients (38,39). Two articles (21,25) involved separate comparisons of the survival of patients with stage III AEG, and they revealed that patients in the LG group had a survival advantage compared with patients in the OG group. Another major prognostic issue in AEG surgery is obtaining R0 resection, including the proximal esophagus and distal duodenal margin. Eight of the included studies reported the status of tumor resection margins, and only one (24) reported positive resection margins, but there was no significant difference between LG and OG in patients with AEG. There are several possible explanations for the benefit of laparoscopy on OS. First, a lower stress response and a higher level of immune function in patients undergoing minimally invasive surgery may contribute to the long-term survival advantage of laparoscopic rectal surgery over open surgery (40). In addition, improved recovery after laparoscopic surgery may allow for earlier initiation of adjuvant therapy. Timely postoperative adjuvant therapy may improve the survival of patients with GC (41).
Our meta-analysis has some limitations. First, all included studies were retrospective studies, and there was a lack of RCTs. Second, all the study centers were from Asia, and most of them were concentrated in China, so the results of the study lack broad applicability. In addition, the included studies. did not reach a consensus on surgical approach, LN dissection range, digestive tract reconstruction and other aspects, which also brought some heterogeneity to the data statistics.
In recent years, with the continuous promotion of multi-disciplinary treatment (MDT) diagnosis and treatment modes, the comprehensive treatment of AEG has gradually become standardized, and individualized. For advanced AEG, neoadjuvant therapy has become the treatment strategy of most surgeons. However, there are still no unified standards. The choice of surgical methods, the technical points of laparoscopy surgery, digestive reconstruction mode, short- and long-term safety of patients after neoadjuvant therapy have become hot topics of controversy and discussion in recent years. In 2012, the first RCT comparing open surgery and totally laparoscopic surgery for esophageal cancer after neoadjuvant therapy was conducted in Europe, and the results showed that thoracoscopic surgery had better short-term outcomes, but there was no significant difference in 3-year OS and DFS (42). Similar results were obtained in another French trial comparing conventional open surgery with a hybrid approach (laparoscopic plus open thoracotomy) for the treatment of esophageal cancer of the middle or lower third (43). Although there are no similar trials to provide enough evidence-based medical evidence for Siewert II/III AEG after neoadjuvant therapy, these trials also point out the direction for us to further explore the feasibility and safety of laparoscopic technology. With the wide application of neoadjuvant therapy in clinical practice, clinical trials involving gastrointestinal surgery, thoracic surgery, oncology and other disciplines will provide practitioners such as myself a better reference for the treatment of AEG.
Our meta-analysis is the first to compare the effect of LG and OG on the number of dissected LNs in AEG patients in subgroups. Considering the different extent of LN dissection in proximal gastrectomy and TG, we compared them separately. We compared the number of dissected LNs between LG and OG in patients with Siewert type II AEG for the first time. Previous studies (44,45) have just compared the effect of LG vs. OG on the number of dissected LNs in AEG patients, the extent of LN dissection varies with tumor location and the extent of surgical resection. In addition, we extracted survival data from the Kaplan-Meier curves and calculated HR from the original articles, and then performed the meta-analysis. Although this meta-analysis (44) compared long-term outcomes, no HR was calculated, and only two studies were included in the analysis of DFS, thus the results were not highly credible.
Conclusions
TG is a safe and reliable method for the treatment of AEG patients, and has certain advantages in postoperative recovery and long-term prognosis. However, more high-quality studies are needed to prove these points.
Acknowledgments
Funding: This work was supported by the Key Research and Development Program of Shandong Province (No. 2021CXGC011104, to L.L.), the Special Foundation for Taishan Scholars Program of Shandong Province (No. ts20190978, to L.L.), and the National Natural Science Foundation of China (No. 82203854, to L.S.).
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://ales.amegroups.com/article/view/10.21037/ales-23-39/rc
Peer Review File: Available at https://ales.amegroups.com/article/view/10.21037/ales-23-39/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://ales.amegroups.com/article/view/10.21037/ales-23-39/coif). L.S. reported that this work was supported by the National Natural Science Foundation of China (No. 82203854). L.L. reported that this work was supported by the Key Research and Development Program of Shandong Province (No. 2021CXGC011104) and the Special Foundation for Taishan Scholars Program of Shandong Province (No. ts20190978). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Global Burden of Disease Cancer Collaboration. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol 2019;5:1749-68. [Crossref] [PubMed]
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Dubecz A, Solymosi N, Stadlhuber RJ, et al. Does the Incidence of Adenocarcinoma of the Esophagus and Gastric Cardia Continue to Rise in the Twenty-First Century?-a SEER Database Analysis. J Gastrointest Surg 2013; Epub ahead of print. [Crossref] [PubMed]
- Hashimoto T, Kurokawa Y, Mori M, et al. Surgical Treatment of Gastroesophageal Junction Cancer. J Gastric Cancer 2018;18:209-17. [Crossref] [PubMed]
- Hasegawa S, Yoshikawa T. Adenocarcinoma of the esophagogastric junction: incidence, characteristics, and treatment strategies. Gastric Cancer 2010;13:63-73. [Crossref] [PubMed]
- Kitano S, Iso Y, Moriyama M, et al. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc 1994;4:146-8. [PubMed]
- Lan H, Zhu N, Lan Y, et al. Laparoscopic gastrectomy for gastric cancer in China: an overview. Hepatogastroenterology 2015;62:234-9. [PubMed]
- Kim W, Kim HH, Han SU, et al. Decreased Morbidity of Laparoscopic Distal Gastrectomy Compared With Open Distal Gastrectomy for Stage I Gastric Cancer: Short-term Outcomes From a Multicenter Randomized Controlled Trial (KLASS-01). Ann Surg 2016;263:28-35. [Crossref] [PubMed]
- Yu J, Huang C, Sun Y, et al. Effect of Laparoscopic vs Open Distal Gastrectomy on 3-Year Disease-Free Survival in Patients With Locally Advanced Gastric Cancer: The CLASS-01 Randomized Clinical Trial. JAMA 2019;321:1983-92. [Crossref] [PubMed]
- Chen XD, He FQ, Chen M, et al. Incidence of lymph node metastasis at each station in Siewert types II/III adenocarcinoma of the esophagogastric junction: A systematic review and meta-analysis. Surg Oncol 2020;35:62-70. [Crossref] [PubMed]
- Gholami S, Janson L, Worhunsky DJ, et al. Number of Lymph Nodes Removed and Survival after Gastric Cancer Resection: An Analysis from the US Gastric Cancer Collaborative. J Am Coll Surg 2015;221:291-9. [Crossref] [PubMed]
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603-5. [Crossref] [PubMed]
- Hong L, Han Y, Jin Y, et al. The short-term outcome in esophagogastric junctional adenocarcinoma patients receiving total gastrectomy: laparoscopic versus open gastrectomy--a retrospective cohort study. Int J Surg 2013;11:957-61. [Crossref] [PubMed]
- Huang CM, Lv CB, Lin JX, et al. Laparoscopic-assisted versus open total gastrectomy for Siewert type II and III esophagogastric junction carcinoma: a propensity score-matched case-control study. Surg Endosc 2017;31:3495-503. [Crossref] [PubMed]
- Shi Y, Li L, Xiao H, et al. Feasibility of laparoscopic gastrectomy for patients with Siewert-type II/III adenocarcinoma of the esophagogastric junction: A propensity score matching analysis. PLoS One 2018;13:e0203125. [Crossref] [PubMed]
- Zhang P, Zhang X, Xue H. Long-term results of hand-assisted laparoscopic gastrectomy for advanced Siewert type II and type III esophagogastric junction adenocarcinoma. Int J Surg 2018;53:201-5. [Crossref] [PubMed]
- Jia J, Li L, Li J, et al. Comparison of the efficacies of laparoscopic-assisted and open proximal gastrectomy for the treatment of Siewert type II and III adenocarcinoma of the esophagogastric junction. Chinese Journal of Clinical Oncology 2018;45:780-4.
- Wang J, Wang JC, Song B, et al. Comparative study of laparoscopic-assisted and open total gastrectomy for Siewert Types II and III adenocarcinoma of the esophagogastric junction. J Cell Physiol 2019;234:11235-9. [Crossref] [PubMed]
- Zhao Y, Zhang J, Yang D, et al. Feasibility of laparoscopic total gastrectomy for advanced Siewert type II and type III esophagogastric junction carcinoma: A propensity score-matched case-control study. Asian J Surg 2019;42:805-13. [Crossref] [PubMed]
- Lee Y, Min SH, Park KB, et al. Long-term Outcomes of Laparoscopic Versus Open Transhiatal Approach for the Treatment of Esophagogastric Junction Cancer. J Gastric Cancer 2019;19:62-71. [Crossref] [PubMed]
- Sugita S, Kinoshita T, Kuwata T, et al. Long-term oncological outcomes of laparoscopic versus open transhiatal resection for patients with Siewert type II adenocarcinoma of the esophagogastric junction. Surg Endosc 2021;35:340-8. [Crossref] [PubMed]
- Zhang B, Liu X, Ma F, et al. Laparoscopic-assisted versus open proximal gastrectomy with double-tract reconstruction for Siewert type II-III adenocarcinomas of esophago-gastric junction: a retrospective observational study of short-term outcomes. J Gastrointest Oncol 2021;12:249-58. [Crossref] [PubMed]
- Lin X, Wan J, Li Z, et al. Surgical and survival outcomes after laparoscopic and open gastrectomy for serosa-invasive Siewert type II/III esophagogastric junction carcinoma: a propensity score matching analysis. Surg Endosc 2022;36:5055-66. [Crossref] [PubMed]
- Song QY, Li XG, Zhang LY, et al. Laparoscopic-assisted vs open transhiatal gastrectomy for Siewert type II adenocarcinoma of the esophagogastric junction: A retrospective cohort study. World J Gastrointest Surg 2022;14:304-14. [Crossref] [PubMed]
- Buas MF, Vaughan TL. Epidemiology and risk factors for gastroesophageal junction tumors: understanding the rising incidence of this disease. Semin Radiat Oncol 2013;23:3-9. [Crossref] [PubMed]
- Edgren G, Adami HO, Weiderpass E, et al. A global assessment of the oesophageal adenocarcinoma epidemic. Gut 2013;62:1406-14. [Crossref] [PubMed]
- Yang HK, Hyung WJ, Han SU, et al. Comparison of surgical outcomes among different methods of esophagojejunostomy in laparoscopic total gastrectomy for clinical stage I proximal gastric cancer: results of a single-arm multicenter phase II clinical trial in Korea, KLASS 03. Surg Endosc 2021;35:1156-63. [Crossref] [PubMed]
- Katai H, Mizusawa J, Katayama H, et al. Single-arm confirmatory trial of laparoscopy-assisted total or proximal gastrectomy with nodal dissection for clinical stage I gastric cancer: Japan Clinical Oncology Group study JCOG1401. Gastric Cancer 2019;22:999-1008. [Crossref] [PubMed]
- Liu F, Huang C, Xu Z, et al. Morbidity and Mortality of Laparoscopic vs Open Total Gastrectomy for Clinical Stage I Gastric Cancer: The CLASS02 Multicenter Randomized Clinical Trial. JAMA Oncol 2020;6:1590-7. [Crossref] [PubMed]
- Siewert JR, Stein HJ. Classification of adenocarcinoma of the oesophagogastric junction. Br J Surg 1998;85:1457-9. [Crossref] [PubMed]
- Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer 2021;24:1-21.
- Li X, Gong S, Lu T, et al. Proximal Gastrectomy Versus Total Gastrectomy for Siewert II/III Adenocarcinoma of the Gastroesophageal Junction: a Systematic Review and Meta-analysis. J Gastrointest Surg 2022;26:1321-35. [Crossref] [PubMed]
- Yoo CH, Kim HO, Hwang SI, et al. Short-term outcomes of laparoscopic-assisted distal gastrectomy for gastric cancer during a surgeon's learning curve period. Surg Endosc 2009;23:2250-7. [Crossref] [PubMed]
- Wu XN, Liu CQ, Tian JY, et al. Prognostic significance of the number of lymph nodes examined in node-negative Siewert type II esophagogastric junction adenocarcinoma. Int J Surg 2017;41:6-11. [Crossref] [PubMed]
- Yamashita H, Seto Y, Sano T, et al. Results of a nation-wide retrospective study of lymphadenectomy for esophagogastric junction carcinoma. Gastric Cancer 2017;20:69-83. [Crossref] [PubMed]
- Kurokawa Y, Hiki N, Yoshikawa T, et al. Mediastinal lymph node metastasis and recurrence in adenocarcinoma of the esophagogastric junction. Surgery 2015;157:551-5. [Crossref] [PubMed]
- Wang JB, Lin MQ, Li P, et al. The prognostic relevance of parapyloric lymph node metastasis in Siewert type II/III adenocarcinoma of the esophagogastric junction. Eur J Surg Oncol 2017;43:2333-40. [Crossref] [PubMed]
- Del Rio P, Viani L, Bertocchi E, et al. The prognostic role of tumor size in patients with gastric cancer. Ann Ital Chir 2017;88:478-84. [PubMed]
- Veenhof AA, Vlug MS, van der Pas MH, et al. Surgical stress response and postoperative immune function after laparoscopy or open surgery with fast track or standard perioperative care: a randomized trial. Ann Surg 2012;255:216-21. [Crossref] [PubMed]
- Ozden S, Ozgen Z, Ozyurt H, et al. Survival in gastric cancer in relation to postoperative adjuvant therapy and determinants. World J Gastroenterol 2015;21:1222-33. [Crossref] [PubMed]
- Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 2012;379:1887-92. [Crossref] [PubMed]
- Mariette C, Markar SR, Dabakuyo-Yonli TS, et al. Hybrid Minimally Invasive Esophagectomy for Esophageal Cancer. N Engl J Med 2019;380:152-62. [Crossref] [PubMed]
- Wu M, Zhang W, Song YY. Laparoscopic versus Open Approach for Siewert Type II/III Adenocarcinoma of the Esophagogastric Junction: A Systematic Review and Meta-Analysis. Dig Surg 2022;39:210-23. [Crossref] [PubMed]
- Liao C, Feng Q, Xie S, et al. Laparoscopic versus open gastrectomy for Siewert type II/III adenocarcinoma of the esophagogastric junction: a meta-analysis. Surg Endosc 2021;35:860-71. [Crossref] [PubMed]
Cite this article as: Teng Q, Ma C, Du F, Shang L, Li L. Outcomes of laparoscopic vs. open gastrectomy for Siewert type II/III adenocarcinoma of the esophagogastric junction: a systematic review and meta-analysis. Ann Laparosc Endosc Surg 2024;9:2.