
Colon polyp characterization (morphology and mucosal patterns): clinical application and techniques
Introduction
Background
Colonoscopy enables not only the detection but also the endoscopic resection of colon polyps. Historically, most of the gastroenterologist’s job involved detecting and removing such polyps, fulfilling the gold standard for colorectal cancer (CRC) screening (1). However, recent advances allow the endoscopic pre-diagnosis of multiple lesion types (i.e., hyperplastic, adenomatous, serrated adenoma, and early CRC), as well as the ability to offer curative en bloc endoscopic resection (2). Relatively large yet shallow lesions can be treated successfully with methods such as endoscopic submucosal dissection (ESD), if not with endoscopic mucosal resection (EMR), oftentimes averting the potential for surgery and preserving important histologic information.
Rationale and knowledge gap
Recently, what could be performed with colonoscopy has dramatically broadened. Many reports have demonstrated different ideas about its usage and importance in the accuracy of what is diagnosed and treated. In this article, we provide an overview of and discuss the current endoscopic strategies to diagnose and treat colon polyps by various modalities.
Objective
The purpose of this review is to tackle the diagnostic tools and treatment of colon polyps from a variety of perspectives.
Tools and treatments
White light imaging (WLI)
Conventional endoscopic evaluation of the colon performed with WLI allows for characterization of polyps by location, size, and morphology. Proximal colon lesions have been associated with increased risk during resection, especially in the cecum, where the risk of post-procedure complications is highest (3). The European Society of Gastrointestinal Endoscopy recommends referral to expert centers for removal of lesions in a diverticulum, the appendix, or the ileocecal valve (4). Prior studies indicate that polyp size directly relates to cancer risk, with larger polyps harboring a higher risk of malignancy (5). Larger polyps may not be amenable to en bloc resection. Size of the lesion therefore may guide endoscopic resection techniques during colonoscopy, though assessment of lesion size during endoscopy is subject to high interobserver variability (6). At present, many endoscopists estimate lesion size by comparison with an open snare of a known diameter or biopsy forceps with a known length during endoscopic resection (7).
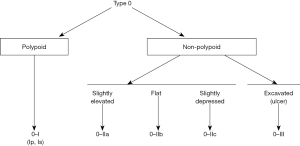
Paris classification
WLI also allows for the description of lesions by morphology. In 1998, a classification to describe the superficial endoscopic appearance of gastric lesions was devised, which was quickly applied to other lesions of the gastrointestinal (GI) tract including the colon (8). A multidisciplinary group later established a new classification scheme for polyp morphology known as the Paris classification (Figure 1) (9). The Paris classification broadly categorizes lesions on whether they are polypoid or non-polypoid. It further classifies polypoid lesions as pedunculated (0–Ip) or sessile (0–Is) and non-polypoid lesions as slightly elevated (0–IIa), flat (0–IIb), slightly depressed (0–IIc), or excavated (0–III) (10). Using the Paris classification during examination of lesions under WLI may predict the prognosis of lesions. The United States Multi-Society Task Force (USMSTF) suggests using the Paris classification to furnish a common nomenclature for polyp surface morphology and recommends that every lesion larger than one cm in size is photo-documented prior to resection; the post polypectomy site should also be photo-documented (11). There is conflicting data on whether non-polypoid lesions confer a greater risk of high-grade dysplasia or early CRC (10,12-14). Other work has shown that, while rare, depressed (0–IIc) or excavated (0–III) lesions are associated with a greater risk of invasive malignancy (15). Larger, left-sided colon polyps, Paris Is–IIa, such as “...a dominant nodule within a lateral spreading tumor [LST]” have a higher risk of early invasive cancer (16). For this reason, as well as to ensure negative margins, such a lesion would necessitate the use of ESD over EMR.
Chromoendoscopy
Kudo’s pit pattern
Olympus Corporation’s (Tokyo, Japan) release of the first magnifying colonoscope on a commercial scale transformed endoscopists’ in vivo ability to inspect mucosal surfaces and scrutinize lesions of concern, especially those normally less apparent to the naked eye with traditional colonoscopes. Initially, 10× magnification capability was introduced (17). Reaping the benefits of the magnifying colonoscope and focusing on the relationship of pit pattern to pathological results, Kudo’s pit pattern emerged as a method of making an optical diagnosis (18). This system would eventually be recognized as an accurate way to predict the pathology and estimate the depth of a lesion (19,20).
Pit patterns comprise crypt openings and microvasculature. The Kudo Classification encompasses seven distinct pit patterns: types I–V, VI, and VN. The first two types constitute a normal or benign appearance (non-neoplastic), whereas types III and IV are regarded as adenomatous. Finally, types VI and VN are considered neoplastic/cancerous (18). Accordingly, the indications for endoscopic resection include types II, IIIs, IIIL, and IV. We will point out that mild irregularity in VI and small regionality in advanced VI may also qualify for resection (21).
In order to better identify the pit pattern, dyes such as crystal violet (CV) may be sprayed intraluminally or applied directly to stain the mucosa (22,23). Interestingly, the original dye used by Kudo and colleagues was cresyl violet for Nissl staining, distinct from crystal or gentian violet (24,25). However, CV staining is uncommon in the U.S., given the existence of alternatives such as topical and per oral methylene blue (26), indigo carmine dye, and acetic acid (27).
A randomized trial done in individuals at average risk of CRC demonstrated that pan-colonic chromoendoscopy with 0.4% indigo carmine spraying significantly increased the overall detection rate for adenomas, flat adenomas, and serrated lesions, as compared to standard colonoscopy, while adding nominally to procedure time. However, potential pitfalls to acknowledge are the additional training needed to skillfully implement the technique and the laborious nature of the application process (28).
Dye-based chromoendoscopy is recommended for inflammatory bowel disease (IBD) surveillance as it improves the visualization of mucosal abnormalities (29,30). When used with magnification endoscopy, it enables a better view of the mucosal crypt architecture and facilitates the application of the Kudo pit pattern classification to ultimately tell apart neoplastic and non-neoplastic lesions (29,31). Methylene blue has been employed in a randomized controlled trial as a tool for early detection of intraepithelial neoplasms and colitis-associated colon carcinomas in patients with ulcerative colitis for at least 8 years. With the aid of the modified pit pattern classification, the sensitivity and specificity of discrimination of non-neoplastic vs. neoplastic lesions were both 93% (32). In a prospective, randomized controlled trial of long-standing ulcerative colitis patients in remission, segmental spraying and inspection with 0.1% methylene blue did not differ significantly from narrow band imaging (NBI) in the detection of colitis-associated neoplasia (33). In a meta-analysis of the diagnostic yield of chromoendoscopy for detecting dysplasia in patients with colonic IBD, adding 11 minutes of procedure time to conduct topical dye spraying increased the yield of dysplasia or cancer detection by 7%. This finding was in relation to white light endoscopy and is consistent with an increased rate of the targeted yield of dysplastic lesions via chromoendoscopy; therefore, chromoendoscopy could, in turn, flag EMR-eligible lesions but does not negate the need to avoid missed dysplastic lesions via random biopsy (34).
In Japan, exceptionally high resolution, magnifying endoscopes are available and suitable for discerning Kudo’s pit pattern. In contrast, such endoscopes for optical high magnification are generally unavailable or seldom used in the U.S. (24,35,36). However, Olympus released “Dual Focus” 190 series colonoscopes (CF-HQ190L/I) in the U.S. for conducting magnifying endoscopy with EMR and/or ESD, though these colonoscopes are incapable of 80× magnification. If a non-pedunculated lesion that is two cm or more in size is seen, the USMSTF recommends EMR and that the endoscopist is seasoned in the art of advanced polypectomy (11). On the other hand, ESD supplies histopathologic information about early cancers, if present, within a polyp that would simply be unattainable after piecemeal resection.
With moderate quality evidence, the USMSTF suggests becoming proficient in electronic (i.e., NBI), dye-based (i.e., chromoendoscopy), or “…image-enhanced endoscopy techniques to apply optical diagnosis classifications for colorectal lesion histology” (11). NBI, a form of image-enhanced endoscopy diagnosis, is more popular than chromoendoscopy in the U.S. (35).
Image-enhanced endoscopy (virtual chromoendoscopy)
Flexible spectral imaging color enhancement (FICE)
Fujifilm Corporation (Tokyo, Japan) developed FICE as a software that uses electronic optical filters during endoscopy to expose subtle details of the mucosa, as processed images display relatively high contrast between a neoplasm and its surrounding normal mucosa (37). As a form of virtual chromoendoscopy, FICE also can be used in the surveillance of IBD. The authors of a prospective, parallel arm, pragmatic trial of patients who underwent surveillance of ulcerative colitis set out to better define the role of FICE in correctly characterizing a neoplastic vs. non-neoplastic lesion; they found that white light endoscopy was less accurate than FICE with the modified Kudo classification at distinguishing visible lesions. FICE accurately identified 97% of non-neoplastic lesions and 93% of neoplastic lesions, and had a higher specificity as compared to white light endoscopy (29). However, a systematic review with network-meta-analysis found, with low certainty, that full spectrum high-definition white-light endoscopy had higher odds of detecting dysplastic lesions during IBD surveillance than FICE. Overall, this analysis of 2,638 patients concluded that chromoendoscopy, high-definition white-light endoscopy, NBI, autofluorescence, FICE, and full spectrum high-definition white-light endoscopy are possibly comparable options for dysplasia surveillance in IBD (38).
i-SCAN
Another image-enhanced endoscopy technology, i-SCAN, out of PENTAX (HOYA Corporation, Tokyo, Japan), also uses contrast enhancement (CE) to demarcate polyps, but has two additional possible modes for image enhancement: surface enhancement (SE) and tone enhancement (TE). i-SCAN can help conduct a detailed inspection of mucosal structures at the push of a button without magnifying endoscopy, although it can be used adjunctively to determine the need for magnified observation, dye-spraying, or biopsy (39). A recent meta-analysis and systematic review included five studies on the impact of i-SCAN on adenoma detection rate and found that colonoscopies performed with i-SCAN with SE and CE led to a higher adenoma detection rate compared to high-definition colonoscopy. However, adenoma per subject, polyp detection rate, and polyps per subject did not reach statistical significance (40).
NBI
NBI was launched by Olympus in 2005 and since then has been used increasingly during endoscopy to further classify colon polyps (41). At present, the majority of Olympus endoscopes have the ability to perform NBI easily (42). Studies evaluating the use of NBI have been mixed, with some showing no significant differences in the detection of colorectal polyps compared to white-light endoscopy (43) while others have shown a significant increase in the detection of polyps with the use of NBI, especially for right-sided colon lesions (42,44).
NBI is a technique in which the wavelengths of light used for visualization are limited to a specific band. Blue and green wavelengths are specifically selected, while red light is eliminated. This technique provides improved visualization of the mucosal architecture (42,45,46). With the use of NBI, a lesion is first evaluated for a demarcation line (DL). If a DL is present, the lesion is then evaluated for irregularities in the microsurface or microvascular pattern (45). Various categorizations using NBI have been developed, including the NBI International Colorectal Endoscopic (NICE) classification and the Japan NBI Experts Team (JNET) classification, which we discuss below.
NICE classification
The NICE classification was proposed to both simplify colorectal tumor categorization and attempt to standardize it worldwide. This classification was the first NBI classification that did not mandate the use of magnifying endoscopy. Additionally, it has been used with other image-enhanced endoscopy methods in a complementary manner (47-49). It entails a unique method of diagnosis that focuses on lesion color, surface pattern, and arrangement of vessels. Color and surface pattern are not only specific but also fairly simple for endoscopists to discern (47). Vessel morphology, in particular, is of salience because angiogenesis is known to correlate with tumor growth (50,51). These three features are grouped into type 1, 2, or 3 to aid endoscopic assessment. Each type is indicative of specific pathology. Type 1 lesions are most likely hyperplastic or sessile serrated polyps, while type 2 are probable adenomas, and type 3 likely reflect deep submucosal invasive cancer (47).
A large, multicenter prospective study examined the accuracy of the NICE classification with respect to the detection of deep lesions not amenable to endoscopic resection and greater than 10 mm in size. When paired with morphologic features, the NICE classification was found to have a specificity of at least 96% even when used by non-experts for the detection of deep lesions. Depressed, nodular mixed type, pedunculated, and ulcerated lesions, on the other hand, interfered with accuracy (52). Though useful, the NICE classification was subject to several issues raised by clinical studies, for example, redundancy of similar or identical terms for magnifying findings. Experts subsequently converged to establish the more unified JNET classification (47).
JNET classification
To address the disadvantages of the NICE classification, the JNET classification was developed. The classification derives from its predecessor, NICE, and also shares similarities with the Kudo pit pattern in that it hones in on both surface pattern and vessel pattern. It delineates four types which pertain to the histological features of polyps: JNET type 1, type 2A, type 2B, and type 3 (Figure 2). All types except type 3 may be considered indications for ESD. Type 1 can generally be left to follow up. Notably, the most likely histology associated with type 2B includes shallow submucosal invasive cancers and high grade intramucosal neoplasia (47). Therefore, it is thought that in comparison to the other types, type 2B is not as strong of an indicator of high-grade dysplasia and superficial submucosal carcinoma due to the heterogeneous nature of the histology it envelopes. Subtyping for type 2B has been proposed as a way to possibly improve its performance and diagnostic utility (54).
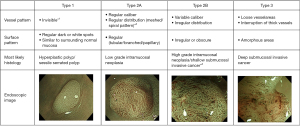
Overall, JNET undoubtedly remains a useful tool to visually evaluate tumor pathology and depth. Furthermore, its diagnostic yield and diagnostic accuracy have been thoroughly studied in the clinical setting (55-57). Additionally, the JNET classification is both reliable and validated (58,59). For these reasons, we view JNET as the final answer to questions about NBI classification.
Other modalities for diagnosis
Artificial intelligence (AI)
Of late, AI has garnered attention for its real time use in colon polyp detection and potential for decreasing the rate of CRCs. In 2020, a 14% absolute increase in adenoma detection rate was reported in a multicenter randomized controlled trial of computer-aided detection in colorectal neoplasia using Medtronic’s GI Genius (60). More recently, the first ever multicenter, multi country randomized crossover trial studying adenoma miss rates (AMRs) also employed GI Genius and found that AI-assisted colonoscopy reduced AMR by almost 50% (61). Additionally, computer aided diagnosis (CADx) systems have been developed to help endoscopists implement the NICE and JNET Classifications. In these ways, AI shows promise in assisting with diagnosis of colorectal lesions (62). The body of research on AI vs. physicians is nascent and presently limited, but it is already apparent that AI has the potential to match or exceed performance of human doctors in colon polyp detection and diagnostic accuracy, or at least serve as a beneficial adjunct (63-66).
CADx has been applied to white-light endoscopy, magnifying NBI, magnifying chromoendoscopy, and various other technological targets to characterize polyps (67). One possible function of CADx could be to lessen the time needed by beginner endoscopists to detect neoplastic lesions (68). Even experienced proceduralists, unaided, can have trouble identifying CRCs with deep submucosal invasion (classified as T1b), which carry a risk of metastasis (69). This is because T1b lesions on plain endoscopic images may be indiscernible from those with superficial invasion or mucosal CRCs (70,71). The USMSTF suggests that proceduralists become proficient in endoscopic recognition of deep submucosal invasion (11). In one study, a CADx system was designed to tell apart T1b from Tis/T1a lesions on plain endoscopy and its performance compared to endoscopists of varying levels (ranging from trainees to experts). Overall, it performed “slightly inferior to experts” (69). No specific trends were identified with respect to diagnostic values determined from readings by expert endoscopists on the basis of morphology and lesion size. The CADx system demonstrated higher sensitivity for polypoid morphology than for flat morphology, but this difference was non-significant (69).
Conclusions
Ultimately, the main goal of polypectomy is to entirely remove colorectal lesions to prevent the development of CRC, and proceduralists should select evidence based techniques that are “…the safest, most complete, and efficient” to perform such resections (11). In patients with IBD, the current practice of colonoscopic surveillance entails identifying and removing precursor lesions of colorectal neoplasia to attenuate the additional CRC risk posed by this condition, though undoubtedly resource-intensive. As others have astutely pointed out, there remains a continued need to evaluate the practices of chromoendoscopy and taking random biopsies, as well as the efficacy of ESD in people diagnosed with IBD (72). As for the average risk population, once technologies such as CADx are studied with more randomized controlled trials, this will enable physicians to make confident predictions about lesions and cut down on the needless removal of minute, noncancerous polyps (73,74). It is forecasted that, in the coming years, AI will be swiftly deployed into colonoscopy practice and continue to impact the landscape of the endoscopic diagnosis and management of polyps (67).
Recent advancements in colonoscopy, imaging modalities, and AI have facilitated high-quality examination of colon lesions. Our review highlights recent updates on these modalities for detecting colorectal lesions, as well as summarizes current knowledge on their diagnostic performance. It is important to understand the features of each test and to apply them appropriately in clinical practice for optimal patient care.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Terry L. Jue) for the series “A U.S. Perspective on Endoscopic Resection of Neoplastic Lesions of the Gastrointestinal Tract” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Peer Review File: Available at https://ales.amegroups.com/article/view/10.21037/ales-23-10/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://ales.amegroups.com/article/view/10.21037/ales-23-10/coif). The series “A U.S. Perspective on Endoscopic Resection of Neoplastic Lesions of the Gastrointestinal Tract” was commissioned by the editorial office without any funding or sponsorship. MN is a consultant for Boston Scientific and Olympus America and receives consultant fees and payment for lectures from them. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rex DK, Boland CR, Dominitz JA, et al. Colorectal Cancer Screening: Recommendations for Physicians and Patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol 2017;112:1016-30. [Crossref] [PubMed]
- Tholoor S, Tsagkournis O, Basford P, et al. Managing difficult polyps: techniques and pitfalls. Ann Gastroenterol 2013;26:114-21. [PubMed]
- Burgess NG, Metz AJ, Williams SJ, et al. Risk factors for intraprocedural and clinically significant delayed bleeding after wide-field endoscopic mucosal resection of large colonic lesions. Clin Gastroenterol Hepatol 2014;12:651-61.e1-3.
- Ferlitsch M, Moss A, Hassan C, et al. Colorectal polypectomy and endoscopic mucosal resection (EMR): European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy 2017;49:270-97. [Crossref] [PubMed]
- Odom SR, Duffy SD, Barone JE, et al. The rate of adenocarcinoma in endoscopically removed colorectal polyps. Am Surg 2005;71:1024-6. [Crossref] [PubMed]
- Rex DK, Rabinovitz R. Variable interpretation of polyp size by using open forceps by experienced colonoscopists. Gastrointest Endosc 2014;79:402-7. [Crossref] [PubMed]
- Vleugels JLA, Hazewinkel Y, Dekker E. Morphological classifications of gastrointestinal lesions. Best Pract Res Clin Gastroenterol 2017;31:359-67. [Crossref] [PubMed]
- Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma - 2nd English Edition -. Gastric Cancer 1998;1:10-24. [Crossref] [PubMed]
- The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc 2003;58:S3-43. [Crossref] [PubMed]
- Tsuda S, Veress B, Tóth E, et al. Flat and depressed colorectal tumours in a southern Swedish population: a prospective chromoendoscopic and histopathological study. Gut 2002;51:550-5. [Crossref] [PubMed]
- Kaltenbach T, Anderson JC, Burke CA, et al. Endoscopic Removal of Colorectal Lesions: Recommendations by the US Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol 2020;115:435-64. [Crossref] [PubMed]
- Rembacken BJ, Fujii T, Cairns A, et al. Flat and depressed colonic neoplasms: a prospective study of 1000 colonoscopies in the UK. Lancet 2000;355:1211-4. [Crossref] [PubMed]
- Kil Lee S, Il Kim T, Kwan Shin S, et al. Comparison of the clinicopathologic features between flat and polypoid adenoma. Scand J Gastroenterol 2008;43:1116-21. [Crossref] [PubMed]
- Park DH, Kim HS, Kim WH, et al. Clinicopathologic characteristics and malignant potential of colorectal flat neoplasia compared with that of polypoid neoplasia. Dis Colon Rectum 2008;51:43-9; discussion 49. [Crossref] [PubMed]
- Moss A, Bourke MJ, Williams SJ, et al. Endoscopic mucosal resection outcomes and prediction of submucosal cancer from advanced colonic mucosal neoplasia. Gastroenterology 2011;140:1909-18. [Crossref] [PubMed]
- Burgess NG, Hourigan LF, Zanati SA, et al. Risk Stratification for Covert Invasive Cancer Among Patients Referred for Colonic Endoscopic Mucosal Resection: A Large Multicenter Cohort. Gastroenterology 2017;153:732-742.e1. [Crossref] [PubMed]
- Kudo SE, Misawa M. Progress in magnifying colonoscopy: Road to optical biopsy. Dig Endosc 2022;34:91-4. [Crossref] [PubMed]
- Kudo S, Hirota S, Nakajima T, et al. Colorectal tumours and pit pattern. J Clin Pathol 1994;47:880-5. [Crossref] [PubMed]
- Li M, Ali SM, Umm-a-OmarahGilani S, et al. Kudo's pit pattern classification for colorectal neoplasms: a meta-analysis. World J Gastroenterol 2014;20:12649-56. [Crossref] [PubMed]
- Wada Y, Kashida H, Kudo SE, et al. Diagnostic accuracy of pit pattern and vascular pattern analyses in colorectal lesions. Dig Endosc 2010;22:192-9. [Crossref] [PubMed]
- Matsuda T, Fujii T, Saito Y, et al. Efficacy of the invasive/non-invasive pattern by magnifying chromoendoscopy to estimate the depth of invasion of early colorectal neoplasms. Am J Gastroenterol 2008;103:2700-6. [Crossref] [PubMed]
- Suzuki T, Hara T, Kitagawa Y, et al. Magnified endoscopic observation of early colorectal cancer by linked color imaging with crystal violet staining (with video). Gastrointest Endosc 2016;84:726-9. [Crossref] [PubMed]
- Zhang JJ, Gu LY, Chen XY, et al. Endoscopic diagnosis of invasion depth for early colorectal carcinomas: a prospective comparative study of narrow-band imaging, acetic acid, and crystal violet. Medicine (Baltimore) 2015;94:e528. [Crossref] [PubMed]
- Buchner AM. The Role of Chromoendoscopy in Evaluating Colorectal Dysplasia. Gastroenterol Hepatol (N Y) 2017;13:336-47. [PubMed]
- Kudo S, Tamura S, Nakajima T, et al. Diagnosis of colorectal tumorous lesions by magnifying endoscopy. Gastrointest Endosc 1996;44:8-14. [Crossref] [PubMed]
- Repici A, Wallace MB, East JE, et al. Efficacy of Per-oral Methylene Blue Formulation for Screening Colonoscopy. Gastroenterology 2019;156:2198-2207.e1. [Crossref] [PubMed]
- Longcroft-Wheaton G, Duku M, Mead R, et al. Acetic acid spray is an effective tool for the endoscopic detection of neoplasia in patients with Barrett's esophagus. Clin Gastroenterol Hepatol 2010;8:843-7. [Crossref] [PubMed]
- Pohl J, Schneider A, Vogell H, et al. Pancolonic chromoendoscopy with indigo carmine versus standard colonoscopy for detection of neoplastic lesions: a randomised two-centre trial. Gut 2011;60:485-90. [Crossref] [PubMed]
- Cassinotti A, Fociani P, Duca P, et al. Modified Kudo classification can improve accuracy of virtual chromoendoscopy with FICE in endoscopic surveillance of ulcerative colitis. Endosc Int Open 2020;8:E1414-22. [Crossref] [PubMed]
- Iacucci M, Kaplan GG, Panaccione R, et al. A Randomized Trial Comparing High Definition Colonoscopy Alone With High Definition Dye Spraying and Electronic Virtual Chromoendoscopy for Detection of Colonic Neoplastic Lesions During IBD Surveillance Colonoscopy. Am J Gastroenterol 2018;113:225-34. [Crossref] [PubMed]
- Nagata S, Tanaka S, Haruma K, et al. Pit pattern diagnosis of early colorectal carcinoma by magnifying colonoscopy: clinical and histological implications. Int J Oncol 2000;16:927-34. [Crossref] [PubMed]
- Kiesslich R, Fritsch J, Holtmann M, et al. Methylene blue-aided chromoendoscopy for the detection of intraepithelial neoplasia and colon cancer in ulcerative colitis. Gastroenterology 2003;124:880-8. [Crossref] [PubMed]
- Bisschops R, Bessissow T, Joseph JA, et al. Chromoendoscopy versus narrow band imaging in UC: a prospective randomised controlled trial. Gut 2018;67:1087-94. [Crossref] [PubMed]
- Subramanian V, Mannath J, Ragunath K, et al. Meta-analysis: the diagnostic yield of chromoendoscopy for detecting dysplasia in patients with colonic inflammatory bowel disease. Aliment Pharmacol Ther 2011;33:304-12. [Crossref] [PubMed]
- Saito Y, Ono A, García VAJ, et al. Diagnosis and treatment of colorectal tumors: Differences between Japan and the West and future prospects. DEN Open 2022;2:e66. [Crossref] [PubMed]
- Nishimura M. ESD and Pit Pattern Diagnosis: Lessons from a Japanese Endoscopist Working in the United States. Clin Colon Rectal Surg 2020;33:329-34. [Crossref] [PubMed]
- Akarsu M, Akarsu C. Evaluation of New Technologies in Gastrointestinal Endoscopy. JSLS 2018;22:e2017.00053.
- Iannone A, Ruospo M, Palmer SC, et al. Systematic review with network meta-analysis: endoscopic techniques for dysplasia surveillance in inflammatory bowel disease. Aliment Pharmacol Ther 2019;50:858-71. [Crossref] [PubMed]
- Kodashima S, Fujishiro M. Novel image-enhanced endoscopy with i-scan technology. World J Gastroenterol 2010;16:1043-9. [Crossref] [PubMed]
- Aziz M, Ahmed Z, Haghbin H, et al. Does i-scan improve adenoma detection rate compared to high-definition colonoscopy? A systematic review and meta-analysis. Endosc Int Open 2022;10:E824-31. [Crossref] [PubMed]
- Gono K. Narrow Band Imaging: Technology Basis and Research and Development History. Clin Endosc 2015;48:476-80. [Crossref] [PubMed]
- Uraoka T, Higashi R, Saito Y, et al. Impact of narrow-band imaging in screening colonoscopy. Dig Endosc 2010;22:S54-6. [Crossref] [PubMed]
- Nagorni A, Bjelakovic G, Petrovic B. Narrow band imaging versus conventional white light colonoscopy for the detection of colorectal polyps. Cochrane Database Syst Rev 2012;1:CD008361. [Crossref] [PubMed]
- Inoue T, Murano M, Murano N, et al. Comparative study of conventional colonoscopy and pan-colonic narrow-band imaging system in the detection of neoplastic colonic polyps: a randomized, controlled trial. J Gastroenterol 2008;43:45-50. [Crossref] [PubMed]
- Rey JF, Lambert RESGE Quality Assurance Committee. ESGE recommendations for quality control in gastrointestinal endoscopy: guidelines for image documentation in upper and lower GI endoscopy. Endoscopy 2001;33:901-3. [Crossref] [PubMed]
- Barbeiro S, Libânio D, Castro R, et al. Narrow-Band Imaging: Clinical Application in Gastrointestinal Endoscopy. GE Port J Gastroenterol 2018;26:40-53. [Crossref] [PubMed]
- Sano Y, Hirata D, Saito Y. Japan NBI Expert Team classification: Narrow-band imaging magnifying endoscopic classification of colorectal tumors. Dig Endosc 2018;30:543-5. [Crossref] [PubMed]
- Wu CH, Chen TH, Hsu CM, et al. Linked-color imaging combined with the NICE classification system for optical diagnosis of colon polyps: new image-enhanced endoscopic technology for pathological prediction. Ther Clin Risk Manag 2017;13:1317-21. [Crossref] [PubMed]
- Pigò F, Bertani H, Manno M, et al. i-Scan high-definition white light endoscopy and colorectal polyps: prediction of histology, interobserver and intraobserver agreement. Int J Colorectal Dis 2013;28:399-406. [Crossref] [PubMed]
- Konerding MA, Fait E, Gaumann A. 3D microvascular architecture of pre-cancerous lesions and invasive carcinomas of the colon. Br J Cancer 2001;84:1354-62. [Crossref] [PubMed]
- Iwatate M, Hirata D, Sano Y. NBI International Colorectal Endoscopic (NICE) Classification. In: Chiu PWY, Sano Y, Uedo N, et al. editors. Endoscopy in Early Gastrointestinal Cancers, Volume 1: Diagnosis. Singapore: Springer Singapore; 2021:69-74.
- Puig I, López-Cerón M, Arnau A, et al. Accuracy of the Narrow-Band Imaging International Colorectal Endoscopic Classification System in Identification of Deep Invasion in Colorectal Polyps. Gastroenterology 2019;156:75-87. [Crossref] [PubMed]
- Sano Y, Tanaka S, Kudo SE, et al. Narrow-band imaging (NBI) magnifying endoscopic classification of colorectal tumors proposed by the Japan NBI Expert Team. Dig Endosc 2016;28:526-33. [Crossref] [PubMed]
- Sumimoto K, Tanaka S, Shigita K, et al. Diagnostic performance of Japan NBI Expert Team classification for differentiation among noninvasive, superficially invasive, and deeply invasive colorectal neoplasia. Gastrointest Endosc 2017;86:700-9. [Crossref] [PubMed]
- Kobayashi S, Yamada M, Takamaru H, et al. Diagnostic yield of the Japan NBI Expert Team (JNET) classification for endoscopic diagnosis of superficial colorectal neoplasms in a large-scale clinical practice database. United European Gastroenterol J 2019;7:914-23. [Crossref] [PubMed]
- Suzuki H, Yamamura T, Nakamura M, et al. An International Study on the Diagnostic Accuracy of the Japan Narrow-Band Imaging Expert Team Classification for Colorectal Polyps Observed with Blue Laser Imaging. Digestion 2020;101:339-46. [Crossref] [PubMed]
- Okamoto Y, Oka S, Tanaka S, et al. Effect of educational lecture on the diagnostic accuracy of Japan NBI Expert Team classification for colorectal lesions. BMC Gastroenterol 2021;21:110. [Crossref] [PubMed]
- Sakamoto T, Takamaru H, Sekiguchi M, et al. Reliability of Japan Narrow-Band Imaging Expert Team Classification for the Diagnosis of Colorectal Neoplasms: A Pilot Study. Digestion 2020;101:638-43. [Crossref] [PubMed]
- Iwatate M, Sano Y, Tanaka S, et al. Validation study for development of the Japan NBI Expert Team classification of colorectal lesions. Dig Endosc 2018;30:642-51. [Crossref] [PubMed]
- Repici A, Badalamenti M, Maselli R, et al. Efficacy of Real-Time Computer-Aided Detection of Colorectal Neoplasia in a Randomized Trial. Gastroenterology 2020;159:512-520.e7. [Crossref] [PubMed]
- Wallace MB, Sharma P, Bhandari P, et al. Impact of Artificial Intelligence on Miss Rate of Colorectal Neoplasia. Gastroenterology 2022;163:295-304.e5. [Crossref] [PubMed]
- Okamoto Y, Yoshida S, Izakura S, et al. Development of multi-class computer-aided diagnostic systems using the NICE/JNET classifications for colorectal lesions. J Gastroenterol Hepatol 2022;37:104-10. [Crossref] [PubMed]
- Li MD, Huang ZR, Shan QY, et al. Performance and comparison of artificial intelligence and human experts in the detection and classification of colonic polyps. BMC Gastroenterol 2022;22:517. [Crossref] [PubMed]
- Gross S, Trautwein C, Behrens A, et al. Computer-based classification of small colorectal polyps by using narrow-band imaging with optical magnification. Gastrointest Endosc 2011;74:1354-9. [Crossref] [PubMed]
- Chen PJ, Lin MC, Lai MJ, et al. Accurate Classification of Diminutive Colorectal Polyps Using Computer-Aided Analysis. Gastroenterology 2018;154:568-75. [Crossref] [PubMed]
- Misawa M, Kudo SE, Mori Y, et al. Accuracy of computer-aided diagnosis based on narrow-band imaging endocytoscopy for diagnosing colorectal lesions: comparison with experts. Int J Comput Assist Radiol Surg 2017;12:757-66. [Crossref] [PubMed]
- Kudo SE, Mori Y, Abdel-Aal UM, et al. Artificial intelligence and computer-aided diagnosis for colonoscopy: where do we stand now? Transl Gastroenterol Hepatol 2021;6:64. [Crossref] [PubMed]
- Misumi Y, Nonaka K, Takeuchi M, et al. Comparison of the Ability of Artificial-Intelligence-Based Computer-Aided Detection (CAD) Systems and Endoscopists to Detect Colorectal Neoplastic Lesions on Endoscopy Video. J Clin Med 2023;12:4840. [Crossref] [PubMed]
- Nakajima Y, Zhu X, Nemoto D, et al. Diagnostic performance of artificial intelligence to identify deeply invasive colorectal cancer on non-magnified plain endoscopic images. Endosc Int Open 2020;8:E1341-8. [Crossref] [PubMed]
- Saitoh Y, Obara T, Watari J, et al. Invasion depth diagnosis of depressed type early colorectal cancers by combined use of videoendoscopy and chromoendoscopy. Gastrointest Endosc 1998;48:362-70. [Crossref] [PubMed]
- Horie H, Togashi K, Kawamura YJ, et al. Colonoscopic stigmata of 1 mm or deeper submucosal invasion in colorectal cancer. Dis Colon Rectum 2008;51:1529-34. [Crossref] [PubMed]
- Wijnands AM, Mahmoud R, Lutgens MWMD, et al. Surveillance and management of colorectal dysplasia and cancer in inflammatory bowel disease: Current practice and future perspectives. Eur J Intern Med 2021;93:35-41. [Crossref] [PubMed]
- Barua I, Wieszczy P, Kudo S, et al. Real-time artificial intelligence-based optical diagnosis of neoplastic polyps during colonoscopy. NEJM Evid 2022;1:EVIDoa2200003.
- Rex DK, Kahi C, O'Brien M, et al. The American Society for Gastrointestinal Endoscopy PIVI (Preservation and Incorporation of Valuable Endoscopic Innovations) on real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest Endosc 2011;73:419-22. [Crossref] [PubMed]
Cite this article as: Syed KA, Koseki M, Satoi S, Park E, Simoes P, Nishimura M. Colon polyp characterization (morphology and mucosal patterns): clinical application and techniques. Ann Laparosc Endosc Surg 2023;8:30.


