Detection and surveillance of neoplastic lesions of the esophagus: application of guidelines and techniques
Introduction
Background
Esophageal cancer is a global health problem, with more than 572,000 new cases diagnosed per year worldwide and 20,640 new cases per year in the United States (1,2). Esophageal adenocarcinoma (EAC) and esophageal squamous cell carcinoma (ESCC) are the two main histological subtypes of esophageal cancer. Globally, ESCC constitutes 85% of all esophageal cancers, with EAC constituting the remainder (15%). There has been a shift in the epidemiology of esophageal cancer in the West, with a dramatically rising incidence rate of EAC (600%) over the past 3 to 4 decades (3).
Rationale and knowledge gap
Despite advances in management techniques, esophageal cancer continues to have a grim prognosis because it is often detected after the emergence of alarm symptoms. The rationale behind screening and surveillance is to decrease morbidity and mortality through early detection and management of precursor lesions and cancer.
Objective
We aim to perform a comprehensive review to highlight the recent screening and surveillance strategies for the detection of esophageal cancer.
EAC
Barrett’s esophagus (BE)
BE is a metaplastic transformation of the distal esophageal mucosa from normal squamous non-keratinized epithelium to specialized columnar epithelium with intestinal metaplasia (IM). BE is seen in approximately 5–15% of patients experiencing gastroesophageal reflux disease (GERD) (4). BE is the well-known precursor to EAC, with progressive malignant transformation from non-dysplastic BE (NDBE) to low-grade dysplasia (LGD), high-grade dysplasia (HGD), and ultimately invasive adenocarcinoma. Outcomes of EAC remain poor, with a 5-year survival rate of only around 20% (5). This grim prognosis is attributed to paucity of symptoms at early stages and an aggressive growth pattern with early metastasis due to lymphatic abundance in the submucosa. Substantially improved outcomes have been observed with the identification and treatment of early-stage EAC (5-year survival rates greater than 80%) (6).
A recent meta-analysis reported that only 12% of EAC patients had a prior diagnosis of BE, but concurrent BE was found in 57% of cases at the time of evaluation, reflecting a considerable missed opportunity for BE screening (7). However, capturing the target population who would be most appropriate for endoscopic screening and surveillance has been a dilemma partly due to a low incidence of EAC at the population level, with a global incidence rate of 0.7 per 100,000 person-years (8). Furthermore, the minimal progression rate from BE to EAC, with an annual risk of 0.12–0.5%, disregards the application of surveillance due to concerns of potential economic burden (4,9). A systematic review and meta-analysis by Codipilly et al. showed that surveillance might improve the detection of EAC at early stage with a better survival rate but could be potentially confounded by length and lead time biases (10). A randomized study is in progress to evaluate the efficacy of surveillance in reducing mortality related to EAC (11). With the advancement in BE risk prediction tools, minimally invasive, cost-effective screening devices, and novel endoscopic treatment modalities, this equation has changed favorably.
Indications for BE screening
Screening in the general population is not recommended but can be considered for high-risk individuals (Table 1) (12-17). Current guidelines recommend a single screening endoscopy for patients with chronic GERD symptoms (symptoms occurring > once/week for more than 5 years) and three or more additional risk factors for BE, including male sex, age >50 years, White race, current or past smoking, obesity, and family history of BE or EAC in a first-degree relative (12).
Table 1
| GI society | Screening guidelines |
|---|---|
| ACG | Patients with chronic GERD (defined as weekly symptoms for ≥5 years) and 3 or more additional risk factors for BE, including male sex, age >50 years, white race, tobacco smoking, obesity, and family history of BE or EAC in a first-degree relative (12) |
| AGA | Individuals with at least 3 established risk factors, including male sex, age >50 years, non-Hispanic white, smoking history, chronic GERD, obesity and a family history of BE/EAC (13) |
| ASGE | Screen “at-risk” population: Individuals with Family history of EAC or BE (high risk) or those with GERD plus at least 1 other risk factor for EAC (moderate risk) |
| Cited risk factors include age >50 years, obesity/central adiposity, history of smoking, or male gender (14) | |
| ACP | Men over the age of 50 years with chronic GERD symptoms (>5 years) and additional risk factors, such as nocturnal reflux symptoms, hiatal hernia, obesity, tobacco use, and intra-abdominal distribution of fat (15) |
| ESGE | High risk individuals with long-standing GERD symptoms (>5 years) and multiple risk factors (age ≥50 years, white race, male sex, obesity, or first-degree relative with BE or EAC) (16) |
| BSG | Chronic GERD symptoms and multiple risk factors (at least three of the following: age ≥50 years, white race, male sex, and obesity) |
| Threshold of multiple risk factors should be lowered in the presence of family history, including at least one first-degree relative with BE or EAC (17) |
BE, Barrett’s esophagus; GI, gastrointestinal; ACG, American College of Gastroenterology; GERD, gastroesophageal reflux disease; EAC, esophageal adenocarcinoma; AGA, American Gastroenterological Association; ASGE, American Society of Gastrointestinal Endoscopy; ACP, American College of Physicians; ESGE, European Society of Gastrointestinal Endoscopy; BSG, British Society of Gastroenterology.
A meta-analysis reported the prevalence of BE with known risk factors as follows: age >50 years (6.1%), male sex (6.8%), obesity (1.9%), family history of BE/EAC (23%), and GERD (2.3%). Interestingly, individuals with GERD and one additional risk factor had a higher prevalence (12.2%) than GERD alone (3.0%). Each additional risk factor was associated with a 1.2% increase in the prevalence of BE (18). Screening for BE is generally not advised in women or men younger than 50 years with chronic GERD but may be considered based on the presence of multiple risk factors.
Despite these societal guidelines, BE screening rates have remained low. The potential explanations include lack of knowledge about BE, the patient underreporting heartburn symptoms because of being mild or availability of effective empiric treatment, or hesitancy with physician ordering or patients proceeding with endoscopy. Challenging access to sedated endoscopy may be another factor (19,20).
To better target the high-risk population, BE/EAC risk assessment tools have been developed that incorporate various risk factors including age, sex, waist-hip ratio, and smoking history into numerical scores (Gerson, Locke, Thrift, M-BERET, HUNT, and Kunzmann tools). These tools have shown improved but modest accuracy [area under the receiver operating characteristic curve (AUROC), 0.66–0.69] in stratifying BE/EAC risk (21).
BE screening modalities
Sedated endoscopy
Conventional sedated per-oral endoscopy is the gold standard and widely used method for BE screening with excellent accuracy for diagnosing BE in combination with histopathology findings. Despite the high accuracy of novel endoscopic techniques discussed above, the widespread application of endoscopy remains limited due to the required expertise, invasiveness, and associated cost (22).
Unsedated transnasal endoscopy (uTNE)
It is an alternative to conventional esophagogastroduodenoscopy (EGD) for the diagnosis of BE, with a sensitivity of 91% and specificity of 96% for detecting IM (23). Unfortunately, it has not been widely adopted for BE screening, possibly because of physician (lack of working channel for biopsy, missing short segment BE lesions) and patient-related barriers (gagging, discomfort, nasal pain) (24). To overcome these limitations, various safe, cost-effective, minimally invasive non-endoscopic techniques for BE screening have been developed.
Capsule sponge/balloon cytology
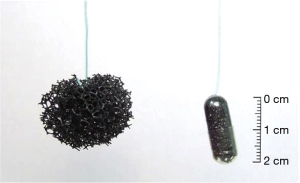
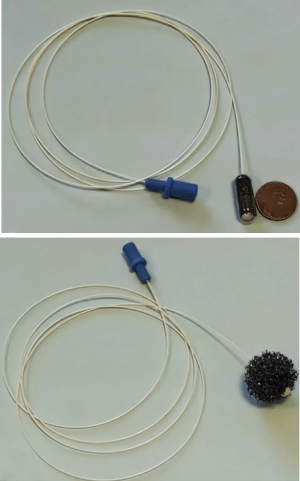
These are ingestible capsules containing a compressed polyurethane foam attached to a cord or suture or inflatable balloons. The capsule is swallowed, and once in the stomach, the outer shell dissolves, releasing a spherical piece of foam. The spherical foam is withdrawn through the mouth by traction on the attached string while obtaining esophageal cytology samples (Figures 1-3). These samples are then assessed for an immunohistochemistry (IHC) based marker [trefoil factor 3 (TFF3) or methylated DNA markers (MDMs)] to predict the presence of BE (25-27). A randomized control trial showed that the patients with chronic reflux who underwent the Cytosponge-TFF3 test had a significantly higher likelihood (10-fold) of being diagnosed with BE by confirmatory endoscopy (2% BE prevalence) in comparison to individuals who underwent EGD based on the provider’s discretion (28). Adverse events reported were mild gagging, throat discomfort, and a rare detachment of the string from the sponge.
Exhaled volatile organic compounds testing
Individuals exhale volatile organic compounds as a product of gut bacterial metabolism that can be detected using electronic nose devices. A disease altering the normal gut flora can be detected using this device using “breath prints”, which are unique to diseases. A recent single-center data suggest that these devices can detect BE with a sensitivity of 91% and specificity of 74% independent of proton pump inhibitor (PPI) use, the presence of hiatal hernia, and reflux (29).
Diagnosis of BE
The criteria for BE diagnosis include (Table 2):
Table 2
| Guidelines | Length of columnar metaplasia in esophagus | Histological criteria |
|---|---|---|
| ACG | ≥1 cm | IM |
| AGA | None | IM |
| ASGE | None | IM |
| BSG | ≥1 cm | Columnar metaplasia |
| ESGE | ≥1 cm | IM |
BE, Barrett’s esophagus; GI, gastrointestinal; ACG, American College of Gastroenterology; AGA, American Gastroenterological Association; ASGE, American Society of Gastrointestinal Endoscopy; BSG, British Society of Gastroenterology; ESGE, European Society of Gastrointestinal Endoscopy; IM, intestinal metaplasia.
- Endoscopic evidence of salmon-colored BE mucosa measuring at least 1 cm above the gastroesophageal junction (GEJ) (measured endoscopically as the top of the gastric folds). Studies have demonstrated an exceedingly low risk of progression to dysplasia and EAC with columnar segments <1 cm (30). A study of 102 patients exhibiting an irregular Z line, who were followed for a median of 70 months, demonstrated only 2 patients developing LGD as the most advanced pathology. However, 8.8% of them were later diagnosed with short-segment BE (defined as the columnar metaplasia ≥1 cm and <3 cm in the tubular esophagus) (31).
- Histopathological evidence of IM characterized by the presence of goblet cells. Most international society guidelines require the presence of IM for diagnosis of BE due to the recognized high risk of EAC. However, guidelines from the British Society of Gastroenterology do not require the presence of IM but do state that only IM-positive BE cases require surveillance (17). A comprehensive study involving 487 subjects of which 86 patients had IM of the GEJ and 401 patients had BE with a median of 8 years, showed no progression to HGD or cancer in the IM-GEJ group. In contrast, the BE group exhibited a 10-year cumulative risk of progression of 7% and an increased mortality risk from EAC with a standardized mortality ratio of 9.62 (32).
Best practices for BE surveillance
The effectiveness of endoscopic surveillance is compromised by the uneven distribution of dysplasia in BE mucosa and suboptimal adherence with surveillance biopsy recommendations. Therefore, best practices for BE surveillance have been discussed, which involve: (I) high-quality endoscopic examination of the BE segment; (II) appropriate and effective sampling; and (III) following appropriate surveillance intervals.
- Endoscopic examination. High-quality endoscopic examination is critical to an effective surveillance program. Using a transparent distal attachment cap at the tip of the endoscope enables better visualization by stabilizing the mucosa and counteracting esophageal motility (33). Basics of endoscopic surveillance should be followed, which include cleaning the mucosa of any debris and mucus, adopting a systematic approach to inspect the mucosa (distal to proximal), and devoting adequate time for this purpose. Ensuring adequate patient sedation and insufflation of the esophagus is also important to allow careful inspection. High-definition white-light endoscopy (WLE) and virtual chromoendoscopy (VC) should be used during the evaluation of BE segment along with the antegrade and retrograde assessment of the GEJ to improve the detection of subtle lesions with dysplasia and carcinoma (34). During initial evaluation, it is essential to identify and document key landmarks, including GEJ, diaphragmatic hiatus, and squamo-columnar junction (35). The Prague classification is the standard description method for the BE segment which involves documenting measurements pertaining to the circumferential extent (C value) and maximum extent (M value) from the proximal margin of the gastric folds and assists in treatment planning (36).
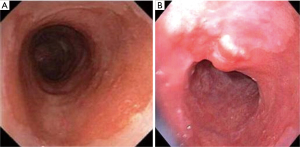
- Appropriate and effective sampling. Surveillance biopsies are recommended if there is endoscopic evidence of BE, measuring at least 1 cm. Any visible lesions observed should be sampled separately, either through biopsy or resection (Figure 4) and remaining BE mucosa should be biopsied using the Seattle protocol. It entails four quadrant biopsies at intervals of every 2 cm (in the absence of no known or suspected dysplasia) or 1 cm (with presence of mucosal irregularities or prior dysplasia) (37). The rationale is to detect more dysplasia by reducing sampling error and missing lesions with dysplasia that might be occult, focal, or variably distributed in Barrett’s segment. Data supporting this comes from a study done by Harrison et al. that suggested that eight random biopsies analyzed with conventional hematoxylin and eosin (H&E) staining are optimum to diagnose benign IM with a yield of 67.9% (38). In instances where obtaining eight biopsies is not feasible, at least four biopsies per cm of circumferential BE and one biopsy per cm in tongues of BE should be obtained.
- Surveillance interval. The primary objective of endoscopic surveillance is to identify presence of IM and closely monitor for progression to dysplasia (LGD/HGD) and early EAC, enabling endoscopic interventions with improved outcomes (12). The recommended surveillance intervals are based on the severity of dysplasia (Table 3) considering the variation in progression to cancer. The annual risk of progression in NDBE is relatively low, estimated at 0.33% compared to LGD, which ranges from 0.7% to 1%. The highest risk of progression is observed with HGD, estimated to be 7% to 8% (39-41).
Table 3
Recommendations for management of patients with BE stratified by dysplasia grade and risk of progression (12)Grade of dysplasia Risk of progression to HGD/EAC Recommendation for endoscopic surveillance No dysplasia 0.33% per year Endoscopic surveillance: <3 cm length every 3 years ≥3 cm length every 5 years Indefinite for dysplasia – Repeat EGD within 6 months after increasing PPI to twice daily If repeat EGD yields NDBE or LGD, manage using the specific algorithm If repeat EGD shows BE indefinite for dysplasia, EGD annually LGD 0.7% to 1.0% per year Confirm diagnosis by expert gastrointestinal pathologist Discuss endoscopic ablation Endoscopic surveillance at 6 months, 12 months and annually thereafter HGD 8% per year Confirm diagnosis by expert gastrointestinal pathologist Refer for endoscopic therapy to center with expertise for Endoscopic resection of visible lesions Endoscopic ablation After successful EET, surveillance EGD at 3 months, 6 months, 12 months, and annually thereafter T1a (mucosal) adenocarcinoma NA Refer for potentially endoscopic therapy to center with expertise After successful EET, surveillance EGD at 3 months, 6 months, 12 months, and annually thereafter T1b (submucosal) adenocarcinoma NA Refer for staging and evaluation by multidisciplinary (gastrointestinal, thoracic surgery, and oncology) team BE, Barrett’s esophagus; HGD, high-grade dysplasia; EAC, esophageal adenocarcinoma; EGD, esophagogastroduodenoscopy; PPI, proton pump inhibitor; NDBE, non-dysplastic Barrett’s esophagus; LGD, low-grade dysplasia; EET, endoscopic eradication therapy; NA, not applicable.
NDBE
Most gastroenterology society guidelines recommend that surveillance should be performed every 3–5 years (12,14,17). Increasing BE segment length is associated with a significantly increased risk of progression to HGD/EAC (42,43). The most recent American College of Gastroenterology (ACG) guidelines recommend patients diagnosed with NDBE undergo endoscopic surveillance every 5 years (with short-segment BE) and every 3 years (with long-segment BE) (12). Additionally, PPI therapy is recommended to prevent symptoms, heal esophagitis and reduce the risk of HGD and EAC (44).
BE with indefinite dysplasia (IND)
BE with IND is observed in approximately 4.3–8.4% of BE biopsies, which may reflect the pathological feature of inflammation-related atypia overlapping with dysplasia (45). Confirmed cases should be treated with anti-reflux therapy to address any underlying esophagitis (12). Follow-up endoscopy should be performed in 6 months to assess for regression to NDBE or progression to LGD, determining subsequent surveillance intervals based on those findings. If IND is observed on repeat endoscopy, surveillance should be performed every 12 months until the resolution of dysplasia.
BE with LGD
LGD should be confirmed with a second experienced pathologist due to interobserver variability among pathologists and the implications related to dysplasia diagnosis in terms of the need for endoscopic eradication therapy (EET) or more frequent surveillance (46). Radiofrequency ablation (RFA) can reduce the likelihood of progression to HGD/EAC and achieve complete eradication of IM (47). However, considering the potential adverse events of ablation, shared decision-making is recommended to determine the most appropriate management (48). The recent ACG guidelines suggest EET for confirmed LGD. An alternate approach is to pursue surveillance with repeat endoscopy every 6 months for 1 year followed by annual surveillance (12).
BE with HGD
Confirmed HGD patients should undergo resection of visible lesions for therapeutic and better diagnostic accuracy, followed by ablation of remaining BE (12). EET is advised over esophagectomy, considering no significant difference in terms of achieving complete eradication and overall mortality (12,49). After successful endoscopic therapy, surveillance is recommended at 3, 6, and 12 months, followed by annual surveillance (12).
Quality metrics for BE surveillance
Quality benchmarks have been proposed in BE endoscopy for effective surveillance, decreasing rates of missed dysplasia, and improving outcomes. These include adherence to defining landmarks and extent of BE, not obtaining biopsies in the setting of a normal-appearing squamocolumnar junction, refraining from obtaining biopsies if mucosa appears normal, following Seattle biopsy protocol, and performing surveillance endoscopy in patients with NDBE at an appropriate interval of 3–5 years. Similar to the validated adenoma detection rate in colonoscopy, the neoplasia detection rate (NDR) has emerged as a potential quality metric in BE surveillance. A recent cohort study reported NDR (rate of HGD/ EAC detection during initial surveillance endoscopy) of 4.9% [95% confidence interval (CI), 3.8–6.4%] with 3.1% of patients with HGD, 1.8% with EAC. Notably, this NDR has been linked to a significantly lower rate of missed dysplasia (50). A systemic review and metanalysis by Hamade et al. found a statistically significant inverse correlation between NDR and post-endoscopy Barrett’s neoplasia (rate of HGD/EAC on repeat endoscopy within 1 year of an index screening examination revealing NDBE/LGD) (51).
Limitations of current dysplasia detection strategies
Despite the advancement in the surveillance of BE, its effectiveness remains limited due to numerous issues, such as the patchy distribution of dysplasia in the BE segment and non-adherence to the Seattle protocol and surveillance best practices (52).
Even complete compliance with the Seattle protocol targets only 5–10% of the entire BE mucosa, particularly in those with LSBE, subtle or patchy lesions resulting in missed dysplasia. Recently, the concept of post-endoscopy esophageal cancer (PEEC) rate has been studied which is defined as the diagnosis of BE-related HGD and EAC within a year of an endoscopic surveillance evaluation that was negative for dysplasia. A multicenter study reported the pooled proportion of PEEC to be 26% (95% CI: 19–34%, I2=93.4%) (53). Missed lesions during endoscopy, incomplete resection or ablation, and rapidly progressive cancer are potential explanations for PEEC.
To decrease the rate of PEEC, an expert panel proposed recommendations, including the use of high-definition WLE/chromoendoscopy, allocating sufficient time for a thorough inspection, standardized reporting using the Prague classification and adhering to the Seattle biopsy protocol. Additionally, the panel suggested a reevaluation of surveillance intervals for BE to identify populations at risk for PEEC (54).
Strategies to improve detection of dysplasia in BE
Advanced imaging to enhance visualization of mucosal abnormalities
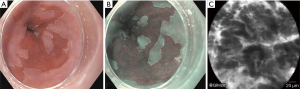
Despite careful inspection using WLE, subtle lesions might be missed (Figure 5). Numerous imaging modalities have been explored to enhance the endoscopic detection of dysplasia in BE. Chromoendoscopy uses dye such as acetic acid or methylene blue to enhance the vasculature and mucosal patterns. Acetic acid application leads to whitening of BE mucosa (“aceto-whitening”), and dysplasia/neoplasia can be identified by early loss of the whitening effect. However, it is cumbersome and time-consuming, considering the need to spray and suction dye.
VC techniques employing optical imaging technologies like narrow-band imaging (NBI) (Olympus, Tokyo, Japan), i scan (Pentax, Tokyo, Japan), or blue-laser imaging (Fujinon, Tokyo, Japan) have become more prevalent. They offer more convenience due to integration into the endoscope and activation via buttons, eliminating the need for additional dyes and spray catheters). NBI in particular has been utilized to develop a classification system aimed at enhancing dysplasia detection in patients with BE (Table 4) (55).
Table 4
| Classification | Mucosal pattern | Vascular patterns |
|---|---|---|
| Regular | Circular, ridged, villous, or tubular regular | Regular blood vessel appearance along or between mucosal ridges with normal, long-branching patterns |
| Irregular | Absent or irregular | Focally or diffusely distributed vessels not following normal mucosal architecture |
NBI, narrow-band imaging; BE, Barrett’s esophagus.
Qumseya et al. demonstrated a 33% increase in the detection of dysplasia using of any of these adjunctive imaging modalities (56). NBI targeted biopsies have proven to be equally effective as the Seattle protocol in dysplasia detection in BE patients and also in screening for BE in GERD patients (57,58). Confocal laser endomicroscopy (CLE) is an imaging modality that enables real-time mesoscopic histology evaluation, allowing differentiation between normal and dysplastic tissue (59). Another advanced imaging technology volumetric lase endomicroscopy (VLE), utilizes optical coherence tomography to generate a three-dimensional (3D), microscopic, cross-sectional scan of the esophageal wall, including subsurface layers (60). This technology is unfortunately not available commercially at this time.
Recent guidelines strongly recommend the use of VC with high-definition white light endomicroscopy for all BE patients undergoing screening/surveillance endoscopy (12,14).
Novel sampling techniques
A recent development is wide-area transepithelial sampling-3D (WATS-3D). This method serves as an adjunct to forceps biopsies (FBs) and involves computer-assisted biopsy using an abrasive cytology brush. It allows for sampling deeper layer of the BE mucosa, reaching depths of up to 150 mm, and covers a wider area for sampling. The samples are then reconstructed as 3D images using a neural network system which highlights areas with high-risk features. As an adjunct to FB, WATS improved the absolute detection rate of BE by 16% and HGD/EAC by 2% compared to FB alone (61). A recent cost-effectiveness analysis using a decision analytic model showed that screening for BE in a reference 60-year-old white male GERD patient was more cost-effective when WATS-3D is used adjunctively to the Seattle protocol (62).
Biomarkers as adjuncts to dysplasia detection
LGD is associated with variable rates of malignant progression, dependent on confirmation by an expert pathologist. In a Dutch study, 73% of suspected LGD patients were down-staged, but confirmed cases had a substantial risk (9.1% per patient-year) of progressing to HGD/EAC (63). A risk stratification biomarker is crucial to enhance the efficacy of BE surveillance given the low incidence of EAC in the general population and the variable risk of progression in those with known histopathological diagnosis of BE.
Aberrant expression of p53 has been shown to be associated with both prevalent and incident HGD/EAC. A large meta-analysis revealed that aberrant p53 immunostaining was associated with a 4–17-fold increased risk of progression to HGD or EAC (64).
Chromosomal abnormalities have also been associated with the development of BE. A recent study revealed aneuploidy in 7% of NDBE, 68% of HGD, and 96% of EAC. Key abnormalities included gains in 1q, 12q, and 20q, losses in 9p and 17p, and 8q gains in NDBE and 8q24 gains specifically in dysplasia (65). These abnormalities were demonstrated on endoscopic brushing specimens using next-generation sequencing technology.
Fluorescent in situ hybridization (FISH) has been investigated to detect chromosomal abnormalities and dysplasia within BE. FISH successfully differentiated HGD/EAC when >10% of the cells exhibited polysomy (>2 signals per probe), with a sensitivity of 80% and specificity of 88 % (66). Despite promising results, its use is limited due to the requirement for manual interpretation of findings, and limited set of specific genes.
The TissueCypher pathology assay analyzes several protein-based biomarkers and tissue morphology to generate a risk score [0–10] to prognosticate the risk for progression to HGD/EAC within 5 years (67,68). Future research would help validate and implement these tools to stratify the population better and individualize treatment based on the risk of progression.
Artificial intelligence (AI) in the detection of BE dysplasia
Deep-learning computer-aided systems algorithms have been developed to assist in detecting neoplasia in patients with BE (69). Highlighting areas which are suspicious for dysplasia using AI could assist in directed sampling of mucosa and improve dysplasia detection. A computer-assisted detection model developed by Struyvenberg et al. demonstrated a remarkable 89% accuracy, 90% sensitivity, and 88% specificity in identifying NDBE and BE-related neoplasia (70). The promising results of AI have sparked ongoing research to explore the potential implications of incorporating machine learning into BE surveillance.
ESCC
While EAC dominates in the United States, globally, ESCC is the more prevalent type of esophageal cancer. ESCC makes up about 90% of esophageal cancer worldwide and is particularly prevalent in the region known as the “esophageal cancer belt”—which spans from China to Northern Iran and Turkey in Central Asia (71). Analogous to BE, the development of ESCC is preceded by low-grade intraepithelial neoplasia (LGIN), progressing to high-grade intraepithelial neoplasia (HGIN) and ESCC.
Most patients remain asymptomatic until the cancer progresses to advanced stages, resulting in dysphagia and weight loss. In contrast to EAC, there are no established protocols for screening ESCC in the US or most parts of the world. ESCC has a poor prognosis, and survival dramatically depends on the disease stage at the time of diagnosis. According to a study that analyzed the SEER database from 2001–2007, the 5-year survival for those with localized, regional, and distant disease were 37%, 18%, and 3%, respectively. In contrast, the 5-year survival for stage T1 ESCC (86%) was significantly better (72). Favorable outcomes have been reported with the detection of early-stage ESCC due to lower rates of lymph node involvement and amenable to successful resection (72). Early screening and diagnosis significantly improved 5-year survival in patients with secondary ESCC compared to those who were diagnosed after symptoms onset (60.4% vs. 0.0%, P<0.01) (73). With a relative risk of up to 28.3 for ESCC, moderate and high-grade squamous dysplasia is an ideal target for screening and endoscopic therapy (74). Poor prognosis and asymptomatic presentation at the early stages of ESCC make it critical to identify and screen the high-risk population to improve outcomes.
Risk factors and screening
Besides the traditional risk factors, including alcohol and tobacco, multiple synergistic risks have been identified with the development of ESCC, which include hot beverages, low fruit intake, nitrosamines compounds, inflammatory conditions including Lichen planus, and genetic conditions including tylosis (Table 5) (75). In an endemic population in China, a risk prediction model incorporating >10 variables successfully predicted the occurrence of severe squamous dysplasia (AUROC, 0.62–0.85), with age emerging as the most significant predictor (76).
Table 5
| Risk factor | Increase in risk (vs. general population) |
|---|---|
| Alcohol | 2–9× |
| Tobacco | 2–4× |
| Consumption of hot beverages | 1.5–2.5× |
| Low fruit intake | 2× |
| High pickled vegetables | 2× |
| Low socio-economic status | 2–4× |
| Esophageal Lichen planus | Up to 6.1% of all affected |
| Tylosis | Up to 80% of all affected |
ESCC, esophageal squamous cell carcinoma.
Screening for ESCC is challenging given the variability in incidence and overall risk, even in different regions of the same country. There is limited evidence demonstrating the effectiveness and cost-benefit of screening to reduce ESCC related mortality. However, there is a growing inclination to consider ESCC screening in specific populations with pre-existing conditions associated with high-risk or poor prognosis in relation to ESCC, such as prior head and neck squamous cell carcinoma, tylosis, achalasia, and caustic ingestion. The strongest evidence supporting ESCC screening comes from studies conducted in endemic regions of China. One-time screening endoscopy with Lugol’s iodine stain and subsequent therapy for dysplasia led to a lower incidence of ESCC (4.17% vs. 5.92%) and improved mortality (3.35% vs. 5.05%) after 10 years (77). A cost-effectiveness analysis study indicated that one-time screening EGD is a viable strategy in high-risk regions, and other approaches like screening endoscopy every 10 years from age 40 years were also deemed cost-effective (78). However, these studies are region specific and have not been replicated in the rest of the world, limiting their generalizability.
Diagnosis of ESCC
Patients may be diagnosed through screening programs in high-risk populations or as a workup for symptoms suspected of ESCC, including dysphagia, hematemesis, persistent heartburn, or dyspepsia. Suspected patients should undergo a biopsy/resection of the lesion seen on endoscopic evaluation. Diagnosis is confirmed by histopathologic evidence of squamous dysplasia, and grading is performed based on the extent of nuclear atypia (such as enlargement, pleomorphism, and hyperchromasia), cellular atypia, and loss of normal tissue maturation without any basement membrane invasion. Worsening grades of dysplasia seen in histology have been associated with a higher risk of developing ESCC (Table 6) (79). Confirmed cases of squamous dysplasia/ESCC should be followed with EUS and computed tomography to evaluate the regional lymph node and distant metastatic spread. Patients should be subjected to surveillance to monitor progression and endoscopic therapy for dysplastic lesions.
Table 6
| Dysplasia grade | WHO classification | OR (95% CI) |
|---|---|---|
| Basal cell hyperplasia | Intra-epithelial neoplasia | 2.1 (0.4–0.8) |
| Mild dysplasia | Low-grade intra-epithelial neoplasia | 2.2 (0.7–7.5) |
| Moderate dysplasia | 15.8 (5.9–42.2) | |
| Severe dysplasia | High-grade intra-epithelial neoplasia | 72.6 (29.8–176.9) |
ESCC, esophageal squamous cell carcinoma; WHO, World Health Organization; OR, odds ratio; CI, confidence interval.
While there is some evidence that screening ESCC reduces mortality, the implementation of screening and surveillance for ESCC at the population level has been limited due to the high cost, invasiveness, and varying efficacy of current modalities. However, efforts are ongoing to develop novel strategies, imaging techniques and biomarkers to enable more cost-effective, accurate and accessible screening for a larger population.
Minimally invasive cytology devices
Novel non-endoscopic devices, such as balloons, meshes and sponges have been investigated to enable comprehensive sampling of exfoliated cells from esophageal mucosa. Early studies conducted in high-risk populations from China demonstrated a sensitivity (≤45%) and specificity (≤82%) of diagnosing dysplasia and cancer using cytology collected with these devices (80,81).
Promising MDMs have been developed for the non-endoscopic detection of esophageal squamous cancer using cytology specimens obtained by swallowed cell collection devices) (82,83). Studies in the United States optimized the use of highly discriminant MDM biomarkers for diagnosing ESCC across three geographic regions with varying incidences of ESCC (84,85). The TBX 15 marker demonstrated high accuracy in detecting in US, Iranian, and Chinese tissues, with area under the curve (AUC) of 0.99, 0.93, and 0.93, respectively. Additionally, the US model was cross-validated in the Iranian and Chinese tissues with AUCs of 0.90 and 0.87, respectively. The levels of MDMs increased with the severity of dysplasia (86).
Endoscopic imaging techniques to detect squamous dysplasia and carcinoma
Standard WLE may have limitations in detecting dysplastic lesions, as these lesions are often flat or minimally raised/depressed and may not be clearly distinguishable from surrounding tissue. Advanced endoscopy techniques using Lugol’s chromoendoscopy/VC are the most widely accepted technique for evaluating ESCC (87). This technique involves spraying Lugol’s iodine solution to the esophageal surface, which stains the glycogen-containing squamous epithelium brown and facilitates detection and targeted biopsies of abnormal squamous epithelium, as evidenced by unstained lesions. Chromoendoscopy is a reliable method for detecting esophageal squamous dysplasia with a sensitivity of 92–100% and specificity of 37–82% (88,89). Methylene blue and indigo carmine are alternate dyes that have been used in chromoendoscopy. Minor side effects such as nausea, chest pain, and allergic reactions have been seen with Lugol’s iodine (90).
Similar to BE, endoscopy techniques have been evolving to better characterize squamous dysplasia. These include NBI, CLE, and high-resolution microendoscopy (HRM).
NBI
NBI had similar sensitivity to Lugol’s chromoendoscopy (94% and 98%) in per-lesion analysis to diagnose HGD and SCC and superior in terms of specificity (65% and 37%). In the per-patient analysis, the AUROC for NBI (0.961) was similar compared to Lugol chromoendoscopy (0.956) (91).
CLE
CLE allows for enhanced and magnified visualization of esophageal squamous epithelium and vascular networks. In a small prospective cohort study, it was associated with sensitivity and specificity were 100% and 87%, respectively, for the diagnosis of SCC (92).
HRM
HRM is a cost-effective alternative that utilizes a fiber-optic micro endoscope probe to depict cellular features after the application of a topical fluorescent agent (93). A prospective screening trial found that HRM markedly enhanced the accuracy of detecting squamous dysplasia compared to Lugol’s alone, demonstrating higher specificity (88% vs. 48%, P<0.001) and positive predictive value (45% vs. 22%, P<0.0001) (94).
Conclusions
In summary, the prognosis of esophageal cancer remains poor due to the limitations in screening and surveillance practices. It is critical to validate non-endoscopic tools for application at the population level and implement adherence to high-quality surveillance protocols, including the use of high-definition WLE with adjunct chromoendoscopy imaging, following systemic sampling protocol with adequate surveillance intervals. Future implementation of minimally invasive screening tools, identification of the prognostic clinical and biomarker tools, and adherence to the quality metrics for BE detection will potentially result in significant improvement in the mortality and morbidity related to esophageal cancer.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Terry L. Jue) for the series “A U.S. Perspective on Endoscopic Resection of Neoplastic Lesions of the Gastrointestinal Tract” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Peer Review File: Available at https://ales.amegroups.com/article/view/10.21037/ales-23-11/prf
Conflicts of Interest: All authors have completed the ICMJE disclosure form (available at https://ales.amegroups.com/article/view/10.21037/ales-23-11/coif). The series “A U.S. Perspective on Endoscopic Resection of Neoplastic Lesions of the Gastrointestinal Tract” was commissioned by the editorial office without any funding or sponsorship. PGI reports that he received research grants and consulting fees from Exact Sciences, Pentax Medical Corporation, CDx Medical, and Castle Biosciences, as well as consulting fees from Ambu. PGI has applied for patents with publication numbers: 20230190243 and 20220071605. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Thrift AP. Global burden and epidemiology of Barrett oesophagus and oesophageal cancer. Nat Rev Gastroenterol Hepatol 2021;18:432-43. [Crossref] [PubMed]
- National Cancer Institute. Surveillance, Epidemiology and ERP. Cancer Stat Facts: Esophageal Cancer. Accessed February 10, 2020; assessed Jan 16, 2023. Available online: https://seer.cancer.gov/statfacts/html/esoph.html
- Rubenstein JH, Shaheen NJ. Epidemiology, Diagnosis, and Management of Esophageal Adenocarcinoma. Gastroenterology 2015;149:302-17.e1. [Crossref] [PubMed]
- Shaheen NJ, Richter JE. Barrett's oesophagus. Lancet 2009;373:850-61. [Crossref] [PubMed]
- Haiyu Z, Xiaofeng P, Xiangqiong M, et al. Incidence and Survival Changes in Patients with Esophageal Adenocarcinoma during 1984-2013. Biomed Res Int 2019;2019:7431850. [Crossref] [PubMed]
- Prasad GA, Wu TT, Wigle DA, et al. Endoscopic and surgical treatment of mucosal (T1a) esophageal adenocarcinoma in Barrett's esophagus. Gastroenterology 2009;137:815-23. [Crossref] [PubMed]
- Tan MC, Mansour N, White DL, et al. Systematic review with meta-analysis: prevalence of prior and concurrent Barrett's oesophagus in oesophageal adenocarcinoma patients. Aliment Pharmacol Ther 2020;52:20-36. [Crossref] [PubMed]
- Coleman HG, Xie SH, Lagergren J. The Epidemiology of Esophageal Adenocarcinoma. Gastroenterology 2018;154:390-405. [Crossref] [PubMed]
- Hvid-Jensen F, Pedersen L, Drewes AM, et al. Incidence of adenocarcinoma among patients with Barrett's esophagus. N Engl J Med 2011;365:1375-83. [Crossref] [PubMed]
- Codipilly DC, Chandar AK, Singh S, et al. The Effect of Endoscopic Surveillance in Patients With Barrett's Esophagus: A Systematic Review and Meta-analysis. Gastroenterology 2018;154:2068-86.e5. [Crossref] [PubMed]
- Old O, Moayyedi P, Love S, et al. Barrett's Oesophagus Surveillance versus endoscopy at need Study (BOSS): protocol and analysis plan for a multicentre randomized controlled trial. J Med Screen 2015;22:158-64. [Crossref] [PubMed]
- Shaheen NJ, Falk GW, Iyer PG, et al. Diagnosis and Management of Barrett's Esophagus: An Updated ACG Guideline. Am J Gastroenterol 2022;117:559-87. [Crossref] [PubMed]
- Muthusamy VR, Wani S, Gyawali CP, et al. AGA Clinical Practice Update on New Technology and Innovation for Surveillance and Screening in Barrett's Esophagus: Expert Review. Clin Gastroenterol Hepatol 2022;20:2696-706.e1. [Crossref] [PubMed]
- ASGE Standards of Practice Committee. ASGE guideline on screening and surveillance of Barrett's esophagus. Gastrointest Endosc 2019;90:335-59.e2. [Crossref] [PubMed]
- Shaheen NJ, Weinberg DS, Denberg TD, et al. Upper endoscopy for gastroesophageal reflux disease: best practice advice from the clinical guidelines committee of the American College of Physicians. Ann Intern Med 2012;157:808-16. [Crossref] [PubMed]
- Weusten B, Bisschops R, Coron E, et al. Endoscopic management of Barrett's esophagus: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy 2017;49:191-8. [Crossref] [PubMed]
- Fitzgerald RC, di Pietro M, Ragunath K, et al. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett's oesophagus. Gut 2014;63:7-42. [Crossref] [PubMed]
- Qumseya BJ, Bukannan A, Gendy S, et al. Systematic review and meta-analysis of prevalence and risk factors for Barrett's esophagus. Gastrointest Endosc 2019;90:707-17.e1. [Crossref] [PubMed]
- Menezes A, Tierney A, Yang YX, et al. Adherence to the 2011 American Gastroenterological Association medical position statement for the diagnosis and management of Barrett's esophagus. Dis Esophagus 2015;28:538-46. [Crossref] [PubMed]
- Pohl H, Robertson D, Welch HG. Repeated upper endoscopy in the Medicare population: a retrospective analysis. Ann Intern Med 2014;160:154. [Crossref] [PubMed]
- Rubenstein JH, McConnell D, Waljee AK, et al. Validation and Comparison of Tools for Selecting Individuals to Screen for Barrett's Esophagus and Early Neoplasia. Gastroenterology 2020;158:2082-92. [Crossref] [PubMed]
- Moriarty JP, Shah ND, Rubenstein JH, et al. Costs associated with Barrett's esophagus screening in the community: an economic analysis of a prospective randomized controlled trial of sedated versus hospital unsedated versus mobile community unsedated endoscopy. Gastrointest Endosc 2018;87:88-94.e2. [Crossref] [PubMed]
- Shariff MK, Bird-Lieberman EL, O'Donovan M, et al. Randomized crossover study comparing efficacy of transnasal endoscopy with that of standard endoscopy to detect Barrett's esophagus. Gastrointest Endosc 2012;75:954-61. [Crossref] [PubMed]
- Sami SS, Iyer PG, Pophali P, et al. Acceptability, Accuracy, and Safety of Disposable Transnasal Capsule Endoscopy for Barrett's Esophagus Screening. Clin Gastroenterol Hepatol 2019;17:638-46.e1. [Crossref] [PubMed]
- Iyer PG, Taylor WR, Johnson ML, et al. Highly Discriminant Methylated DNA Markers for the Non-endoscopic Detection of Barrett's Esophagus. Am J Gastroenterol 2018;113:1156-66. [Crossref] [PubMed]
- Iyer PG, Taylor WR, Johnson ML, et al. Accurate Nonendoscopic Detection of Barrett's Esophagus by Methylated DNA Markers: A Multisite Case Control Study. Am J Gastroenterol 2020;115:1201-9. [Crossref] [PubMed]
- Iyer PG, Taylor WR, Slettedahl SW, et al. Validation of a methylated DNA marker panel for the nonendoscopic detection of Barrett's esophagus in a multisite case-control study. Gastrointest Endosc 2021;94:498-505. [Crossref] [PubMed]
- Fitzgerald RC, di Pietro M, O'Donovan M, et al. Cytosponge-trefoil factor 3 versus usual care to identify Barrett's oesophagus in a primary care setting: a multicentre, pragmatic, randomised controlled trial. Lancet 2020;396:333-44. [Crossref] [PubMed]
- Peters Y, Schrauwen RWM, Tan AC, et al. Detection of Barrett's oesophagus through exhaled breath using an electronic nose device. Gut 2020;69:1169-72. [Crossref] [PubMed]
- Thota PN, Vennalaganti P, Vennelaganti S, et al. Low Risk of High-Grade Dysplasia or Esophageal Adenocarcinoma Among Patients With Barrett's Esophagus Less Than 1 cm (Irregular Z Line) Within 5 Years of Index Endoscopy. Gastroenterology 2017;152:987-92. [Crossref] [PubMed]
- Itskoviz D, Levi Z, Boltin D, et al. Risk of Neoplastic Progression Among Patients with an Irregular Z Line on Long-Term Follow-Up. Dig Dis Sci 2018;63:1513-7. [Crossref] [PubMed]
- Jung KW, Talley NJ, Romero Y, et al. Epidemiology and natural history of intestinal metaplasia of the gastroesophageal junction and Barrett's esophagus: a population-based study. Am J Gastroenterol 2011;106:1447-55; quiz 1456. [Crossref] [PubMed]
- Kolb JM, Wani S. Barrett's esophagus: current standards in advanced imaging. Transl Gastroenterol Hepatol 2021;6:14. [Crossref] [PubMed]
- Sami SS, Subramanian V, Butt WM, et al. High definition versus standard definition white light endoscopy for detecting dysplasia in patients with Barrett's esophagus. Dis Esophagus 2015;28:742-9. [Crossref] [PubMed]
- Gorrepati VS, Sharma P. How Should We Report Endoscopic Results in Patient's with Barrett's Esophagus? Dig Dis Sci 2018;63:2115-21. [Crossref] [PubMed]
- Sharma P, Dent J, Armstrong D, et al. The development and validation of an endoscopic grading system for Barrett's esophagus: the Prague C & M criteria. Gastroenterology 2006;131:1392-9. [Crossref] [PubMed]
- Levine DS, Haggitt RC, Blount PL, et al. An endoscopic biopsy protocol can differentiate high-grade dysplasia from early adenocarcinoma in Barrett's esophagus. Gastroenterology 1993;105:40-50. [Crossref] [PubMed]
- Harrison R, Perry I, Haddadin W, et al. Detection of intestinal metaplasia in Barrett's esophagus: an observational comparator study suggests the need for a minimum of eight biopsies. Am J Gastroenterol 2007;102:1154-61. [Crossref] [PubMed]
- Desai TK, Krishnan K, Samala N, et al. The incidence of oesophageal adenocarcinoma in non-dysplastic Barrett's oesophagus: a meta-analysis. Gut 2012;61:970-6. [Crossref] [PubMed]
- Singh S, Manickam P, Amin AV, et al. Incidence of esophageal adenocarcinoma in Barrett's esophagus with low-grade dysplasia: a systematic review and meta-analysis. Gastrointest Endosc 2014;79:897-909.e4; quiz 983.e1, 983.e3.
- Rastogi A, Puli S, El-Serag HB, et al. Incidence of esophageal adenocarcinoma in patients with Barrett's esophagus and high-grade dysplasia: a meta-analysis. Gastrointest Endosc 2008;67:394-8. [Crossref] [PubMed]
- Chandrasekar VT, Hamade N, Desai M, et al. Significantly lower annual rates of neoplastic progression in short- compared to long-segment non-dysplastic Barrett's esophagus: a systematic review and meta-analysis. Endoscopy 2019;51:665-72. [Crossref] [PubMed]
- Krishnamoorthi R, Singh S, Ragunathan K, et al. Factors Associated With Progression of Barrett's Esophagus: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol 2018;16:1046-55.e8. [Crossref] [PubMed]
- Chen Y, Sun C, Wu Y, et al. Do proton pump inhibitors prevent Barrett's esophagus progression to high-grade dysplasia and esophageal adenocarcinoma? An updated meta-analysis. J Cancer Res Clin Oncol 2021;147:2681-91. [Crossref] [PubMed]
- Thota PN, Kistangari G, Esnakula AK, et al. Clinical significance and management of Barrett's esophagus with epithelial changes indefinite for dysplasia. World J Gastrointest Pharmacol Ther 2016;7:406-11. [Crossref] [PubMed]
- Montgomery E, Bronner MP, Goldblum JR, et al. Reproducibility of the diagnosis of dysplasia in Barrett esophagus: a reaffirmation. Hum Pathol 2001;32:368-78. [Crossref] [PubMed]
- Phoa KN, van Vilsteren FG, Weusten BL, et al. Radiofrequency ablation vs endoscopic surveillance for patients with Barrett esophagus and low-grade dysplasia: a randomized clinical trial. JAMA 2014;311:1209-17. [Crossref] [PubMed]
- Harrison M, Allen JE, Gorrepati VS, et al. Management of Barrett's esophagus with low-grade dysplasia. Dis Esophagus 2018; [Crossref] [PubMed]
- Standards of Practice Committee. Endoscopic eradication therapy for patients with Barrett's esophagus-associated dysplasia and intramucosal cancer. Gastrointest Endosc 2018;87:907-31.e9. [Crossref] [PubMed]
- Dhaliwal L, Codipilly DC, Gandhi P, et al. Neoplasia Detection Rate in Barrett's Esophagus and Its Impact on Missed Dysplasia: Results from a Large Population-Based Database. Clin Gastroenterol Hepatol 2021;19:922-9.e1. [Crossref] [PubMed]
- Hamade N, Kamboj AK, Krishnamoorthi R, et al. Systematic review with meta-analysis: neoplasia detection rate and post-endoscopy Barrett's neoplasia in Barrett's oesophagus. Aliment Pharmacol Ther 2021;54:546-59. [Crossref] [PubMed]
- Wani S, Williams JL, Komanduri S, et al. Endoscopists systematically undersample patients with long-segment Barrett's esophagus: an analysis of biopsy sampling practices from a quality improvement registry. Gastrointest Endosc 2019;90:732-41.e3. [Crossref] [PubMed]
- Desai M, Lieberman DA, Kennedy KF, et al. Increasing prevalence of high-grade dysplasia and adenocarcinoma on index endoscopy in Barrett's esophagus over the past 2 decades: data from a multicenter U.S. consortium. Gastrointest Endosc 2019;89:257-63.e3. [Crossref] [PubMed]
- Wani S, Yadlapati R, Singh S, et al. Post-endoscopy Esophageal Neoplasia in Barrett's Esophagus: Consensus Statements From an International Expert Panel. Gastroenterology 2022;162:366-72. [Crossref] [PubMed]
- Sharma P, Bergman JJ, Goda K, et al. Development and Validation of a Classification System to Identify High-Grade Dysplasia and Esophageal Adenocarcinoma in Barrett's Esophagus Using Narrow-Band Imaging. Gastroenterology 2016;150:591-8. [Crossref] [PubMed]
- Qumseya BJ, Wang H, Badie N, et al. Advanced imaging technologies increase detection of dysplasia and neoplasia in patients with Barrett's esophagus: a meta-analysis and systematic review. Clin Gastroenterol Hepatol 2013;11:1562-70.e1-2.
- Sharma P, Hawes RH, Bansal A, et al. Standard endoscopy with random biopsies versus narrow band imaging targeted biopsies in Barrett's oesophagus: a prospective, international, randomised controlled trial. Gut 2013;62:15-21. [Crossref] [PubMed]
- Elsheaita A, El-Bially MA, Shamseya MM, et al. Seattle protocol vs narrow band imaging guided biopsy in screening of Barrett's esophagus in gastroesophageal reflux disease patients. Medicine (Baltimore) 2020;99:e19261. [Crossref] [PubMed]
- Sharma P, Meining AR, Coron E, et al. Real-time increased detection of neoplastic tissue in Barrett's esophagus with probe-based confocal laser endomicroscopy: final results of an international multicenter, prospective, randomized, controlled trial. Gastrointest Endosc 2011;74:465-72. [Crossref] [PubMed]
- Smith MS, Cash B, Konda V, et al. Volumetric laser endomicroscopy and its application to Barrett's esophagus: results from a 1,000 patient registry. Dis Esophagus 2019;32:doz029. [Crossref] [PubMed]
- Suresh Kumar VC, Harne P, Patthipati VS, et al. Wide-area transepithelial sampling in adjunct to forceps biopsy increases the absolute detection rates of Barrett's oesophagus and oesophageal dysplasia: a meta-analysis and systematic review. BMJ Open Gastroenterol 2020;7:e000494. [Crossref] [PubMed]
- Singer ME, Smith MS. Wide Area Transepithelial Sampling with Computer-Assisted Analysis (WATS3D) Is Cost-Effective in Barrett's Esophagus Screening. Dig Dis Sci 2021;66:1572-9. [Crossref] [PubMed]
- Duits LC, Phoa KN, Curvers WL, et al. Barrett's oesophagus patients with low-grade dysplasia can be accurately risk-stratified after histological review by an expert pathology panel. Gut 2015;64:700-6. [Crossref] [PubMed]
- Snyder P, Dunbar K, Cipher DJ, et al. Aberrant p53 Immunostaining in Barrett's Esophagus Predicts Neoplastic Progression: Systematic Review and Meta-Analyses. Dig Dis Sci 2019;64:1089-97. [Crossref] [PubMed]
- Konda VJA, Ellison A. The Utility of Biomarkers for Risk Stratification in Barrett’s Esophagus. Foregut 2021;1:41-7. [Crossref]
- Poneros JM, Faye AS, Barr Fritcher EG, et al. A Multicenter Study of a Fluorescence In Situ Hybridization Probe Set for Diagnosing High-Grade Dysplasia and Adenocarcinoma in Barrett's Esophagus. Dig Dis Sci 2017;62:1216-22. [Crossref] [PubMed]
- Prichard JW, Davison JM, Campbell BB, et al. TissueCypher(™): A systems biology approach to anatomic pathology. J Pathol Inform 2015;6:48. [Crossref] [PubMed]
- Critchley-Thorne RJ, Duits LC, Prichard JW, et al. A Tissue Systems Pathology Assay for High-Risk Barrett's Esophagus. Cancer Epidemiol Biomarkers Prev 2016;25:958-68. [Crossref] [PubMed]
- Struyvenberg M, Kahn A, Fleischer D, et al. Expert assessment on volumetric laser endomicroscopy full scans in Barrett's esophagus patients with or without high grade dysplasia or early cancer. Endoscopy 2021;53:218-25. [Crossref] [PubMed]
- Struyvenberg MR, de Groof AJ, van der Putten J, et al. A computer-assisted algorithm for narrow-band imaging-based tissue characterization in Barrett's esophagus. Gastrointest Endosc 2021;93:89-98. [Crossref] [PubMed]
- Abnet CC, Arnold M, Wei WQ. Epidemiology of Esophageal Squamous Cell Carcinoma. Gastroenterology 2018;154:360-73. [Crossref] [PubMed]
- Wang GQ, Jiao GG, Chang FB, et al. Long-term results of operation for 420 patients with early squamous cell esophageal carcinoma discovered by screening. Ann Thorac Surg 2004;77:1740-4. [Crossref] [PubMed]
- Murakami S, Hashimoto T, Noguchi T, et al. The utility of endoscopic screening for patients with esophageal or head and neck cancer. Dis Esophagus 1999;12:186-90. [Crossref] [PubMed]
- Wang GQ, Abnet CC, Shen Q, et al. Histological precursors of oesophageal squamous cell carcinoma: results from a 13 year prospective follow up study in a high risk population. Gut 2005;54:187-92. [Crossref] [PubMed]
- Codipilly DC, Wang KK. Squamous Cell Carcinoma of the Esophagus. Gastroenterol Clin North Am 2022;51:457-84. [Crossref] [PubMed]
- Liu M, Liu Z, Cai H, et al. A Model To Identify Individuals at High Risk for Esophageal Squamous Cell Carcinoma and Precancerous Lesions in Regions of High Prevalence in China. Clin Gastroenterol Hepatol 2017;15:1538-46.e7. [Crossref] [PubMed]
- Wei WQ, Chen ZF, He YT, et al. Long-Term Follow-Up of a Community Assignment, One-Time Endoscopic Screening Study of Esophageal Cancer in China. J Clin Oncol 2015;33:1951-7. [Crossref] [PubMed]
- Yang J, Wei WQ, Niu J, et al. Cost-benefit analysis of esophageal cancer endoscopic screening in high-risk areas of China. World J Gastroenterol 2012;18:2493-501. [Crossref] [PubMed]
- Dawsey SM, Lewin KJ, Wang GQ, et al. Squamous esophageal histology and subsequent risk of squamous cell carcinoma of the esophagus. A prospective follow-up study from Linxian, China. Cancer 1994;74:1686-92. [Crossref] [PubMed]
- Pan QJ, Roth MJ, Guo HQ, et al. Cytologic detection of esophageal squamous cell carcinoma and its precursor lesions using balloon samplers and liquid-based cytology in asymptomatic adults in Llinxian, China. Acta Cytol 2008;52:14-23. [Crossref] [PubMed]
- Roth MJ, Liu SF, Dawsey SM, et al. Cytologic detection of esophageal squamous cell carcinoma and precursor lesions using balloon and sponge samplers in asymptomatic adults in Linxian, China. Cancer 1997;80:2047-59. [Crossref] [PubMed]
- Chettouh H, Mowforth O, Galeano-Dalmau N, et al. Methylation panel is a diagnostic biomarker for Barrett's oesophagus in endoscopic biopsies and non-endoscopic cytology specimens. Gut 2018;67:1942-9. [Crossref] [PubMed]
- Moinova HR, LaFramboise T, Lutterbaugh JD, et al. Identifying DNA methylation biomarkers for non-endoscopic detection of Barrett's esophagus. Sci Transl Med 2018;10:eaao5848. [Crossref] [PubMed]
- Qin Y, Wu CW, Taylor WR, et al. Discovery, Validation, and Application of Novel Methylated DNA Markers for Detection of Esophageal Cancer in Plasma. Clin Cancer Res 2019;25:7396-404. [Crossref] [PubMed]
- Kisiel JB, Taylor WR, Yab TC, et al. Accurate site prediction of gastrointestinal cancer by novel methylated DNA markers: Discovery & Validation. Cancer Res 2015;75:abstr 4252.
- Qin Y, Taylor W, Bamlet WR, et al. Methylated DNA Markers of Esophageal Squamous Cancer and Dysplasia: An International Study. Cancer Epidemiol Biomarkers Prev 2020;29:2642-50. [Crossref] [PubMed]
- Dawsey SM, Fleischer DE, Wang GQ, et al. Mucosal iodine staining improves endoscopic visualization of squamous dysplasia and squamous cell carcinoma of the esophagus in Linxian, China. Cancer 1998;83:220-31. [Crossref] [PubMed]
- Carvalho R, Areia M, Brito D, et al. Diagnostic accuracy of lugol chromoendoscopy in the oesophagus in patients with head and neck cancer. Rev Esp Enferm Dig 2013;105:79-83. [Crossref] [PubMed]
- Dubuc J, Legoux J. Endoscopic screening for esophageal squamous-cell carcinoma in high-risk patients: a prospective study conducted in 62 French endoscopy centers. Endoscopy 2006;38:690-5. [Crossref] [PubMed]
- Park JM, Seok Lee I, Young Kang J, et al. Acute esophageal and gastric injury: complication of Lugol's solution. Scand J Gastroenterol 2007;42:135-7. [Crossref] [PubMed]
- Morita FH, Bernardo WM, Ide E, et al. Narrow band imaging versus lugol chromoendoscopy to diagnose squamous cell carcinoma of the esophagus: a systematic review and meta-analysis. BMC Cancer 2017;17:54. [Crossref] [PubMed]
- Pilonis ND, Januszewicz W, di Pietro M. Confocal laser endomicroscopy in gastro-intestinal endoscopy: technical aspects and clinical applications. Transl Gastroenterol Hepatol 2022;7:7. [Crossref] [PubMed]
- Pierce M, Yu D, Richards-Kortum R. High-resolution fiber-optic microendoscopy for in situ cellular imaging. J Vis Exp 2011;2306. [PubMed]
- Protano MA, Xu H, Wang G, et al. Low-Cost High-Resolution Microendoscopy for the Detection of Esophageal Squamous Cell Neoplasia: An International Trial. Gastroenterology 2015;149:321-9. [Crossref] [PubMed]