
Endoluminal wound vacuum therapy for gastrointestinal leaks: current state and future directions
Introduction
Background
Anastomotic leak after gastrointestinal (GI) and oropharyngeal surgery is a serious complication with significant morbidity and mortality (1,2). These often-devastating situations can occur in 0.7–19% of patients undergoing surgery on the GI tract (3-5). Although classically these catastrophic events were addressed with emergent surgery which would entail either primary repair, diversion, or additional resection with or without reconstruction and intentional fistulae (1), today multiple modalities have been developed to treat these challenging complications including endoscopic suture repair, clip placement, stenting, radiographic drainage or diversion and/or most invasively, surgical salvage (6). In the search for the optimal treatment, endoluminal vacuum (EVAC) therapy was developed in Germany in the early 2000s and has seen increasing uptake in the United States (7-10). This novel technique was initially described as treatment for anastomotic leaks after low anterior resection (8) but is now being applied throughout the GI tract from the oropharynx to the rectum.
Mechanism of action
The mechanism of EVAC therapy is similar to that of superficial wound vacuum therapy (11). A porous sponge is placed over the area of injury and negative pressure is applied. This prevents further contamination, evacuates fluid, and removes necrotic and infected tissue. The local debridement and negative pressure promote neovascularization and new tissue growth. This contrasts with radiographic drainage and/or endoscopic stenting where control of leaking contents is the main goal. The consistent control of leakage with suction, in combination with tissue debridement and granulation allow for a more efficient and effective therapy for GI defects. For best effect, sponges should be exchanged every 3–5 days. These principles now used for decades in open wounds, now can be translated, and safely applied to anastomotic and other oropharyngeal and GI defects with excellent success rates.
Rationale & objectives
Although it has been nearly 20 years since its initiation EVAC is still only an emerging technique in the United States with only several centers routinely using this technology (7-10). EVAC therapy has the potential to drastically change patient outcomes, a truly comprehensive review exploring existing research and presenting surgical technique can both fill a lacuna in the literature and serve as a resource for proper implementation of the technique. As such, in this paper, we aim to present the basic method of EVAC assisted therapy, review the current literature around EVAC utilization, and present our experience with the technique for various anatomic locations in order to address these gaps. Furthermore, we present considerations and lessons learned for programs that wish to investigate this technique for possible future utilization in their own practice. Below we describe our modified technique as well as systemic review of application of the EVAC technology.
EVAC technique, application and discussion
General review of the technique
Endoluminal wound vacuum therapy begins with an endoscopic assessment in either the operating room or endoscopy suite. Upper GI defects may require intubation while lower GI defects can often be managed with sedation alone. Carbon dioxide insufflation is preferred as air insufflation leads to persistent intraperitoneal and/or intrathoracic air.
At the initial endoscopy, the location, extent, defect size and associated cavity should be assessed. Defects larger than 1 cm allow passage of the endoscope through the defect and into the cavity to remove leaked fluid and debride necrotic tissue. The distance from the anal verge or incisors is noted and recorded to recognize unintentional sponge displacement. If debridement of necrotic tissue is necessary, it can be done with the large, rat-tooth type grasping forceps (Raptor 360, Steris, Mentor, OH, USA). This should be done anytime there is significant necrotic or white, fibrinous tissue (similar to what is seen with superficial wound vacuum devices) that will delay the formation of granulation tissue. Debridement can be done at the initial presentation and placement as well as latter exchanges. Narrow luminal defects leading to larger extraluminal cavities can be dilated to 10 mm (to allow passage of a standard, adult endoscope) with through-the-scope dilating balloons to allow thorough debridement and fluid evacuation.
Once the perforation has been identified and characterized, the placement location and sponge size should be determined. Defects >1 cm with an associated extraluminal cavity are typically managed with extraluminal placement of the sponge. It is unnecessary to fill the cavity with the sponge as the suction will reduce the size significantly. Instead, continue extraluminal placement until the cavity is less than 5 mm in depth and avoid placing sponges larger than the diameter of the defect and approximately half the depth of the cavity.
The EVAC sponge is created with a 16 or 18 Fr nasogastric tube (NGT), a small black foam sponge (KCI, San Antonio, TX, USA) and 0-Ethibond sutures (Ethicon, Johnson & Johnson, USA) (Figure 1A). For esophageal defects in which the NGT was secured in the nose, the uncut NGT was placed via the nares and into the hypopharynx, grasped with a Magill forceps in the back of the throat and withdrawn through the mouth prior to cutting and suturing the sponge to it. This is not needed for colorectal placement. The black foam is cut to length of the defect (approximately 1–5 cm) with a diameter between 1–3 cm in a cylindrical shape (Figure 1B). Colorectal sponges can be made relatively wide (3 cm) if needed; however, esophageal sponges larger than 2 cm in diameter are exceedingly difficult to ‘drag’ down the esophagus and should be avoided.
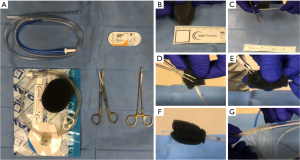
Once the sponge is cut to the appropriate size, the tip of the NGT is cut to match the length of the sponge, ensuring that all holes are covered (Figure 1C). The NGT is then ‘tunneled’ within the sponge and secured with one suture at the base, ensuring the suture included both walls of the NGT as well as the sponge (Figure 1D). A small air knot is created at the tip to assist with positioning that can be grasped with the rat tooth grasper (Figure 1E). Utilizing a monofilament suture (Monocryl, Ethicon Inc., Raritan, NJ, USA) can facilitate release of the suture from the rat tooth grasper as the braided Ethibond (Ethicon Inc., Raritan, NJ, USA) can be difficult to ‘let go of’ when coated with mucus. At least 1 additional suture is placed at the base, and between 0 and 2 sutures placed in between as needed, to avoid sponge displacement from the NGT (Figure 1F). These sutures are placed in a ‘stick-tie fashion’, completely wrapping around the sponge and tubing to avoid sponge dislodgement. In preparation for suction initiation, the ‘lily pad’ portion is cut and discarded so that tubing itself inserts into the clear NGT channel and reinforced with clear pad adhesive plastic from the wound vac package. This connection can be further sealed with a small tegaderm (3MTM TegadermTM, St. Paul, MN, USA) to avoid leakage or inadvertent disconnection (Figure 1G). The blue, sump port, on the NGT is plugged or knotted.
To place the sponge, a small amount of lubrication is placed on the sponge itself and the air knot grasped with the forceps or a snare running through the working channel of the endoscope. Excess suture (from the air knot) is withdrawn into the working channel so that the sponge laid tightly alongside the endoscope itself without obstructing the view. The tip of the endoscope and sponge are then inserted ‘as one’ into the rectum or the hypopharynx with plenty of lubricating jelly along the endoscope and NGT. Gentle traction on the NGT helps reduce looping while dragging it with a grasper or snare. Additional lubricating jelly should be applied as the NGT enters the nare externally to reduce ‘drag’.
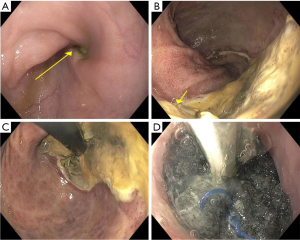
Once past the cricopharyngeus or anal sphincter, the sponge is positioned either within the cavity (for large defects) or within the GI tract lumen (Figure 2). Once appropriately positioned, suction (125 mmHg) is initiated, and the grasper released. To avoid accidental displacement after the air knot is released, we utilize a twisting/rotational motion to minimize friction between the rubber scope housing and the NGT. If the defect is angulated or difficult to visualize, concomitant fluoroscopy can be used to ensure sponge overlap of the defect by marking the proximal and distal margins with the endoscope and externally with radio-opaque markers (paper clips) and then ensuring the radio-opaque line within the NGT overlaps both clips.
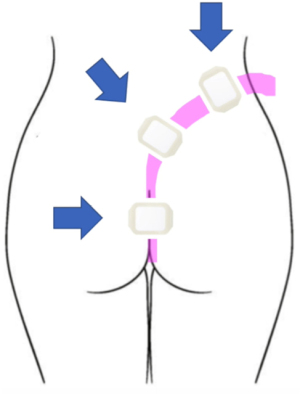
For esophageal defects, the NGT is secured with a nasal bridle (Corpak, Medline Industries, Northfield, IL, USA) taking care to note the distance and informing all staff not to displace or attempt re-positioning of the tube in the event of accidental displacement. Avoiding intentional displacement by patients can be done by asking which nare is preferred. For lower GI tract placement, the tube was brought posteriorly up the gluteal cleft and then over the hip to either side. In an outpatient setting, securing the tube with suture in two places is advised. For inpatients, the clear adhesive wound vac dressing is adequate and should be applied generously around the tube beginning at the top of the cleft and anteriorly around the hip (Figure 3). Patients can ambulate as desired but must always maintain suction.
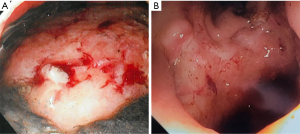
We exchange the sponge at least twice per week, typically every 3–5 days, or when it is apparent that the system has dislodged, becomes clogged, or fails due to loss of suction. Clinical decompensation warrants endoscopic evaluation and replacement as often the sponge is clogged or displaced even if it hasn’t been expelled completely. Frequent endoscopic exchanges also allow monitoring and photo-documentation of healing which is especially important when multiple endoscopists are involved (Figure 4).
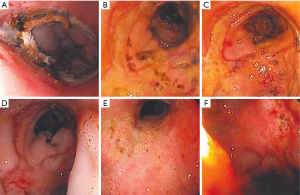
For low colon-rectal defects, less than 10 cm from the anal verge and/or a defect less than 1 cm in size, we typically recommend limited activity initially as the risk of displacement is high. However, these defects can often be sealed in 2 exchanges, so bedrest is only needed for 1 week or less. Lower GI tract patients should also be kept nil per os (NPO), with total parenteral nutrition (TPN), until the defect is well granulated (Figure 5) and the patient’s labs are normalizing (typically 5–10 days, 1–3 sponge exchanges) if they are not proximally diverted. Upper GI tract patients are nourished with TPN or via feeding access distal to the EVAC. Feeding access can be achieved with a tube alongside the NGT and sponge that extends distally. Alternatively, a dual lumen tube can be used for both feeding and suctioning (12). Sponge exchanges can also be done alongside percutaneous gastrostomy and/or gastro-jejunostomy placement to allow ongoing feeding independent of EVAC therapy (13). EVAC therapy is deemed complete when the defect is fully sealed to the retroperitoneum, mediastinum, or surrounding tissue, even before full granulation tissue. Once the leak is contained, the EVAC can be discontinued. Full granulation tissue and/or mucosal coverage is unnecessary. We routinely confirm leak containment with a contrasted study after EVAC removal.
Systemic review of EVAC application based on anatomic location
Pharynx and larynx
Perhaps the most recent application of EVAC therapy has been reported in head and neck surgery. The utilization of this technique was first documented in 2019 in post laryngectomy fistulas by a group from Hamburg Germany, where a fistula was closed successfully after 14 days of treatment (14). Another publication from 2021 by Steinbichler et al. describes closure of pharyngocutaneous fistulas in 5 of 6 patients with an average therapy duration of 18.5 days/4 vac exchanges (15). At our institution we have utilized EVAC in seven patients after complicated head and neck surgery. Healing was achieved in all patients after average of 3 exchanges (range, 2–5) and after 17 days (range, 7–30). We had one death in this group which was unrelated to EVAC therapy.
Esophagus
The first published report of successful healing of esophageal injuries with EVAC therapy is again from our German colleagues. Wedemeyer et al., a group in Hannover, Germany reported successful defect closure in two patients in their initial study from 2008 (7). Since then multiple other authors have described utilization EVAC in esophageal injuries or leaks. A group from Dallas led by Dr. Leeds has shown that the EVAC technology is effective in management of esophageal leaks from multiple different etiologies such as spontaneous perforations, iatrogenic injuries, or surgical leaks (16). Shalaby et al. reported analysis of 17 studies with total of 276 patients where [95% confidence interval (CI): 80.1–90.5] with median duration of EVAC was 47 days (range, 64.6–87.2). Their conclusion is that EVAC therapy is highly effective in healing injuries and is a safe option in appropriately selected patients (10). Scognamiglio et al. in a similar study reviewed five studies including 274 patients, comparing EVAC and self-expanding metallic (SEM) stents in patients with anastomotic leak after esophageal surgery and found that EVAC was associated with higher closure rate, shorter duration of therapy and lower mortality rate compared to SEM stents (17). Jung et al. reviewed 29 studies with upper GI tract defects involving 498 participants and demonstrated a successful closure rate with EVAC of 85%, three times higher than with SEM stents (18). A unique use of EVAC therapy was published by a group from Switzerland and the United Kingdom where preemptive EVACs were placed after esophagectomy. After its use in 67 patients, anastomotic leaks were encountered in just 7.5% of patients and 1 of those had an uncontained leak because of proximal gastric conduit necrosis (19).
Clear benefits of the EVAC technology are easier and better fixation in place. Further we feel that this technology is very well suited for defects larger than 1 cm. Nevertheless, complications of EVAC therapy have to be recognized and these, although rarely reported range from minor such as NGT related discomfort to major complications such as bleeding and strictures, and erosions of the sponge into nearby vessels (20-22).
Our experience is similar. We have managed 12 patients with an 92% success rate with average of 3.4 wound vac exchanges per patient over average of 14 days (range, 5–26). Specific tips for esophageal or gastric conduit leaks include taking care not to oversize the sponge as diameters >3 cm can be very difficult to maneuver and are often unnecessary. Additional, rotational torque can be applied to the scope during withdrawal to avoid the plastic-on-plastic binding with the nasogastric tubing that can lead to inadvertent sponge displacement.
Non-bariatric stomach
A relatively small group of patients have undergone EVAC therapy for leaks after non-bariatric surgery. Smallwood et al. described two patients that were successfully treated after iatrogenic gastric perforation or repair of traumatic injury. The first required 36 days of treatment and 8 vac changes; the second required 27 days of treatment and 5 changes to achieve healing (9). A group from Korea reported success in 100% of 11 patients with leaks after gastrectomy for gastric cancer. In this study four patients were treated with primary EVAC therapy and the remaining seven had failed SEMS treatment. When comparing outcomes to SEMS, they reported a significantly shortly duration of therapy (11 vs. 19.5 days, P=0.043) and lower failure rate, when including patients converted to EVAC therapy (0% vs. 26.7%, P=0.089). In this study one of the drawbacks to EVAC therapy after gastrectomy was stated as inability to commence enteral feedings shortly after initiation as can be done in cases of SEMS. Nevertheless, the authors felt that this did not affect the outcomes adversely (23).
Bariatric stomach surgery
Several studies have demonstrated resolution of staple line leaks after Roux-en Y (RY) gastric bypass surgeries as well sleeve gastrostomies, treated with endoscopic stenting (24-27). In 2016, Leeds et al. published 100% successful healing in 9 patients with staple line leaks after sleeve gastrectomy (28). More contemporary data from the same group described success in 21 out of 26 bariatric patients (81%) (29). Based on the available data, EVAC therapy results are very promising and perhaps eventually we can move away from surgical exploration in complex bariatric patients.
Duodenum
To date, there are several small case series or reports describing the successful management of duodenal leaks with EVAC therapy. A team from Tubingen, Germany describes successful utilization of EVAC therapy combined with antibiotic therapy in two patients who presented with spontaneous duodenal diverticulum perforation, a very rare yet life-threatening complication. In this report the team mentions that both patients healed after 20 days of only EVAC therapy and four vacuum changes. Both patients had healing documented with radiologic imaging (30). The largest study is by a combined group from Switzerland and Germany who documented success in 80% of 10 patients with duodenal perforations of various etiologies (iatrogenic perforations during endoscopies or surgical procedures, duodenal ulcer perforations, and anastomotic leaks). In this study the patients were treated only with EVAC therapy and antibiotics. No other endoscopic techniques where used, but the one patient who did not heal with EVAC underwent surgical exploration, repair, and drainage. The average therapy duration was 9 days (range, 7–31) (31).
Another group from Milan, Italy describes closure of duodenal stumps after subtotal or total gastrectomy’s with RY reconstructions in seven consecutive patients. In this study, due to technical difficulty owing to location required accessing the site of injury through retrograde endoscopic technique and using long, large bore suction tubing (French 14–18). Procedures were performed using a pediatric colonoscopy under fluoroscopic guidance. A guidewire was used to position the vac system correctly with appropriate tension from both the enteric and cutaneous aspects of the fistula. Healing was achieved in all patients with 12±5 days of therapy (32). All these results, despite more technical difficulties, are very encouraging.
At our institution, we managed a patient who suffered an iatrogenic duodenal injury after endoscopic resection of a large tubular adenoma on the posterior wall of the duodenum near the ampulla of Vater. Numerous modalities were attempted prior to EVAC therapy including surgical exploration with wide drainage, percutaneous biliary drainage, and repeated radiographic drainage. The patient continued to decline and was offered EVAC therapy as an alternate to a salvage pancreaticoduodenectomy. The defect required multiple debridement’s and prolonged EVAC therapy due to constant exposure to bile but successfully healed over 31 days and 6 exchanges (33).
Small bowel
A very interesting technique for accessing the small bowel has been described by Krajinovic et al. This team described utilization of a “rendezvous technique” where EVAC placement is assisted by means of a pullback string, where a string is inserted through the anastomotic leak, captured by the endoscope, and pulled back though the area of injure placing the sponge in the correct site under endoscopic guidance. This technique led to the complete closure of an anastomotic leak after 27 days and 7 changes (34).
However, cases in which a patient’s anatomy has been altered surgically can present an opportunity to access small bowel endoscopically in a similar fashion as the upper GI tract. We utilized this feature when we successfully treated a patient with an anastomotic leak after pancreaticojejunostomy. Leak was diagnosed on postoperative day four and initially treated though exploratory laparotomy, repair, and drainage, this failed, thus endoscopic clipping combined with imaging guided drainage was attempted. Unfortunately, these maneuvers failed again, thus the team attempted an endoscopic vac therapy. Placement of the EVAC was done via the roux limb. This was aided using a pediatric colonoscope. Oxygen tubing was utilized in place of a NGT to achieve the additional length for sponge placement. After 6 weeks of treatment and eight vac changes the fistula had healed (unpublished data). The biggest draw back in this case was the difficulty of placing the vac device in the correct space which required special equipment as well as advanced endoscopic skills.
Colorectal
The first description of EVAC therapy by Weidenhagen et al. was for the treatment of anastomotic leaks after low anterior resection. This group reported 97% successfully closure of anastomotic leak in 29 with an average therapy duration of 34 days (8). Borstlap et al. reported in 2017 healing of 16 out of 30 (53%) anastomotic leaks after total mesorectal excision (TME) for cancer. In this study all patients had been diverted via an ileostomy either during the index procedure or once the leak was identified though imaging or endoscopy. The average duration of therapy was 13 days (range, 5–21) and included average of 3.5 (range, 2–15) system changes. In interesting fact, 22 of the patients had undergone neoadjuvant radiation therapy (35). Another study from France describes successful healing of colorectal leaks in 26/47 patients (55%). The mean number of changes was 6.6 and the days of treatment was 27. The results also showed that patients where EVAC therapy was primary had better success (73%) versus patients where EVAC was used as recure therapy (33%) (36). Finally, a large metanalysis involving review of seventeen studies totaling 384 patients was recently published in Endoscopy International Open Journal. This study describes a calculated pooled rate of clinical success at 84.99% and pooled rate of adverse events at 7.6%. The conclusion of this large analysis is the EVAC therapy appears to be safe an effective modality to treat colorectal anastomotic leaks with high success and fairly low level of complications (37).
We have successfully managed several patients with anastomotic leak after colorectal surgery. The more distal the leak, for example a leak at an ileo-rectal anastomosis, the more difficult to manage as the sponge can be easily displaced with movement. If the anastomosis is 10 cm or more proximal from the anal verge, there is less likelihood of inadvertent displacement and, after securing the tubing posteriorly via the gluteal cleft, allow patients to ambulate as tolerated during therapy. We also do not find proximal diversion a requirement for EVAC therapy and find a brief period of NPO through the first exchange is often all that is required to evacuate leaked GI contents and achieve initial sealing of the defect.
Pediatric surgery
Several reports of application of EVAC technique for pediatric surgeries are available (38-40). A group from Boston Children’s Hospital described the first use of EVAC in pediatric patients in 2018. Manfredi et al. describes treatment of 17 patients with esophageal atresia who required therapy for esophageal perforation. The cumulative success rate was 88% in contrast to success in just 63% (15/24) of perforations treated with endoscopically placed stents (38). Another small study from Munich, Germany also describes use of EVAC closure of 4 out 5 esophageal injuries either from endoscopic perforations during dilation (1), perforation with an NGT after GI surgery, and anastomotic leaks after various surgeries (3). Of the injuries that healed two developed strictures which required dilations. One of the patients failed to heal the persistent fistula and required surgical intervention. The average time of therapy in this group was 19.6 days (range, 11–30) (39). Another group from Germany recently reported application of EVAC technology in four infants with esophageal perforations due to endoscopy or NGT placement. All four patients achieved complete healing after an average 22 days (range, 7–39) and an average 4.5 (range, 1–12) changes. Of note is that the youngest and smallest patient was a 24-day infant born in the 31 week of pregnancy and weighed only 980 g (40).
Discussion of EVAC technique and caveats
Learning curve
To develop an EVAC therapy program access, it is necessary to have access to proficient endoscopists either from surgical or GI medicine teams, but still there is a learning curve to proficiently manage these patients. In a 2019 study from Surgical Endoscopy, Ward et al. also described the time to develop proficiency for an advanced endoscopist is about 10 cases (41). We agree with the sentiment of Leeds et al. and have now ‘trained’ approximately 5 surgeons to perform EVAC therapy with exposure to just 5–10 cases. The regular use of an endoscope whether it be simply “checking” post-operative anastomoses or having a regular day of ‘scopes’ in the endoscopy suite—facilitates the rapid acquisition of EVAC skills. Even without regular use, the mandatory training in endoscopy that all general surgical graduates obtain is not completely lost in the age of minimally invasive surgery and can often be translated rapidly in successful sponge creation, manipulation, and placement.
EVAC complications
EVAC appears to be a safe and effective technique, nevertheless complications have been reported. Although based on our experience and available literature healing was achieved in most cases, non-healing required other techniques including, but not limited to salvage surgery have been reported. Mortality has been reported at 0–12.5%, but this perhaps should be attributed to the general clinical condition as a result of the injury and not EVAC use directly. The most common complications reported associated with EVAC have been strictures, which have been generally responsive to endoscopic dilation (20,21,42). It must be mentioned here though, that there have been reports of severe bleeding including potential life-threatening situations such as aorto-esophageal fistulas (43). It is of utmost importance to monitor NGT output for blood as that can be a sentinel event and catching it early potentially could prevent a fatal outcome.
Procedural cost and outpatient EVAC therapy
Procedural cost is an important consideration in the current healthcare marketplace and decreasing costs without impeding results is extremely important. Dr. Leeds and his group in a recent study have reported EVAC therapy performed completely in endoscopy suites as opposed to the operating room, achieving a 60% decrease in cost (41). Further, outpatient EVAC therapy is an emerging adaptation and at our intuition, that can further decrease procedural cost. It has now been successfully applied in two patients with anastomotic leaks after colorectal anastomoses following pelvic radiation. Both patients were diverted proximally. The tubing was secured with suture to the gluteal fold in addition to vac dressing as described above. Patients returned in 3–5 days for scheduled exchanges with sedation. Patient self-limited their activity and no unintentional dislodgements were encountered.
Future directions
This is still novel technology and guidelines have not been developed. For example, the optimal duration of therapy and interval between exchanges have not yet been defined. Initial duration was prolonged as we awaited near complete healing of the defect. We have since learned that adherence of the defect to surrounding tissue (retroperitoneum, pelvis, mediastinal tissue, etc.) that effectively ‘seals’ the leak is often all that is needed. These “contained leaks” can then heal without further intervention.
Another area for improvement is that there is no commercially available device in the United States thus it requires construction by the endoscopist. This is likely a barrier to implementation for many physicians as searching for the products and constructing the device in addition to placement and removal every 3–5 days increases the time required for therapy. In contrast, radiographic drainage, or stent placement, in the optimal setting, can be successful after the initial procedure. The increased uptake of EVAC therapy in Europe is aided not only by its introduction in Germany but also by commercially available products.
A recently published product that includes a stent circumferentially wrapped with black sponge warrants mention as it has the potential to combine the benefits of both EVAC therapy and stenting. In the first case series of 3 patients with upper GI leaks, the VAC (44) was successfully used to close the defects while maintaining oral nutrition. More study is warranted to confirm the success of this new device with larger and more complex defects, however, allowing oral intake during therapy that is not delivered via a concomitant feeding tube (either through the sponge or alongside it) is encouraging.
An interesting application of the EVAC technology is to place the system at the time of the index surgery. There are reports found in literature describing prophylactic use of EVAC technology by surgeons to manage anastomosis at high-risk dehiscence (19,42). This is in interesting novel approach worth further study and analysis.
Lastly, when considering implementation of EVAC therapy versus stenting and/or radiographic drainage; a consideration for cost and effort is important. EVAC therapy is ‘labor-intensive’ requiring repeated procedures every 3–5 days for, on average, 3 exchanges. If a single endoscopist is performing EVAC therapy, this can be severely limiting to the physician as they are now ‘on-call’ until therapy is finished. Stenting requires fewer procedures but utilizes a high-cost implant and close monitoring for migration. We could find no published estimates of device or labor costs comparing these techniques. However, in patients who are septic, rapidly implementing the therapy with the highest chance of success is key, regardless of labor and cost required. More comparative studies are needed but the current data is clear the EVAC therapy is the optimal therapy for closure of GI leaks.
Limitation of the review
EVAC technique is still new and is only finding its way into the operating rooms and endoscopy suite. Although the literature review as well as our own experience is quite promising, still much more research is needed to confirm utility of this promising technique. Further limitation sems to be that EVAC techniques should be used with assistance of experienced endoscopists as clearly the efficacy increases as the team’s experience increases.
Conclusions
EVAC therapy is a novel and versatile endoscopic technique which to treat leaks or perforations throughout the GI tract. Based on our experience and the available literature, EVAC therapy is a safe and effective technique with high rate of success; often allowing avoidance of reoperation and/or diversion. It has been used successfully as a primary treatment of leaks or perforation as well as salvage therapy where other endoscopic and surgical therapies have failed. Given these data, we believe that EVAC therapy should be considered as a first-line treatment when the injury is accessible with an endoscope.
Acknowledgments
Funding: None.
Footnote
Peer Review File: Available at https://ales.amegroups.com/article/view/10.21037/ales-22-79/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://ales.amegroups.com/article/view/10.21037/ales-22-79/coif). KJW has served as expert witness in fields of bariatric, robotic, and general surgery, nothing related to endoscopy or EVAC technology. MDM has received grant funding for unrelated studies from Merck, Inc. and Taiho, Inc./NCCN as well as meal reimbursement from Covidien. He has a patent pending titled enhancing cancer therapy treatment with BH3 mimetics. He also serves on the Debbie’s Dream Foundation Advisory Board. None of these are related to this study. ELJ is a consultant for Boston Scientific. He has also provided expert witness testimony not related to endoluminal wound vacs. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work and in ensuring that questions related to the accuracy or integrity of any part of the work presented have been appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Messmann H, Schmidbaur W, Jäckle J, et al. Endoscopic and surgical management of leakage and mediastinitis after esophageal surgery. Best Pract Res Clin Gastroenterol 2004;18:809-27. [Crossref] [PubMed]
- Thomas MS, Margolin DA. Management of Colorectal Anastomotic Leak. Clin Colon Rectal Surg 2016;29:138-44. [Crossref] [PubMed]
- Alizadeh RF, Li S, Inaba C, et al. Risk Factors for Gastrointestinal Leak after Bariatric Surgery: MBASQIP Analysis. J Am Coll Surg 2018;227:135-41. [Crossref] [PubMed]
- Moghadamyeghaneh Z, Hanna MH, Alizadeh RF, et al. Contemporary management of anastomotic leak after colon surgery: assessing the need for reoperation. Am J Surg 2016;211:1005-13. [Crossref] [PubMed]
- McDermott FD, Heeney A, Kelly ME, et al. Systematic review of preoperative, intraoperative and postoperative risk factors for colorectal anastomotic leaks. Br J Surg 2015;102:462-79. [Crossref] [PubMed]
- Moore CB, Almoghrabi O, Hofstetter W, et al. Endoluminal wound vac: an evolving role in treatment of esophageal perforation. J Vis Surg 2020;6:43. [Crossref]
- Wedemeyer J, Schneider A, Manns MP, et al. Endoscopic vacuum-assisted closure of upper intestinal anastomotic leaks. Gastrointest Endosc 2008;67:708-11. [Crossref] [PubMed]
- Weidenhagen R, Gruetzner KU, Wiecken T, et al. Endoscopic vacuum-assisted closure of anastomotic leakage following anterior resection of the rectum: a new method. Surg Endosc 2008;22:1818-25. [Crossref] [PubMed]
- Smallwood NR, Fleshman JW, Leeds SG, et al. The use of endoluminal vacuum (E-Vac) therapy in the management of upper gastrointestinal leaks and perforations. Surg Endosc 2016;30:2473-80. [Crossref] [PubMed]
- Shalaby M, Emile S, Elfeki H, et al. Systematic review of endoluminal vacuum-assisted therapy as salvage treatment for rectal anastomotic leakage. BJS Open 2018;3:153-60. [Crossref] [PubMed]
- Argenta LC, Morykwas MJ. Vacuum-assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg 1997;38:563-76; discussion 577. [Crossref] [PubMed]
- Lee SY, Kim KW, Lee JI, et al. Esophageal Endoscopic Vacuum Therapy with Enteral Feeding Using a Sengstaken-Blakemore Tube. Korean J Thorac Cardiovasc Surg 2018;51:76-80. [Crossref] [PubMed]
- Tachezy M, Chon SH, Rieck I, et al. Endoscopic vacuum therapy versus stent treatment of esophageal anastomotic leaks (ESOLEAK): study protocol for a prospective randomized phase 2 trial. Trials 2021;22:377. [Crossref] [PubMed]
- Loeck J, von Lücken HJ, Kehrl W, et al. Endoscopic negative pressure therapy (ENPT) of a post-laryngectomy pharyngocutaneous fistula: first report of a new treatment method. HNO 2019;67:77-9. [Crossref] [PubMed]
- Steinbichler TB, Wolfram D, Runge A, et al. Modified vacuum-assisted closure (EndoVAC) therapy for treatment of pharyngocutaneous fistula: Case series and a review of the literature. Head Neck 2021;43:2377-84. [Crossref] [PubMed]
- Still S, Mencio M, Ontiveros E, et al. Primary and Rescue Endoluminal Vacuum Therapy in the Management of Esophageal Perforations and Leaks. Ann Thorac Cardiovasc Surg 2018;24:173-9. [Crossref] [PubMed]
- Scognamiglio P, Reeh M, Karstens K, et al. Endoscopic vacuum therapy versus stenting for postoperative esophago-enteric anastomotic leakage: systematic review and meta-analysis. Endoscopy 2020;52:632-42. [Crossref] [PubMed]
- Jung DH, Yun HR, Lee SJ, et al. Endoscopic Vacuum Therapy in Patients with Transmural Defects of the Upper Gastrointestinal Tract: A Systematic Review with Meta-Analysis. J Clin Med 2021;10:2346. [Crossref] [PubMed]
- Müller PC, Morell B, Vetter D, et al. Preemptive Endoluminal Vacuum Therapy to Reduce Morbidity After Minimally Invasive Ivor Lewis Esophagectomy: Including a Novel Grading System for Postoperative Endoscopic Assessment of GI-Anastomoses. Ann Surg 2021;274:751-7. [Crossref] [PubMed]
- Laukoetter MG, Mennigen R, Neumann PA, et al. Successful closure of defects in the upper gastrointestinal tract by endoscopic vacuum therapy (EVT): a prospective cohort study. Surg Endosc 2017;31:2687-96. [Crossref] [PubMed]
- Virgilio E, Ceci D, Cavallini M. Surgical Endoscopic Vacuum-assisted Closure Therapy (EVAC) in Treating Anastomotic Leakages After Major Resective Surgery of Esophageal and Gastric Cancer. Anticancer Res 2018;38:5581-7. [Crossref] [PubMed]
- De Pasqual CA, Mengardo V, Tomba F, et al. Effectiveness of endoscopic vacuum therapy as rescue treatment in refractory leaks after gastro-esophageal surgery. Updates Surg 2021;73:607-14. [Crossref] [PubMed]
- Choi SI, Park JC, Jung DH, et al. Efficacy of Endoscopic Vacuum-Assisted Closure Treatment for Postoperative Anastomotic Leak in Gastric Cancer. Gut Liver 2020;14:746-54. [Crossref] [PubMed]
- Arterburn DE, Telem DA, Kushner RF, et al. Benefits and Risks of Bariatric Surgery in Adults: A Review. JAMA 2020;324:879-87. [Crossref] [PubMed]
- Al-Sabah S, Ladouceur M, Christou N. Anastomotic leaks after bariatric surgery: it is the host response that matters. Surg Obes Relat Dis 2008;4:152-7; discussion 157-8. [Crossref] [PubMed]
- Chang J, Sharma G, Boules M, et al. Endoscopic stents in the management of anastomotic complications after foregut surgery: new applications and techniques. Surg Obes Relat Dis 2016;12:1373-81. [Crossref] [PubMed]
- Shehab H. Enteral stents in the management of post-bariatric surgery leaks. Surg Obes Relat Dis 2018;14:393-403. [Crossref] [PubMed]
- Leeds SG, Burdick JS. Management of gastric leaks after sleeve gastrectomy with endoluminal vacuum (E-Vac) therapy. Surg Obes Relat Dis 2016;12:1278-85. [Crossref] [PubMed]
- Leeds SG, Mencio M, Ontiveros E, et al. Endoluminal Vacuum Therapy: How I Do It. J Gastrointest Surg 2019;23:1037-43. [Crossref] [PubMed]
- Wichmann D, Jansen KT, Onken F, et al. Endoscopic negative pressure therapy as stand-alone treatment for perforated duodenal diverticulum: presentation of two cases. BMC Gastroenterol 2021;21:436. [Crossref] [PubMed]
- Chevallay M, Lorenz F, Bichard P, et al. Outcome of endoscopic vacuum therapy for duodenal perforation. Surg Endosc 2023;37:1846-53. [Crossref] [PubMed]
- Mutignani M, Dioscoridi L, Massad M, et al. Endoscopic Vacuum Therapy (EVT) for Persistent Duodenal Stump Dehiscence After Upper Gastrointestinal Surgery in Selected Patients: A Tertiary Referral Center Case Series. Surg Laparosc Endosc Percutan Tech 2021;31:502-5. [Crossref] [PubMed]
- Abbitt D, Barnes AL, Hammad HT, et al. Endoluminal vacuum closure of a duodenal perforation. J Surg Case Rep 2021;2021:rjab479. [Crossref] [PubMed]
- Krajinovic K, Reimer S, Kudlich T, et al. “Rendezvous technique” for intraluminal vacuum therapy of anastomotic leakage of the jejunum. Surg Case Rep 2016;2:114. [Crossref] [PubMed]
- Borstlap WAA, Musters GD, Stassen LPS, et al. Vacuum-assisted early transanal closure of leaking low colorectal anastomoses: the CLEAN study. Surg Endosc 2018;32:315-27. [Crossref] [PubMed]
- Abdalla S, Cotte E, Epin A, et al. Short-term and Long-term Outcome of Endoluminal Vacuum Therapy for Colorectal or Coloanal Anastomotic Leakage: Results of a Nationwide Multicenter Cohort Study From the French GRECCAR Group. Dis Colon Rectum 2020;63:371-80. [Crossref] [PubMed]
- Dhindsa BS, Naga Y, Saghir SM, et al. Endo-sponge in management of anastomotic colorectal leaks: a systematic review and meta-analysis. Endosc Int Open 2021;9:E1342-9. [Crossref] [PubMed]
- Manfredi MA, Clark SJ, Staffa SJ, et al. Endoscopic Esophageal Vacuum Therapy: A Novel Therapy for Esophageal Perforations in Pediatric Patients. J Pediatr Gastroenterol Nutr 2018;67:706-12. [Crossref] [PubMed]
- Ritz LA, Hajji MS, Schwerd T, et al. Esophageal Perforation and EVAC in Pediatric Patients: A Case Series of Four Children. Front Pediatr 2021;9:727472. [Crossref] [PubMed]
- Kaczmarek DJ, Heling DJ, Strassburg CP, et al. Management of esophageal perforations in infants by endoscopic vacuum therapy: a single center case series. BMC Gastroenterol 2022;22:282. [Crossref] [PubMed]
- Ward MA, Hassan T, Burdick JS, et al. Endoscopic vacuum assisted wound closure (EVAC) device to treat esophageal and gastric leaks: assessing time to proficiency and cost. Surg Endosc 2019;33:3970-5. [Crossref] [PubMed]
- Adamenko O, Ferrari C, Seewald S, et al. Prophylactic endoluminal vacuum therapy after major gastrointestinal surgery: a systematic review. Updates Surg 2022;74:1177-86. [Crossref] [PubMed]
- Omran S, Ardalani L, Beyer K, et al. Management of Tumor- and Nontumor-related Aorto-esophageal and Aorto-bronchial Fistulas. Ann Vasc Surg 2021;72:419-29. [Crossref] [PubMed]
- Lange J, Dormann A, Bulian DR, et al. VACStent: Combining the benefits of endoscopic vacuum therapy and covered stents for upper gastrointestinal tract leakage. Endosc Int Open 2021;9:E971-6. [Crossref] [PubMed]
Cite this article as: Wikiel KJ, McConnell B, Bollinger D, Abbitt D, Ahrendt SA, McCarter MD, Morton AP, Pierraci FM, Jones EL. Endoluminal wound vacuum therapy for gastrointestinal leaks: current state and future directions. Ann Laparosc Endosc Surg 2023;8:19.


