Esophageal adenocarcinoma after laparoscopic adjustable gastric banding: a case report and literature review
Highlight box
Key findings
• A case of early esophageal adenocarcinoma after gastric banding for morbid obesity with minimally invasive approach.
What is known and what is new?
• The relationship between bariatric procedures and cancer is increasingly recognized.
• This case highlights the importance of investigating symptoms for early diagnosis and not solely attributing them to bariatric procedures.
• Minimally invasive approach remains an option after bariatric procedures.
What is the implication, and what should change now?
• Consideration should be given to performing routine upper endoscopy both prior to LAGB and in the follow-up in the presence of upper GI symptoms.
• After LAGB, minimally invasive approach is feasible and safe.
Introduction
Morbid obesity has been increasing worldwide in recent years, and various techniques, such as laparoscopic adjustable gastric banding (LAGB), have been implemented for bariatric surgery. LAGB is an effective weight loss procedure that was often chosen due to the minimally invasive nature of the operation and the potential for reversal (1). However, long-term outcomes have discouraged its use, and some authors are concerned about its impact on the development of esophageal cancer (2).
The relationship between bariatric procedures and the development of esophagogastric cancer is not well-established (3). Cases of esophagogastric tumors following bariatric surgery were once considered exceptional, but concerns have increased, and national studies are expanding to identify the epidemiological, histological, and clinical factors that characterize these patients. The management of these patients may be complicated by the anatomical alterations from previous bariatric procedures. The available literature on the treatment options for these patients is limited (4).
Herein, we present a case of adenocarcinoma of the esophagus that developed after gastric band placement and was treated with minimally invasive esophagectomy (MIE). To provide context, we reviewed 26 cases of esophageal adenocarcinoma after LAGB, with a focus on treatment options. In contrast to previous reports, which often described palliative treatments for advanced tumors, we present a case of early adenocarcinoma treated with curative MIE. This approach has only been reported in one other case in the literature, and we present our intraoperative findings. We present this case in accordance with the CARE reporting checklist (available at https://ales.amegroups.com/article/view/10.21037/ales-22-62/rc).
Case presentation
We present a 48-year-old woman with a history of LAGB for morbid obesity in a previous hospital. She had no other medical history, and there was no drug or alcohol use. No history of reflux prior to gastric banding has been documented. The patient was followed for 10 years postoperatively with a weight reduction of 26%. During this time, she experienced intermittent vomiting attacks that were relieved by deflating the band. Contrast swallowing was the only diagnostic test performed at that time.
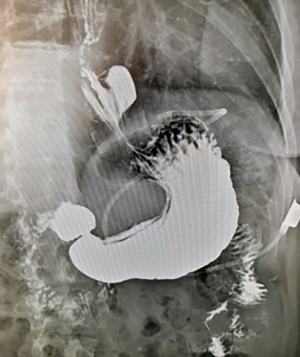
Twelve years after the LAGB, the patient became symptomatic with epigastric pain, dysphagia, and recurrent vomiting, with a body mass index (BMI) of 33.1 kg/m2. A contrast swallow revealed a gastric pouch enlargement with band displacement associated with a hiatal hernia (type 3a of gastric pouch enlargement) (5) and severe gastroesophageal reflux disease (GERD) (Figure 1). Further investigation with an esophagogastroduodenoscopy showed Barrett’s esophagus with an ulcerated lesion in the distal esophagus (at 35 cm from the incisor teeth). Biopsy of the esophageal lesion confirmed a moderately differentiated T1a adenocarcinoma. The patient was referred to our center for further management.
Staging studies revealed a T1N0 lesion on esophageal endoscopy ultrasonography and no evidence of metastatic disease on the computed tomography of the chest and abdomen. Endoscopic resection was attempted but pathological findings observed a pT1 high-grade adenocarcinoma with positive resection margins.
The role of esophagectomy with curative intent was discussed with the patient and she accepted. A minimally invasive Ivor-Lewis esophagectomy was performed.
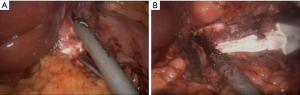
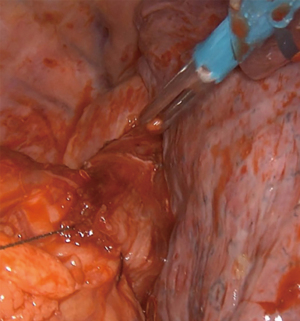
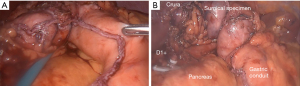
Extensive adhesiolysis was performed and gastric band was exposed (Figure 2). Subsequently, the gastric band and the components of the subcutaneous port were removed. D1+ lymphadenectomy and hiatal dissection were performed. The Akiyama-type plastia was created by stapling along the lesser curvature of the stomach. It was not necessary to modify the normal procedure as there were no visible lesions caused by the band slippage. The distal end of the specimen was sutured to the gastric conduit to facilitate its delivery into the thoracic cavity (Figure 3). The mobilization of the esophagus was completed with a standard lymphadenectomy. An end-to-side intrathoracic circular stapled esophagogastric anastomosis was performed with anterior manual reinforcement (Figure 4). A chest drain was placed.
Postoperatively, a contrast swallow revealed a leak. Upper gastrointestinal (GI) endoscopy did not identify the defect although computed tomography showed a small collection adjacent to the anastomosis with minimal contrast extravasation. A self-expanding stent was placed at the anastomosis site. The closure of the leak was checked in a computed tomography at 15 days. The patient was asymptomatic, and the stent was removed.
Final pathology biopsy revealed an intestinal infiltrative high-grade adenocarcinoma over Barrett’s esophagus, T1bN0 with negative surgical margins. The patient’s epigastric pain, dysphagia, and recurrent vomiting were relieved after surgery. The patient has remained asymptomatic without the need for further treatment, and there has been no recurrence after 39 months of follow-up.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Literature review
A search was conducted on PubMed database from 2000 to December 2022 to compare the management of patients with adenocarcinoma in distal esophagus or esophagogastric junction (EGJ) after gastric banding as the sole treatment for morbid obesity. The keywords “esophagogastric” and “bariatric procedures” were used. The inclusion criteria consisted of systematic reviews, case reports and retrospective and/or prospective cohort studies in the English language. Patients with malignancies other than esophageal/esophagogastric cancer and those who underwent multiple bariatric procedures were excluded. Studies that did not report the treatment of these tumors were also excluded. In the case of systematic reviews, the original studies were analyzed, and duplicate studies were excluded. A total of 26 patients with adenocarcinoma after gastric banding, with described management, were obtained from 10 studies (6 case reports and 4 cohort studies) (Table 1). Of these cases, 53.8% presented metastasis at diagnosis. Curative surgery was performed in 9 cases (34.6%), and only two studies (4,14) specify the procedure performed (Ivor-Lewis esophagectomy). Only one patient’ case was described with a minimally invasive approach.
Table 1
| Author | N | Year | Gender | Age (years) | Previous GERD | Time to diagnosis (years) | Symptoms/BMI at diagnosis (kg/m2) | Localization | TNM or stage | Treatment |
|---|---|---|---|---|---|---|---|---|---|---|
| Snook (6) | 1 | 2003 | Female | 50 | Yes | 8 | Dysphagia/30 | Lower esophagus | IV | Palliative CT + stent |
| Hackert (7) | 1 | 2004 | Female | 62 | NS | 10 | Epigastric pain/29.2 | Cardias | IV | Palliative subtotal gastrectomy |
| Korswagen (8) | 1 | 2009 | Male | 43 | No | 2 | Back pain/16.9 | Lower esophagus | IV | Palliative CT and RT for metastases |
| Stauffer (9) | 1 | 2011 | Male | 66 | NS | 2 | Dysphagia/NS | EGJ | N+ | CT and no surgical treatment due to progression |
| Burton (10) | 4 | 2016 | Male | 57 | Yes | 9 | Reflux/42.3 | EGJ | IV | Palliative care |
| Male | 52 | Yes | 15 | Reflux/52.3 | Lower esophagus | III | Resectional surgery (NS) | |||
| Female | 56 | Yes | 13 | Reflux/24.9 | Lower esophagus | IV | Palliative care | |||
| Male | 59 | Yes | 12 | Haematemesis/36.9 | Lower esophagus | IV | Palliative care | |||
| Maret-Ouda (11) | 4 | 2017 | Male | 71 | NS | 22 | Dysphagia/ 39 | Lower esophagus and cardias | T1N0 | CT + surgery (NS) |
| Male | 64 | NS | 9 | Asymptomatic/NS | Lower esophagus and cardias | T1N0 | Surgery (NS) | |||
| Male | 74 | NS | 19 | Vomiting/44 | Lower esophagus and cardias | M1 | None treatment | |||
| Male | 64 | NS | 8 | Melena/38 | Lower esophagus and cardias | T3N0 | CT-RT + surgery (NS) | |||
| Trautman (12) | 1 | 2018 | Female | 65 | NS | 19 | Vomiting and epigastric pain/NS | EGJ | NS | CT-RT + surgery (NS) |
| Lam (13) | 2 | 2018 | Male | 66 | NS | 10 | Dysphagia/NS | EGJ | IV | Palliative CT + stent |
| Male | 58 | NS | 2 | Dysphagia/NS | Lower esophagus | IV | Palliative CT + stent | |||
| Gehwolf (14) | 7 | 2019 | NS | NS | NS | 14 | NS | EGJ | IA | Ivor-Lewis |
| NS | NS | NS | 17 | NS | EGJ | IV | Palliative CT | |||
| NS | NS | NS | NS | NS | EGJ | IV | Palliative CT | |||
| NS | NS | NS | 5 | NS | Lower esophagus | IV | Palliative CT | |||
| NS | NS | NS | 3 | NS | Lower esophagus | IIIA | Ivor-Lewis | |||
| NS | NS | NS | 14 | NS | EGJ | IIIB | Ivor-Lewis | |||
| NS | NS | NS | 18 | NS | Barret with LGD | 0 | RFA | |||
| Plat (4) | 4 | 2021 | Male | 47 | NS | 14 | NS/30.8 | Lower esophagus | T1N0 | Endoscopic mucosal resection + RFA |
| Male | 47 | NS | 10 | NS/30 | Lower esophagus | M1 | Palliative CT | |||
| Female | 68 | NS | 10 | NS/35 | Lower esophagus | T3N0 | CT-RT + minimally invasive Ivor-Lewis | |||
| Male | 57 | NS | 14 | NS/NS | Lower esophagus | M1 | Palliative CT |
GERD, gastroesophageal reflux disease; BMI, body mass index; TNM, tumor-nodes-metastasis; CT, chemotherapy; NS, not specified; RT, radiotherapy; EGJ, esophagogastric junction; LGD; low grade dysplasia; RFA, radiofrequency ablation.
Discussion
Esophageal adenocarcinoma following LAGB has been reported previously, however, the primary and most significant contribution of this clinical case is the study of symptoms that manifest during the follow-up of LAGB patients. These symptoms, even if they can be attributed to common gastric band complications, are studied to facilitate early diagnosis, and subsequently, an endoscopic or minimally invasive intervention. Literature review focused on the management of this pathology was considered with the aim to highlight these aspects. However, our study is subject to the limitation that among the 63 reported cases of esophagus/gastroesophageal junction (GEJ) neoplasms following gastric band placement for morbid obesity (15), only 26 cases documented the treatment of these tumors.
Most of the previously tumors reported were diagnosed at a locally advanced or metastatic stage (6-10,13). In our case, due to the early performance of the upper endoscopy at the start of symptoms, an early tumor was detected. Follow-up of patients with LAGB is crucial. Unspecified symptoms commonly associated with bariatric procedures such as iron deficiencies, dysphagia, or GERD, as well as suboptimal follow-up, may delay cancer diagnosis. The International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO) recommends performing an upper endoscopy after LAGB based on upper GI symptoms (16).
Time to cancer diagnosis after LAGB varies. In a retrospective global study, Parmar et al., reported a mean diagnosis time of 10.5±5.7 years after LAGB (17). However, in our review, several patients presented with a neoplasm as early as 2 years after LAGB (8,9,13,14). This highlights the importance of routine upper endoscopy prior to bariatric procedures (18).
Obesity is a well-established risk factor for esophageal neoplasm development, likely due to its correlation with hiatal hernia formation and GERD (19). In contrast, bariatric surgery is associated with weight loss and reduced chronic reflux. Although current evidence is limited, no significant differences in cancer incidence have been reported between patients who underwent bariatric surgery and those who did not (4). A recent review revealed that most of the cancer cases after bariatric procedures involved a Roux-en-Y gastric bypass as the primary bariatric surgical procedure, followed by gastric banding (14). A nationwide survey was conducted in Austria with the aim of finding esophageal malignancies after LAGB and the authors described a four to fivefold risk in these patients (15).
Gastric pouch enlargement with band displacement is the most common surgical complication associated with LAGB (5). The band, which acts as an artificial outlet obstruction, leads to low esophageal sphincter distension and subsequent development of a common cavity that includes the gastric pouch and esophagus. The main mechanisms associated with cancer pathogenesis after LAGB are increased intraluminal pressure, band-induced erosion, and increased GERD (20). In fact, cases of carcinoma in the pouch above the gastric band have also been described (14).
However, other factors such as tobacco, alcohol, Helicobacter pylori infection, GERD, or a personal or family history of esophageal malignancies may contribute to the onset of this pathology in patients with normal body mass index (BMI). In our review, previous GERD was not documented in most of the studies (Table 1). Our patient had a BMI of 33.1 kg/m2 and experienced repeated vomiting after band insertion, which has been described as a major risk factor for band slippage (21). She also developed a hiatal hernia and GERD, all of which may have contributed to the development of an esophageal neoplasm.
The Austrian nationwide survey described three cases of esophageal cancer after LAGB that were considered for curative surgical treatment, with the Ivor-Lewis esophagectomy being the preferred surgical option (15). Plat et al. is the only study that describes the minimally invasive approach in a single patient and without postoperative complications (4). This case demonstrates that the minimally invasive Ivor-Lewis esophagectomy is safe and technically feasible for patients with previous LAGB, even with the presence of intraabdominal adhesions. Prior to proceeding with esophagectomy, it is crucial to remove the gastric band and assess gastric length adequacy for reconstruction. These patients with possible complexities and a need for other techniques, such as colonic interposition, require an individualized approach with specialized surgeons in these techniques to improve oncological outcomes.
Conclusions
Esophageal cancer following LAGB is a challenging problem. Band-related symptomatology might be considered for early endoscopic evaluation. Minimally invasive approach is a feasible option in the treatment of these patients.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://ales.amegroups.com/article/view/10.21037/ales-22-62/rc
Peer Review File: Available at https://ales.amegroups.com/article/view/10.21037/ales-22-62/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://ales.amegroups.com/article/view/10.21037/ales-22-62/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Favretti F, Ashton D, Busetto L, et al. The gastric band: first-choice procedure for obesity surgery. World J Surg 2009;33:2039-48. [Crossref] [PubMed]
- Melstrom LG, Bentrem DJ, Salvino MJ, et al. Adenocarcinoma of the gastroesophageal junction after bariatric surgery. Am J Surg 2008;196:135-8. [Crossref] [PubMed]
- Blomain ES, Funderburk LK, Jernigan PL, et al. The relationship between bariatric surgery and esophageal and gastric cancer: a review of the current literature and future research needs. Obes Surg 2015;25:2393-401.
- Plat VD, Kasteleijn A, Greve JWM, et al. Esophageal Cancer After Bariatric Surgery: Increasing Prevalence and Treatment Strategies. Obes Surg 2021;31:4954-62. [Crossref] [PubMed]
- Ponce J, Fromm R, Paynter S. Outcomes after laparoscopic adjustable gastric band repositioning for slippage or pouch dilation. Surg Obes Relat Dis 2006;2:627-31. [Crossref] [PubMed]
- Snook KL, Ritchie JD. Carcinoma of esophagus after adjustable gastric banding. Obes Surg 2003;13:800-2. [Crossref] [PubMed]
- Hackert T, Dietz M, Tjaden C, et al. Band erosion with gastric cancer. Obes Surg 2004;14:559-61. [Crossref] [PubMed]
- Korswagen LA, Schrama JG, Bruins Slot W, et al. Adenocarcinoma of the lower esophagus after placement of a gastric band. Obes Surg 2009;19:389-92. [Crossref] [PubMed]
- Stauffer JA, Mathew J, Odell JA. Esophageal adenocarcinoma after laparoscopic gastric band placement for obesity. Dis Esophagus 2011;24:E8-10. [Crossref] [PubMed]
- Burton PR, Ooi GJ, Laurie C, et al. Diagnosis and Management of Oesophageal Cancer in Bariatric Surgical Patients. J Gastrointest Surg 2016;20:1683-91. [Crossref] [PubMed]
- Maret-Ouda J, Tao W, Mattsson F, et al. Esophageal adenocarcinoma after obesity surgery in a population-based cohort study. Surg Obes Relat Dis 2017;13:28-34. [Crossref] [PubMed]
- Trautman J, Craig SJ. Gastric inlet obstruction from oesophageal cancer with internalized gastric band: a worrisome outcome? J Surg Case Rep 2018;2018:rjy118. [Crossref] [PubMed]
- Lam JJM, Di Maggio F, Lynn W, et al. Oesophageal adenocarcinoma following gastric band surgery in two patients. J Surg Case Rep 2018;2018:rjy293. [Crossref] [PubMed]
- Gehwolf P, Kienzl-Wagner K, Cakar-Beck F, et al. Laparoscopic Adjustable Gastric Banding: an Underestimated Risk Factor for the Development of Esophageal Cancer?-a Nationwide Survey. Obes Surg 2019;29:626-31. [Crossref] [PubMed]
- Parmar C, Pouwels S. Oesophageal and Gastric Cancer After Bariatric Surgery: an Up-to-Date Systematic Scoping Review of Literature of 324 Cases. Obes Surg 2022;32:3854-62. [Crossref] [PubMed]
- Brown WA, Johari Halim Shah Y, Balalis G, et al. IFSO Position Statement on the Role of Esophago-Gastro-Duodenal Endoscopy Prior to and after Bariatric and Metabolic Surgery Procedures. Obes Surg 2020;30:3135-53. [Crossref] [PubMed]
- Parmar C, Zakeri R, Abouelazayem M, et al. Esophageal and gastric malignancies after bariatric surgery: a retrospective global study. Surg Obes Relat Dis 2022;18:464-72. [Crossref] [PubMed]
- Sharaf RN, Weinshel EH, Bini EJ, et al. Endoscopy plays an important preoperative role in bariatric surgery. Obes Surg 2004;14:1367-72. [Crossref] [PubMed]
- Chen Q, Zhuang H, Liu Y. The association between obesity factor and esophageal caner. J Gastrointest Oncol 2012;3:226-31. [PubMed]
- Ebrahimi R, Kermansaravi M, Khalaj A, et al. Gastro-Intestinal Tract Cancers Following Bariatric Surgery: a Narrative Review. Obes Surg 2019;29:2678-94. [Crossref] [PubMed]
- Abdelbaki TN, Abdelsalam WN, ElKayal S. Management modalities in slipped gastric band. Surg Obes Relat Dis 2016;12:714-6. [Crossref] [PubMed]
Cite this article as: Palomares Casasús S, Fernández-Moreno MC, López Mozos F, Barrios Carvajal ME, Martí Obiol R, Ortega J. Esophageal adenocarcinoma after laparoscopic adjustable gastric banding: a case report and literature review. Ann Laparosc Endosc Surg 2023;8:24.