Feasibility and safety of laparoscopic 3-port cholecystectomy using the LiVac retractor: a prospective cohort study
Highlight box
Key findings
• Laparoscopic cholecystectomy with the LiVac retractor is associated with limited exposure of the operative field and only moderate user satisfaction.
What is known and what is new?
• Feasibility of laparoscopic cholecystectomy with the LiVac retractor has already been demonstrated before.
• LiVac use was analyzed for the first time in everyday clinical practice with regard to patient outcomes and user satisfaction.
What is the implication, and what should change now?
• 4-port laparoscopic cholecystectomy remains the gold standard of treatment.
Introduction
Laparoscopic cholecystectomy (LC) is the gold standard in the treatment of both symptomatic cholecystolithiasis and acute cholecystitis (1-3). Usually, the procedure is carried out using a 4-port technique (4). Performing LC with reduced ports (5-7) or as single-incision laparoscopic surgery procedure (SILS) (8,9) is also possible, but of course requires adherence to the same safety standards as laparoscopic 4-port cholecystectomy (10). This includes achieving the critical view of safety (CVS) criteria for safe avoidance of bile duct injury (11,12).
For SILS, a meta-analysis demonstrated a better cosmetic outcome, but on the other hand a fourfold increased risk of incisional hernia compared to the conventional 4-port technique (13). In addition, the performance of SILS is technically more demanding and is accompanied by a prolonged operation time (14). For these reasons, SILS has not been able to gain widespread acceptance to date.
One option for performing a laparoscopic 3-port cholecystectomy is to use the LiVac system, which is a vacuum-based internal liver retractor system. The LiVac system allows LC to be performed as a reduced 3-port operation. The function and clinical application of the LiVac system undergoing cholecystectomy (n=6), primary gastric banding (n=3) and fundoplication (n =1) were first published in 2016 by Gan et al. (15). In the same year, the use of the LiVac device in laparoscopic splenectomy was described, again by Gan (16), who is also the inventor of the system.
Due to the limited number of studies on the LiVac retractor to date, an evaluation of its safety and use in everyday clinical practice is hardly possible. Also, potential local effects of vacuum suction on the liver have hardly been investigated so far.
It is unclear whether there are any relevant clinical benefits for the patient when using the LiVac system and the reduced 3-port procedure, e.g., in terms of reduced postoperative pain.
Thus, the aim of our study was to investigate the safety, perioperative parameters and handling with the LiVac retractor in LC. We present the following article in accordance with the STROCSS reporting checklist (available at https://ales.amegroups.com/article/view/10.21037/ales-22-48/rc).
Methods
Study design
This is a prospective cohort study. Data were prospectively collected from all patients who underwent LC using the 3-port technique with the LiVac retractor during the period October 2018–December 2019 (n=25; group 1).
A retrospective and randomly selected cohort of patients of the same period undergoing conventional laparoscopic 4-port cholecystectomy served as a comparison group (n=50; group 2). This resulted in n=75 finally analyzed patients. A flowchart of patient selection for groups 1 and 2 is shown in Figure 1.
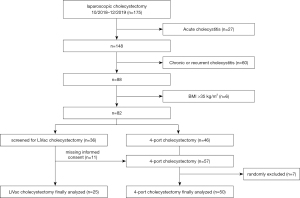
Groups were analyzed per protocol. If the application of the LiVac retractor was unsuccessful in patients of group 1, the procedure was switched to the standard 4-port technique. To avoid attrition bias, these patients were not excluded, but still evaluated as LiVac patients.
In retrospective group 2 there were no missing data in comparison to prospective group 1 (parameters see below).
Length of hospital stay was as a standard at least three days for all patients undergoing LC during the study period. Thus, a three-day follow-up (complications, pain levels, laboratory values; see below) was possible for all patients.
The study was conducted in the Department of General, Abdominal and Thoracic Surgery, German Armed Forces Hospital of Ulm (tertiary care hospital), Germany from October 2018–December 2019.
All data were taken from the digital patient record, surgical reports, and nursing documentation.
Primary and secondary outcomes
Patient safety was set as the primary outcome and evaluated by the rate of postoperative complications (according to Clavien-Dindo; see below).
Postoperative pain scores, laboratory values and surgical handling of the LiVac system were defined as the secondary outcome.
Inclusion and exclusion criteria
Only patients with elective cholecystectomy for symptomatic cholelithiasis (e.g., previous episodes of biliary colic) were included. A minimum age of 18 years and voluntary informed consent were required for inclusion in the study.
Exclusion criteria were the presence of acute or previous cholecystitis, a body mass index (BMI) >35 kg/m2, and an American Society of Anesthesiologists (ASA) score ≥4.
Statistical analysis
Mean values with standard deviation were calculated for all relevant data. Data analysis was performed with SPSS statistics (IBM Corp., Armonk, NY, USA). The chi-squared test was used for group comparison of categorical variables, and the Mann-Whitney U test was used to compare differences of ordinal variables. A P value <0.05 was considered statistically significant.
LiVac retractor
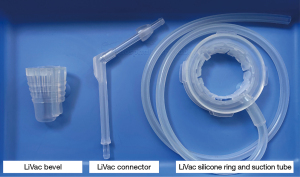
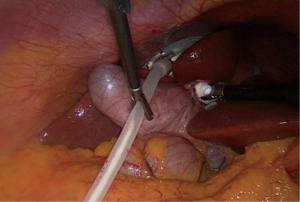
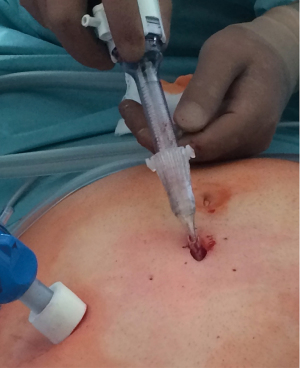
The LiVac system (LiVac Pty Ltd., Australia) consists of a soft silicone ring to which a vacuum can be applied via a suction tube. For LC, the silicone ring is placed between the liver and the diaphragm to expose the gallbladder after vacuum is applied. By means of an additional LiVac bevel, it is possible to insert the tube together with a 12 mm camera port and an adapted LiVac connector (Figures 2,3).
Medical treatment procedures
All laparoscopic cholecystectomies were performed in French position by surgical residents in their third or fourth year of residency under supervision of one out of five consultants, with an experience of at least 200 cholecystectomies. These were the same residents and consultants in both groups. After a supra- or infraumbilical skin incision, the pneumoperitoneum was established using a Veres needle.
In patients with conventional 4-port LC (group 2), two 12-mm (umbilical and epigastric) and two 5-mm ports (right subcostal and lower right hemiabdomen) were used. The patient was placed in anti-Trendelenburg position. The gallbladder was grasped at the fundus and retracted towards the right diaphragm for dissection of Calot’s triangle. The cystic artery and cystic duct were identified, and both structures were then cut between clips. The gallbladder was dissected out and removed using an endobag. After final hemostasis with electrocautery, fascial and wound closure was performed.
In patients with laparoscopic 3-port LiVac cholecystectomy (group 1), two 12-mm ports (umbilical and epigastric) and only one 5-mm port (right subcostal) were used. The umbilical 12-mm port had to be temporarily removed in order to insert the silicone ring with suction tube of the LiVac system. An Allis clamp was used for this purpose. The LiVac bevel was then placed over the temporarily removed 12 mm port, and the LiVac connector was also adapted to the bevel (Figure 2). The liver was elevated with a stick swab, and the LiVac silicone ring was placed between the liver and the right diaphragm (Figure 4). Once the correct position of the silicone ring was achieved, the vacuum could be applied. The further course of the cholecystectomy corresponded to that described above.
Analgesia and venous thromboembolism prophylaxis
All patients received postoperative oral analgesic medication with metamizole (1 g four times daily) and ibuprofen (400 mg three times daily). To prevent venous thromboembolism, enoxaparin 40 mg was subcutaneously given once daily.
Ultrasound examinations
All patients of the LiVac group underwent additional postoperative ultrasound examinations of the liver to detect possible subcapsular hematomas.
Postoperative complications
The recording of postoperative complications was limited to the inpatient stay. Complications were graded according to Clavien-Dindo (17,18).
Postoperative pain level
The postoperative pain level was assessed and documented three times a day by the nursing staff using the numeric ranking scale (NRS; 1–10).
Laboratory values
Alanine and aspartate aminotransferase (ALT, AST), gamma-glutamyl transferase (GGT) and alkaline phosphatase were identified as possible markers for liver damage. White blood count and C-reactive protein (CRP) were used as markers for inflammation. Laboratory values were obtained in all patients on the first preoperative day and on the first and third postoperative days (POD).
LiVac handling and satisfaction
Immediately postoperatively, satisfaction, handling, and difficulties with the LiVac system were recorded by the surgeon using a standardized questionnaire. Satisfaction with handling and exposure of the surgical field were graded on a scale from 1 (very good) to 6 (absolutely insufficient).
Ethics approval
Ethical approval was required for data collection for scientific purposes. The ethics committee of the University of Ulm/Germany gave a positive vote for prospective and retrospective data collection and study conduct (approval number: 225/18). The study was performed in accordance with the Declaration of Helsinki (as revised in 2013), and written informed consent was taken from all individual participants of the prospective patient group (LiVac group).
Results
Patient characteristics
A total of 75 patients were included in the analysis (group 1: n=25; group 2: n=50). The groups were comparable with respect to all characteristics at baseline. There was no statistically significant difference between the groups for any parameter [e.g., age: 48.4 vs. 51.1 years, P=0.52; BMI: 27.4 vs. 26.9 kg/m2, P=0.74; previous abdominal surgery: 44% vs. 48%, P=0.74; previous endoscopic retrograde cholangiopancreaticography (ERCP): 16% vs. 26%, P=0.33; for further parameters and preoperative laboratory values see Table 1].
Table 1
| Variables | LiVac 3-port LC (n=25) | 4-port LC (n=50) | P value |
|---|---|---|---|
| Age (years) (mean ± SD) | 48.4±17 | 51.1±16.6 | 0.52 |
| BMI (kg/m2) (mean ± SD) | 27.4±3.8 | 26.9±3.4 | 0.74 |
| Gender; n [%] | 0.24 | ||
| Male | 13 [52] | 19 [38] | |
| Female | 12 [48] | 31 [62] | |
| Previous abdominal surgery; n [%] | 11 [44] | 24 [48] | 0.74 |
| Preexisting umbilical hernia; n [%] | 1 [4] | 3 [6] | 0.77 |
| Previous ERCP; n [%] | 4 [16] | 13 [26] | 0.33 |
| Smoking; n [%] | 7 [28] | 13 [26] | 0.85 |
| Laboratory values (mean ± SD) | |||
| AST (U/L) | 27.6±20.1 | 26.8±20.6 | 0.51 |
| ALT (U/L)* | 31.8±20.5 | 43.0±64.9 | 0.50 |
| ALP (U/L) | 85.2±45.9 | 95.2±34.6 | 0.76 |
| GGT (U/L) | 97.4±132.9 | 72.8±68.2 | 0.94 |
| CRP (mg/L)* | 0.6±1.8 | 0.4±0.4 | 0.27 |
| WBC (103/µL) | 6.8±2.7 | 6.7±3.4 | 0.59 |
*, variables with deviations between the distributions (Mann-Whitney U test). LC, laparoscopic cholecystectomy; SD, standard deviation; BMI, body mass index; ERCP, endoscopic retrograde cholangiopancreaticography; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; GGT, gamma-glutamyl transferase; CRP, C-reactive protein; WBC, white blood count.
Surgical and perioperative measures
In 15/25 (60%) cases in the LiVac group, there was a switch to conventional 4-port cholecystectomy. Operative time was significantly longer in the LiVac group compared with 4-port LC (99.2 vs. 75.3 minutes; P=0.008).
Primary outcomes
Complications of all grades according to Clavien-Dindo occurred in 16% (LiVac) and 10% (4-port LC) of cases, respectively. This difference was not statistically significant (P=0.45).
In the LiVac group, tangential injury to the common bile duct (CBD) was detected in one patient (4%) postoperatively. This patient underwent ERCP with stent placement and re-laparoscopy with irrigation and drainage placement, and recovered completely thereafter.
In group 2, two patients (4%) required re-laparoscopy (irrigation and drainage placement in each case, once due to a postoperative hematoma, and once due to a postoperative abscess). Grade IV and V complications did not occur in either group.
Secondary outcomes
There were no significant differences between the groups in terms of postoperative pain level according to NRS (NRS on POD 3: 0.82 vs. 0.74 points, P=0.21).
No statistically significant difference between the groups occurred for any of the laboratory values recorded on PODs 1 and 3. ALT was 95.9 vs. 81.7 U/L (P=0.24) on POD 1, and 100.8 vs. 65.6 U/L (P=0.38) on POD 3. Further detailed values and measures are shown in Table 2.
Table 2
| Variables | LiVac 3-port LC (n=25) | 4-port LC (n=50) | P value |
|---|---|---|---|
| Switch to 4-port LC; n [%] | 15 [60] | – | – |
| Operative time (min) (mean ± SD) | 99.2±41.2 | 75.3±27.8 | 0.008 |
| Complications according to Clavien-Dindo; n [%] | |||
| I | 3 [12] | 2 [4] | 0.19 |
| II | 0 [0] | 1 [2] | 0.47 |
| III | 1 [4] | 2 [4] | 1.00 |
| IV | 0 [0] | 0 [0] | – |
| V | 0 [0] | 0 [0] | – |
| All grades I–V | 4 [16] | 5 [10] | 0.45 |
| Pain Scores (NRS) (mean ± SD) | |||
| POD 1* | 2.01±2.01 | 1.54±1.31 | 0.39 |
| POD 3 | 0.82±0.83 | 0.74±1.02 | 0.21 |
| Laboratory values | |||
| POD 1 (mean ± SD) | |||
| AST (U/L) | 70.4±24.5 | 67.2±27.6 | 0.53 |
| ALT (U/L) | 95.9±47.4 | 81.7±47.7 | 0.24 |
| ALP (U/L)* | 81.7±53.6 | 77.4±32.6 | 1.00 |
| GGT (U/L) | 76.8±73.5 | 72.9±58.5 | 0.82 |
| CRP (mg/L) | 5.7±7.9 | 4.1±5.1 | 0.21 |
| WBC (103/µL) | 9.2±3.4 | 9.3±2.7 | 0.34 |
| POD 3 (mean ± SD) | |||
| AST (U/L)* | 60.0±82.5 | 37.4±15.7 | 0.31 |
| ALT (U/L) | 100.8±128.6 | 65.6±35.9 | 0.38 |
| ALP (U/L) | 94.1±53.4 | 82.3±31.0 | 0.69 |
| GGT (U/L) | 124.8±81.2 | 88.9±76.0 | 0.09 |
| CRP (mg/L) | 5.8±7.9 | 9.9±9.9 | 0.77 |
| WBC (103/µL) | 7.4±2.5 | 6.7±2.0 | 0.43 |
*, variables with deviations between the distributions (Mann-Whitney U test). LC, laparoscopic cholecystectomy; SD, standard deviation; NRS, numeric ranking scale; POD, postoperative day; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; GGT, gamma-glutamyl transferase; CRP, C-reactive protein; WBC, white blood count.
The following parameters showed a deviation in the distribution of values: baseline ALT, baseline CRP, NRS on POD 1, ALP on POD 1 and AST on POD 3. Thus, for these parameters one prerequisite for the Mann-Whitney U test was not met.
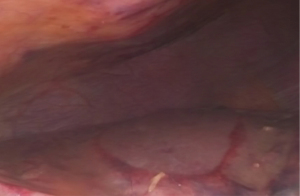
In all patients in the LiVac group, superficial liver hematomas were visible after removal of the silicone ring (Figure 5). However, no subcapsular liver hematoma was detectable in any of the postoperative ultrasound examinations.
Handling of the LiVac system and exposure of the surgical field during its use were not rated as “very good” by any of the surgeons. “Good handling” was attested in 20%, and “good exposure” of the surgical field in 5.9%; 32% of surgeons rated the handling of the LiVac system as “deficient” or “absolutely insufficient”, and 35.3% evaluated the exposure of the surgical field in the same way. “Poor exposure of the operative field” was the most commonly (37.5%) reported limitation of performing surgery with the LiVac system. Details of LiVac evaluation by the surgeons are shown in Table 3.
Table 3
| Variables | n [%] |
|---|---|
| Satisfaction with intraoperative handling | |
| 1= Very good | 0 [0] |
| 2= Good | 5 [20] |
| 3= Satisfactory | 9 [36] |
| 4= Sufficient | 3 [12] |
| 5= Deficient | 2 [8] |
| 6= Absolutely insufficient | 6 [24] |
| Satisfaction with exposure of operative field* | |
| 1= Very good | 0 [0] |
| 2= Good | 1 [5.9] |
| 3= Satisfactory | 5 [29.4] |
| 4= Sufficient | 5 [29.4] |
| 5= Deficient | 4 [23.5] |
| 6= Absolutely insufficient | 2 [11.8] |
| Specification of problems during LiVac placement** | |
| None | 9 [37.5] |
| Increased time required | 8 [33.3] |
| Vacuum cannot be established | 5 [20.8] |
| Liver injury during placement | 2 [8.3] |
| Specification of intraoperative problems with LiVac device** | |
| None | 11 [45.8] |
| Poor exposure of operative field | 9 [37.5] |
| Insufficient retraction of gallbladder | 1 [4.2] |
| Repeated loss of vacuum | 3 [12.5] |
Rating of the LiVac device by the surgeon using a standardized questionnaire with grades 1–6 (1= very good, 2= good, 3= satisfactory, 4= sufficient, 5= deficient, 6= insufficient). *, missing data for eight patients; **, missing data for one patient.
Discussion
The aim of our study was to test the use of the LiVac laparoscopic liver retractor under routine conditions. The focus was on the evaluation of patient safety on the one hand and the standardized assessment of the LiVac system by the surgeon using it on the other. Furthermore, we aimed to investigate whether 3-port cholecystectomy offers patient benefits, such as reduced postoperative pain, compared with 4-port cholecystectomy.
From our results, considering the limitations of our study (see below), the following points are of worthy of note: (I) the use of the LiVac system is associated with a prolonged operative time compared with conventional 4-port LC. This is due to (i) the higher number of required procedural steps (temporary port removal, insertion and positioning, establishment of a vacuum and so on) and (ii) the more difficult performance of the procedure—mainly due to impaired retraction of the gallbladder and thus limited exposure of Calot’s triangle. Of course, cholecystectomy is possible in 3-port technique using LiVac, but it is more difficult and more time consuming. (II) In our LiVac group, one patient experienced a CBD injury. This was a “Class I-injury” according to Stewart Way classification (19) or “Type C injury” according to Hannover classification [Bektas et al. (20)], and could therefore be treated with ERCP and laparoscopic irrigation. However, a CBD injury should always be considered a sentinel event. And although there was no statistically significant difference with regard to complications between both groups, it is well known that suboptimal exposure and tension of Calot’s triangle can promote injuries to the CBD. (III) The LiVac system failed to convince surgeons in terms of handling, and the limited exposure of the surgical field was the most frequently mentioned item of complaint. These conditions led to a switch to a conventional 4-port LC in more than half (60%) of the LiVac patients. (IV) There were no benefits found for patients in the LiVac group in terms of postoperative pain levels, as differences in NRS were statistically not significant.
Despite the mentioned problems with LiVac, it should be also emphasized that its application did not result in sonographically visualizable subcapsular liver hematomas, nor did it cause any remarkable changes in laboratory values.
In contrast to our results, Chiung Ta Lu and Gan found reduced postoperative opioid need in patients with LiVac 3-port versus 4-port LC (21). In the same study, operating time was significantly reduced in the 3-port LiVac group compared with the 4-port LC (58.3 vs. 89.4 minutes; P<0.001). However, they stated that “All surgeons had at least 10 years operative experience as consultant surgeons…” and “…all the 3-port laparoscopic cholecystectomies were performed by one surgeon…”. This setting cannot be compared to our study. At our institution, the vast majority of laparoscopic cholecystectomies are performed by residents under supervision of a consultant. Thus, the longer operating times and poor satisfaction with the LiVac retractor in our study may also reflect a lack of familiarity of surgeons with the device. The possible effect of a learning curve was not investigated in our series.
The strengths of the study consist in the testing of the LiVac under everyday surgical conditions as well as the assessment of the surgical handling by means of a standardized questionnaire.
Limitations of our study are the monocentric design, the small patient cohort and the limited follow-up period of only three PODs, which should be considered when interpreting the results.
For the parameters baseline ALT, baseline CRP, NRS on POD 1, ALP on POD 1 and AST on POD 3, not all the necessary prerequisites for performing the Mann-Whitney U test were fulfilled, so that these test results should be interpreted with particular caution.
Conclusions
It can be concluded that laparoscopic 3-port cholecystectomy with the LiVac retractor is feasible. However, we think that LiVac should only be used by (I) experienced laparoscopic surgeons, that (II) are very familiar with the device, and (III) when standards such as the “Critical View of Safety” are strictly followed. Due to the specifics regarding handling and limited exposure of the operative field with the LiVac system mentioned above, 3-port LC is hardly suitable as a training procedure, and is not appropriate for wide application.
In our own evaluation, the benefits of a standard 4-port LC with a better retraction of the gallbladder and thus better visualization of Calot’s triangle clearly outweighs the supposed advantages of saving a 5 mm port.
A prospective randomized comparative study would be required to reach a definitive assessment.
Acknowledgments
We would like to express our sincere thanks to Ms. Antonie Hellwig for her statistical advice.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROCSS reporting checklist. Available at https://ales.amegroups.com/article/view/10.21037/ales-22-48/rc
Data Sharing Statement: Available at https://ales.amegroups.com/article/view/10.21037/ales-22-48/dss
Peer Review File: Available at https://ales.amegroups.com/article/view/10.21037/ales-22-48/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://ales.amegroups.com/article/view/10.21037/ales-22-48/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The ethics committee of the University of Ulm/Germany gave a positive vote for prospective and retrospective data collection and study conduct (approval number: 225/18). The study was performed in accordance with the Declaration of Helsinki (as revised in 2013), and written informed consent was taken from all individual participants of the prospective patient group (LiVac group).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wakabayashi G, Iwashita Y, Hibi T, et al. Tokyo Guidelines 2018: surgical management of acute cholecystitis: safe steps in laparoscopic cholecystectomy for acute cholecystitis (with videos). J Hepatobiliary Pancreat Sci 2018;25:73-86. [Crossref] [PubMed]
- Shamiyeh A, Wayand W. Current status of laparoscopic therapy of cholecystolithiasis and common bile duct stones. Dig Dis 2005;23:119-26. [Crossref] [PubMed]
- Tazuma S, Unno M, Igarashi Y, et al. Evidence-based clinical practice guidelines for cholelithiasis 2016. J Gastroenterol 2017;52:276-300. [Crossref] [PubMed]
- Bittner R. The standard of laparoscopic cholecystectomy. Langenbecks Arch Surg 2004;389:157-63. [Crossref] [PubMed]
- Asakuma M, Uchiyama K. Reduced-port surgery. Nihon Geka Gakkai Zasshi 2016;117:364-9. [PubMed]
- Berlet M, Jell A, Bulian D, et al. Clinical value of alternative technologies to standard laparoscopic cholecystectomy - single port, reduced port, robotics, NOTES. Chirurgie (Heidelb) 2022;93:566-76. [Crossref] [PubMed]
- Kartal K, Uludag M. Can 4-port laparoscopic cholecystectomy remain the gold standard for gallbladder surgery? Ann Ital Chir 2016;87:13-7. [PubMed]
- Markar SR, Karthikesalingam A, Thrumurthy S, et al. Single-incision laparoscopic surgery (SILS) vs. conventional multiport cholecystectomy: systematic review and meta-analysis. Surg Endosc 2012;26:1205-13. [Crossref] [PubMed]
- Navarra G, Pozza E, Occhionorelli S, et al. One-wound laparoscopic cholecystectomy. Br J Surg 1997;84:695. [PubMed]
- Guidelines for the clinical application of laparoscopic biliary tract surgery. Society of American Gastrointestinal Endoscopic Surgeons. Surg Endosc 2000;14:771-2. [Crossref] [PubMed]
- Strasberg SM, Hertl M, Soper NJ. An analysis of the problem of biliary injury during laparoscopic cholecystectomy. J Am Coll Surg 1995;180:101-25. [PubMed]
- Strasberg SM, Brunt LM. Rationale and use of the critical view of safety in laparoscopic cholecystectomy. J Am Coll Surg 2010;211:132-8. [Crossref] [PubMed]
- Haueter R, Schütz T, Raptis DA, et al. Meta-analysis of single-port versus conventional laparoscopic cholecystectomy comparing body image and cosmesis. Br J Surg 2017;104:1141-59. [Crossref] [PubMed]
- Trastulli S, Cirocchi R, Desiderio J, et al. Systematic review and meta-analysis of randomized clinical trials comparing single-incision versus conventional laparoscopic cholecystectomy. Br J Surg 2013;100:191-208. [Crossref] [PubMed]
- Gan P, Bingham J. A clinical study of the LiVac laparoscopic liver retractor system. Surg Endosc 2016;30:789-96. [Crossref] [PubMed]
- Gan PS. Vacuum Stabilization of the Spleen in Laparoscopic Splenectomy. JSLS 2016;20:e2016.00013.
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 2009;250:187-96. [Crossref] [PubMed]
- Way LW, Stewart L, Gantert W, et al. Causes and prevention of laparoscopic bile duct injuries: analysis of 252 cases from a human factors and cognitive psychology perspective. Ann Surg 2003;237:460-9. [Crossref] [PubMed]
- Bektas H, Schrem H, Winny M, et al. Surgical treatment and outcome of iatrogenic bile duct lesions after cholecystectomy and the impact of different clinical classification systems. Br J Surg 2007;94:1119-27. [Crossref] [PubMed]
- Chiung Ta Lu T, Gan P, Versace V. Fewer Ports Cut Opioid Use and Length of Stay in Elective Laparoscopic Cholecystectomy. JSLS 2021;25:e2020.00093.
Cite this article as: Beltzer C, Burghard A, Kühnert N, Schmidt R. Feasibility and safety of laparoscopic 3-port cholecystectomy using the LiVac retractor: a prospective cohort study. Ann Laparosc Endosc Surg 2023;8:2.