Transanal total mesorectal excision as a natural orifice specimen extraction technique
Introduction
Since open surgery in rectal cancer causes a high rate of morbidity, there is still an ongoing search for minimally invasive methods. The theoretically accepted approach is based on the principle that as the invasiveness of the method is reduced, recovery is faster, and post-operative pain, surgical site infections, and post-operative herniation are decreased.
Although laparoscopic and robotic surgery is a minimally invasive method compared to the open method, it requires an abdominal incision during specimen removal, which limits its potential to reduce complications related to postoperative pain and wound infection rates. Therefore, the natural orifice specimen extraction (NOSE) has become one of the topics of research and interest as a way of eliminating the need for mini-laparotomy for the removal of the resected specimen.
Although transvaginal and transanal approaches were initially only applied for benign diseases, both also found an application area in colorectal cancer over time (1-4). Transanal NOSE with laparoscopic colectomy was performed for the first time in 2009 by Cheung et al. (5). the advantage of transanal specimen extraction over the transvaginal method is that it can be used in both women and men.
The evolution of rectal surgery toward the NOSE technique has occurred for several reasons. Initially, experienced colorectal surgeons performed colorectal local excisions with more rigid and fixed devices, such as transanal endoscopic microsurgery (TEM; RichardWolf Company, Tubingen, Germany) and transanal endoscopic operation (TEO; Karl Storz, Tuttlingen, Germany), and over time the technique allowed for incisions into the lumen, which could be easily closed, leading to the idea of total mesorectal excision (TME). Using this method, presacral dissection was performed under CO2 insufflation more linearly and more easily, with a better and magnified view, ‘down-to-up’ dissection, especially in the narrow male pelvis. This method was called transanal total mesorectal excision (TaTME) and defined as a new NOSE technique.
Initially, TEM and TEO devices were used in TaTME. However, due to the cost burden and difficulty of the learning curve, they were replaced with several different ports over time, with the currently used examples being SILS Port, Covidien, Mansfield, MA, USA; Gel-POINT Path, Applied Medical, Rancho Santa Margarita, CA, USA. Their flexibility and easily disassembly have resulted in a wider use of these devices (6).
TME continues to be the gold standard in rectal cancer surgery (7). However, during this procedure, the specimen must be extracted with sufficient accuracy and integrity. The mesorectum should be removed undamaged and as a whole, and its circumferential and distal borders should be adequately preserved or tumor-free. Theoretically, TaTME offers better visualization of pelvic dissection and draws attention with expectations to improve clinical, oncological, and functional outcomes by providing more accurate TME resection. TaTME is not a completely new concept but a blend of important surgical techniques. It has been developed with the contributions of the transabdominal transanal approach (TATA), as well as TEM and TEO. Since Sylla and Lacy (8,9) reported their early experience with TaTME in 2010, numerous case series have been published with encouraging results in terms of safety and efficacy (10). TaTME has emerged as a safe and viable minimally invasive approach to overcome some of the pitfalls of traditional transabdominal TME (11-16).
The potential advantages of TaTME, which is suitable for a NOSE technique, can be listed as provision of better access, especially to the middle and distal rectum, safer distal and circumferential rectal transection, not subjecting the distal rectum to multiple staple firings, and transanal extraction of the specimen (14,17,18). Although TaTME was initially defined for cancer cases, it has become a method that can also be used in the revision surgery of patients that have developed ulcerative colitis, rectovaginal fistula, rectourethral fistula, and anastomotic insufficiency. This technique also has the advantage of potentially lower morbidity and safer transanal ileal pouch anal anastomosis (19-24).
Can TaTME be performed with pure NOSE? Does it require hybrid approach?
Sylla et al. determined, first in animals and later human cadavers, that the mash NOSE technique was not safe enough for dissection (13,25-28). In 2009, Sylla and Lacy made their first case with the TEO platform with a double team, which is the hybrid method, and standardized it (8,9,15,29). In the hybrid method, the abdominal team can work together with the transanal team after performing certain dissections. This is also the approach we generally prefer. Alternatively, two teams can start dissection at the same time. The hybrid approach seems to be advantageous because it both shortens the operation time and minimizes the requirement of conversion to open surgery (30-34).
How should TaTME learning?
TaTME applications should be adopted by centers with sufficient laparoscopic experience and more than 50 years of laparoscopic colon-rectal surgery experience. Transanal minimal invasive surgery (TAMIS) experience is also recommended. After the simulation training is completed, at least two cadaver dissections are required (35). Subsequently, it is essential to perform this procedure on selected live cases accompanied by a mentor. Mentoring and supervision are vital to the successful implementation of a TaTME program (31,36-38). The learning curve of different procedures is variable, and it is stated that The learning curve of different procedures is variable, and it is stated that experience of approximately 40 cases is required for TaTME (39).
How should patient selection be performed?
TaTME is indicated in male patients with a narrow pelvis, those with middle and especially lower rectal tumors or a swollen mesorectum, and obese patients. Since most of the patient group included in these definitions require TaTME, surgery should be planned by considering these factors. However, although not included in this definition, TaTME may also be sometimes required due to the localization of the tumor or anatomical deterioration caused by neoadjuvant chemotherapy. In our center, we have a ready-made set-up that allows us to perform TaTME at any time in these cases.
Preoperative preparation
Potential stoma sites are marked one day before surgery, and mechanical bowel preparation is performed for all patients. Intravenous cefazolin and metronidazole antibiotic prophylaxis is injected 30 minutes or 1 hour before skin incision to prevent surgical site infections. To avoid venous thromboembolism, prophylactic doses of subcutaneous low molecular weight heparin are administered 2 hours before the operation prior to the induction of general anesthesia. The legs are wrapped in an elastic bandage. Anesthesia should be of sufficient depth. This depth should provide sufficient muscle paralysis and the large transanal area should provide ease of use.
After shoulder and side support is provided on an appropriate adjustable operating table, the patient is placed in the Lloyd-Davies and/or modified lithotomy position. Rectal irrigation is performed with povidone iodine solution, and a urinary catheter is placed using the aseptic technique.
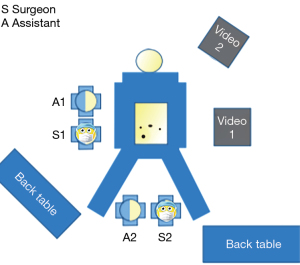
At our center, we routinely use a two-team approach because we consider it to be easier, faster, and safer. In this approach, a separate monitor and tower are required for both teams (Figure 1). The abdominal team uses the equipment utilized in standard laparoscopic colon-rectum operations and accesses the abdominal area with standard four ports. After the camera port is placed in the infraumbilical area, a second 11–12-mm port is placed at the intersection of the right midclavicular line and the SIAC-Umbilicus line. For this purpose, a camera with a 30-degree angle is preferred.
In addition to a laparoscopic tower for the transanal team, we generally use the Gel-POINT patch for transanal access. Cauterization is usually performed with a monopolar hook (Figure 2). Since the area is small and smoke formation makes dissection difficult, a system that continuously removes the smoke formed in the area is preferred (33).

Layout of surgical equipment
The surgeon and camera assistant of the abdominal team should be on the right side of the patient, the second assistant should be on the left side, the nurse should be by the right foot, and the laparoscopic tower should stand on the left of the patient directly opposite the surgeon (Figure 1).
The surgeon and surgeon assistant of the transanal team should be in a seated position between the patient’s legs. The second nurse should stand to their left, and the monitor should be directly opposite the surgeon if possible. We generally position it on the left, close to the patient’s head.
Where to start the operation?
It is recommended that the abdominal team start the operation. This is due to the necessity of examining the abdominopelvic space to exclude carcinomatosis or other unpredictable findings that can prevent radical resection (33,40).
Transabdominal team: abdominal entry, sigmoid colon and splenic flexure mobilization, and pelvis entry
The abdominal team completes abdominal exploration after providing 12–14 mmHg pneumoperitoneum with the closed Veres method. Then, the second port site is appropriately placed in the ileostomy site if it is to be opened. Subsequently, an abdominal set-up is completed by placing a 5-mm port on the left and right wings and four laparoscopic ports in the right iliac fossa (33). If needed, an additional 5-mm trocar can be inserted for the mobilization of the splenic flexure. If the patient is female, the uterus can be suspended. After confirming the absence of peritoneal or liver metastases, both teams can start working simultaneously. The operating table is tilted to the right, and the patient is placed in the Trendelenburg position. The small intestines are pulled cranially from the pelvis. Medio-lateral dissection is started by finding the right common iliac artery and aortic peritoneal tracing. After identification of the left ureter, the inferior mesenteric artery (IMA) is transected 1 cm distal to the aorta by ligating it with a vessel closure device or clip, preserving the hypogastric nerves. Next, the inferior mesenteric vein is ligated and separated from the lower border of the pancreas from the lateral duodenum to the cranial. Then, the left colon is mobilized over Gerota’s fascia up to the colon pelvis. Toldt’s fascia is opened from the left, and the sigmoid, descending colon, and splenic flexure are mobilized from the lower pole of the spleen. Subsequently, the gastrocolic ligament is opened, and the pancreas is opened from the border of the lower-transverse colon-meso to the lower pole of the spleen, and the splenic flexure is completely released. After confirming that the new conduit is formed, upper rectal dissection is started. The ureter is dissected up to the bladder entry point on both sides, ensuring its safety, and then the neurovascular bundle area is dissected (33).
Transanal team: rectal transection and mobilization
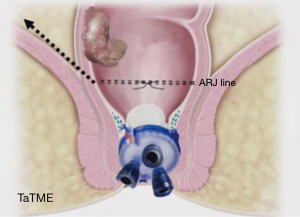
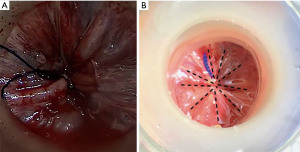
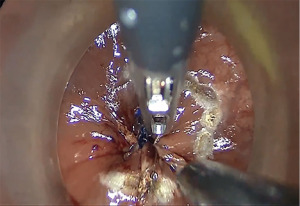
We divide TaTME into two according to the anorectal junction (ARJ) location of the tumor. This classification described by Rullier et al. is made according to the authors’ intersphincteric definition (41,42). If the tumor is located 2 cm above ARJ, the anal region is explored with the Lone Star® Retractor (CooperSurgical, Inc., Trumbull, CT, USA) (Figure 3). If the tumor is too high to be visible on exploration, the GelPOINT® Path Transanal Access Platform (4 cm × 5.5 cm) (Applied Medical Inc., Rancho Santa Margarita, CA, USA) is placed directly and insufflated using rectal AirSeal® (Conmed Inc., Utica, NY, USA). Meanwhile, the abdominal team clamps the distal sigmoid colon to ensure that the gas does not disperse into the entire colon. After the tumor is localized, the lumen should be completely closed with 2/0 polydioxanone (PDS) or Prolene 360 ℃ purse-string sutures at least 1 cm distal to the tumor, which constitutes the most important stage of the procedure. The suture should fully bite into the rectal wall. A needle curve of 26 mm should be preferred, and purse-string bites should be placed 8–12 times circularly in the same plane. When the suture is tightened, it should stand in the middle in the form of a star (Figure 4). Some authors (43,44) recommend making a second suture. During the subsequent pelvic dissection, spilled tumour cells might be scattered as a result of the continuous high-flow insufflation used in the dissection area in TaTME, leading to multifocal local recurrence. After tightening the purse-string and making sure that there is no upward gas leakage, the access channel is removed and the area is washed with 5% povidone iodine to benefit from its bactericidal and tumorocidal effects. The access channel of the GelPOINT patch is re-inserted into the rectum, and the camera and the other two ports are triangulated into the GelPOINT patch. The area is insufflated again using AirSeal. An intraluminal pressure of 12–14 mmHg is desirable (45,46). The mucosa and submucosa are dissected 1 cm distal to the purse-string (Figure 5), and the dissection is completed at 360°. This was followed by the full-thickness cutting of the circular and longitudinal wall muscles. In general, it is recommended to cut the muscles at the 5-7-11-1 o’clock positions. After the muscles are cut at one point, circumferential dissection must be completed. The aim here is to continue the dissection in this area along the cell area, which is the extension of the Holly Plane, called Angel’s Hair. If the dissection is to be continued in the anterior plane, the advantage of this method is that it is planar and generally the same in all patients, but a disadvantage is the urethra being located here in male patients. Although it seems safer to advance in the posterior plane, this can cause undesirable mesorectal dissections, presacral hemorrhage, or rectal perforation due to the plane differences that vary from patient to patient (45).
If the tumor is within 2 cm of ARJ; i.e., in the intersphincteric area, when we directly place the access channel of the GelPOINT patch, the tumor may remain under the access channel. Therefore, in these types of tumors, after the lone-star retractor is placed and before insufflation, either the Ferguson anal retractor or only the end of the access channel is placed in such a way that only the end of the access canal can be seen, the purse-string is removed from 1 cm distal of the tumor, and the area is washed with povidone iodine (Figure 6). In cases where the field is too short, the intersphincteric space is first openly dissected to fit the access channel before the removal of the purse-string. After confirming the dissection of sufficient area to place the access channel, the purse-string is removed, and the process is continued as described above (47,48).
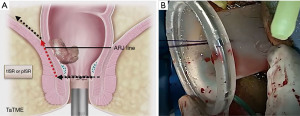
Abdominal dissection margin may depend on many factors, such as the surgeon’s preference, and the difficulty of abdominal and transanal dissections. By opening the posterior rectum and lateral peritoneum, the anterior peritoneal reflection can be opened to help the two planes meet. At this time, the transanal team should ask the abdominal team to retract the rectum upwards while continuing to perform appropriate dissections for access (33,45,46,49).
Meeting of the abdominal and transanal teams: rendezvous
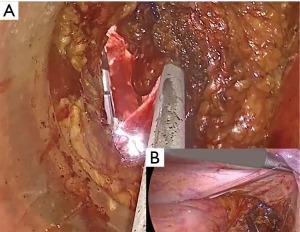
The anterior plane is typically an easier point to enter the peritoneal cavity from below; however, posterior meeting may sometimes be more possible if the posterior dissection is more advanced or anterior dissection is difficult. After the successful meeting of the two teams, the abdominal team (Figure 7) can provide assistance by pulling the anterior peritoneal reflection upwards, continuing to pull the rectum up to facilitate dissection and back into the abdominal cavity where the dissection can be completed (Figure 7). When the entire rectum is removed, the transanal cap is taken out, the table position is stabilized, and the pelvis is rinsed with abundant saline or sterile water from above and allowed to drain transanally. The distal pouch string is then held, and the specimen can often be removed transanally. In the case of a bulky tumor or mesentery that prevents transanal extraction, a Pfannenstiel incision can be used for sample extraction (33,45,50).
Transanal extraction of specimen with NOSE
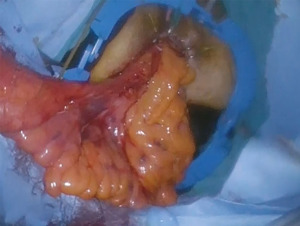
In the case of transanal extraction, the access channel is removed, and the entire rectum and sigmoid colon are taken out through the anus (Figure 8). The proximal of IMA is located. It is recommended to determine the proximal transection margin with indocyanine green angiography imaging. As a team, we visually check the Riolan and Drummond circulation after transection.
How do we perform anastomosis
If the purse-string is located 2 cm above ARJ at the beginning, our choice of anastomosis is with a double purse-string circular stapler. Purse-string suturing is performed in the distal of the descending colon, a circular stapler anvil no 31 or 33 is placed, and a plastic tube is inserted to form an anvil guide and sent back to the pelvic area. Subsequently, the second purse-string is placed on the distal rectal stump. During tightening, the plastic guide is compressed. The abdominal team follows the colon-meso position to avoid any twisting. The plastic guide is pulled, the tip of the anvil is removed from the distal rectum and placed in the stapler socket, and firing is performed. After confirming that the protruding rings have been fully removed, anastomosis control is undertaken (Figure 9) (the full short summary of the operation is presented in Video 1 with voiceover).
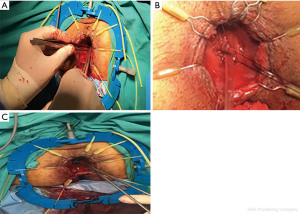
If the lower border of the dissection is in the intersphincteric area, either one-by-one suture with the manual colo-anal anastomosis technique or the two-stage Turnbull-Cutait pull-through technique is preferred. The latter seems to be advantageous because it does not require a protective ileostomy and has low leakage rates (51,52).
Ileostomy
While the transanal team performs the anastomosis, the abdominal team places a pelvic drain through a laparoscopic 5-mm port. A diverting ileostomy is recommended in high-risk patients with prior right lower quadrant radiotherapy, colo-anal anastomosis, obesity, or other risk factors (33).
Benefits of TaTME (4,33,53)
- Direct visualization of intraluminal rectal wall transection;
- Reducing local recurrence due to the increased rate of tumor-free distal and peripheral margins;
- Reducing the possibility of anastomotic leaks by avoiding multiple staple fires
- Preventing permanent stomas;
- Reducing both surgical site infections and the rate of abdominal wall hernias as a NOSE technique;
- Resection of T4 and bulky tumors with a minimally invasive approach;
- Less postoperative pain and shorter hospital stay;
- Providing direct view of surgical plans (mesorectum, autonomic nerves, and surrounding structures);
- Complete resection of the mesorectum (oncological effect in local and distal recurrence);
- Better preservation of urinary and sexual functions.
Transanal NOSE indications (54,55)
- Locally advanced tumor;
- Absence of intestinal obstruction and perforation;
- Tis and T1 tumors not suitable for local excision;
- Presence of T2 or T3 tumor;
- Peripheral specimen diameter <3 cm;
- Body mass index <30 kg/m2.
Oncological outcomes
The largest case series published on TaTME belongs to Roodbeen et al. (56), who obtained data from the international TaTME registry and reviewed 2,803 cases. In that study, the quality of TME was evaluated as intact in 86% of the patients, while a major defect was observed in the mesorectum of 3%, and the rates of circumferential resection margin (CRM) (+) and distal resection margin (DRM) (+) were 5.1% and 1%, respectively. The local recurrence rate within two years was reported to be 4.8%. Furthermore, the rates of DFS and OS at two years were 76.6% and 91.9%, respectively. Similar oncological results were obtained from 1,283 cases of TaTME from China (4,57,58). In the ACOSOG and ALaCaRT trials, the DFS and OS rates were obtained as 81.7% and 82%, respectively in the patients treated using laparoscopic TME compared to 86.9% and 89%, respectively for those that underwent open TME (59,60). In a study by Roodbeen et al. (56), this composite endpoint was achieved in 90% of the patients, suggesting more precise surgery compared with the laparoscopic groups in both of the randomized trials and similar results to those obtained from patients treated using open surgery. The local recurrence rate in the current study is in agreement with the results reported in these large registry-based datasets, suggesting that TaTME provides equivalent or superior local disease control.
Functional outcomes
Although the survival rates in rectal cancer have improved, the quality of life and functional outcomes have become increasingly important. A Dutch group of researchers (61), who performed TME and followed their patients up for 14 years, reported that major low anterior resection syndrome (LARS) continued at a rate of 46%, which they attributed to the risk factors of ≤75 years of age and radiotherapy.
Theoretically, it is widely believed that the better visualization of the pelvic area and safer dissection with the transanal approach will result in better functional outcomes. However, anastomosis being performed at a low level even in middle rectal tumors, especially at the beginning stages, the formation of ‘reversed coning’ during down-to-up dissection, and the access channel’s dilatation effect on the sphincters have caused concerns related to the worsening of functional outcomes. In a study comparing TaTME and Laparoscopic-TME, Li et al. (62) reported similar major-LARS rates in both groups, but noted that TaTME provided significantly better outcomes in terms of sexual and urological functions. In a prospective observational study, de Lacy et al. (63) determined that the rate of major-LARS, which was 13.3% in the pre-operative period, increased to 60% at the third month and decreased to 25% at the 12th month. The authors stated that there was no deterioration in the sexual and urological functions of the patients and they were at a similar level in all measurements.
Experience of our center
We completed our 50 TaTME cases between January 2018 and April 2022. Thirty-eight of these patients were male. TaTME was performed in two patients due to familial adenomatous polyposis (FAP). One patient required abdominoperineal excision (APR). The average distance of the tumor from the anal verge was 6.1 [2–10] cm. Laparoscopic-TaTME was performed in 24 patients because ARJ was 2 cm above, and intersphincteric-TaTME in the remaining 26 patients because it was in the intersphincteric area. The quality of TME was evaluated as complete in 90% of the patients and slightly damaged in 8%. CRM(+) and DRM(+) were observed in two patients each. Local recurrence occurred in one case (2%) at 23 months. Distant organ metastasis developed in the same patient at 33 months. In another patient, distant organ metastasis was detected at 12 months.
The specimens of 30 cases (60%) were extracted by the transanal route in accordance with NOSE without any problems.
Conclusion and future of TaTME
As a NOSE technique, TaTME provides either better or similar results compared to open, laparoscopic, and robotic TME when evaluated from an oncological point of view. From the functional point of view, it is better than other methods in terms of sexual and urological aspects, but the results of other methods are also not unfavorable concerning fecal continence. It is expected that ongoing RCT studies COLOR-III and ETAP-GERCCAR-11 will provide more evidence-based results (64,65). The current impression is that the use of TaTME has evolved into more distal tumors and become a form of sphincter-sparing surgery. de Lacy et al. (66) reported that while the APR rate was 25.6% in the Laparoscopic-TME group, this rate decreased to 2.9% in the TaTME group. When evaluated in light of these findings, every center performing rectal cancer surgery or non-cancer surgery should have this technique in their portfolio.
Acknowledgments
We would like to thank Can Yüksel who voiced the video in English.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Cuneyt Kayaalp) for the series “Natural Orifice Specimen Extraction in Colorectal Surgery” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Peer Review File: Available at https://ales.amegroups.com/article/view/10.21037/ales-22-26/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://ales.amegroups.com/article/view/10.21037/ales-22-26/coif). The series “Natural Orifice Specimen Extraction in Colorectal Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Marks JH, Frenkel JL, D'Andrea AP, et al. Maximizing rectal cancer results: TEM and TATA techniques to expand sphincter preservation. Surg Oncol Clin N Am 2011;20:501-20. viii-ix. [Crossref] [PubMed]
- Torres RA, Orban RD, Tocaimaza L, et al. Transvaginal specimen extraction after laparoscopic colectomy. World J Surg 2012;36:1699-702. [Crossref] [PubMed]
- Franklin ME Jr, Liang S, Russek K. Integration of transanal specimen extraction into laparoscopic anterior resection with total mesorectal excision for rectal cancer: a consecutive series of 179 patients. Surg Endosc 2013;27:127-32. [Crossref] [PubMed]
- Maykel JA, Hahn SJ, Beauharnais CC, et al. Oncologic Outcomes After Transanal Total Mesorectal Excision for Rectal Cancer. Dis Colon Rectum 2022;65:827-36. [Crossref] [PubMed]
- Cheung HY, Leung AL, Chung CC, et al. Endo-laparoscopic colectomy without mini-laparotomy for left-sided colonic tumors. World J Surg 2009;33:1287-91. [Crossref] [PubMed]
- McLemore EC, Weston LA, Coker AM, et al. Transanal minimally invasive surgery for benign and malignant rectal neoplasia. Am J Surg 2014;208:372-81. [Crossref] [PubMed]
- Ridgway PF, Darzi AW. The role of total mesorectal excision in the management of rectal cancer. Cancer Control 2003;10:205-11. [Crossref] [PubMed]
- Sylla P, Rattner DW, Delgado S, et al. NOTES transanal rectal cancer resection using transanal endoscopic microsurgery and laparoscopic assistance. Surg Endosc 2010;24:1205-10. [Crossref] [PubMed]
- Sylla P, Lacy A. NOTES transanal rectal cancer resection using transanal endoscopic microsurgery. European Surgery 2011;43:146-52. [Crossref]
- Penna M, Hompes R, Arnold S, et al. Transanal Total Mesorectal Excision: International Registry Results of the First 720 Cases. Ann Surg 2017;266:111-7. [Crossref] [PubMed]
- Lacy AM, Tasende MM, Delgado S, et al. Transanal Total Mesorectal Excision for Rectal Cancer: Outcomes after 140 Patients. J Am Coll Surg 2015;221:415-23. [Crossref] [PubMed]
- Ma B, Gao P, Song Y, et al. Transanal total mesorectal excision (taTME) for rectal cancer: a systematic review and meta-analysis of oncological and perioperative outcomes compared with laparoscopic total mesorectal excision. BMC Cancer 2016;16:380. [Crossref] [PubMed]
- Suwanabol PA, Maykel JA. Transanal Total Mesorectal Excision: A Novel Approach to Rectal Surgery. Clin Colon Rectal Surg 2017;30:120-9. [Crossref] [PubMed]
- Rouanet P, Mourregot A, Azar CC, et al. Transanal endoscopic proctectomy: an innovative procedure for difficult resection of rectal tumors in men with narrow pelvis. Dis Colon Rectum 2013;56:408-15. [Crossref] [PubMed]
- de Lacy AM, Rattner DW, Adelsdorfer C, et al. Transanal natural orifice transluminal endoscopic surgery (NOTES) rectal resection: "down-to-up" total mesorectal excision (TME)--short-term outcomes in the first 20 cases. Surg Endosc 2013;27:3165-72. [Crossref] [PubMed]
- Chen CC, Lai YL, Jiang JK, et al. The evolving practice of hybrid natural orifice transluminal endoscopic surgery (NOTES) for rectal cancer. Surg Endosc 2015;29:119-26. [Crossref] [PubMed]
- Motson RW, Lacy A. The Rationale for Transanal Total Mesorectal Excision. Dis Colon Rectum 2015;58:911-3. [Crossref] [PubMed]
- Motson RW, Whiteford MH, Hompes R, et al. Current status of trans-anal total mesorectal excision (TaTME) following the Second International Consensus Conference. Colorectal Dis 2016;18:13-8. [Crossref] [PubMed]
- de Buck van Overstraeten A, Wolthuis AM, D'Hoore A. Transanal completion proctectomy after total colectomy and ileal pouch-anal anastomosis for ulcerative colitis: a modified single stapled technique. Colorectal Dis 2016;18:O141-4. [Crossref] [PubMed]
- van Overstraeten A, Wolthuis A, d'Hoore A. Transanal completion proctectomy after total colectomy and ileal pouch-anal anastomosis for ulcerative colitis: a codified single stapled technique. Diseases of the Colon & Rectum 2016;59:E398-9.
- Leo CA, Samaranayake S, Perry-Woodford ZL, et al. Initial experience of restorative proctocolectomy for ulcerative colitis by transanal total mesorectal rectal excision and single-incision abdominal laparoscopic surgery. Colorectal Dis 2016;18:1162-6. [Crossref] [PubMed]
- Gaujoux S, Bretagnol F, Au J, et al. Single port access proctectomy with total mesorectal excision and intersphincteric resection with a primary transanal approach. Colorectal Dis 2011;13:e305-7. [Crossref] [PubMed]
- Park L, Truong A, Zaghiyan K, et al. A Single-Center Comparative Study of Open Transabdominal and Laparoscopic Transanal Ileal Pouch-Anal Anastomosis with Total Mesorectal Excision. Has the Bar Been Raised? J Gastrointest Surg 2022;26:1070-6. [Crossref] [PubMed]
- Coffey JC, Dillon MF, O'Driscoll JS, et al. Transanal total mesocolic excision (taTME) as part of ileoanal pouch formation in ulcerative colitis--first report of a case. Int J Colorectal Dis 2016;31:735-6. [Crossref] [PubMed]
- Carmichael H, Sylla P. Evolution of Transanal Total Mesorectal Excision. Clin Colon Rectal Surg 2020;33:113-27. [Crossref] [PubMed]
- Lee GC, Sylla P. Shifting Paradigms in Minimally Invasive Surgery: Applications of Transanal Natural Orifice Transluminal Endoscopic Surgery in Colorectal Surgery. Clin Colon Rectal Surg 2015;28:181-93. [Crossref] [PubMed]
- Izquierdo KM, Salem JF, Cha E, et al. Transanal Surgery: A History of taTME Ancestry. Clin Colon Rectal Surg 2020;33:128-33. [Crossref] [PubMed]
- Emhoff IA, Lee GC, Sylla P. Transanal colorectal resection using natural orifice translumenal endoscopic surgery (NOTES). Dig Endosc 2014;26:29-42. [Crossref] [PubMed]
- Telem DA, Han KS, Kim MC, et al. Transanal rectosigmoid resection via natural orifice translumenal endoscopic surgery (NOTES) with total mesorectal excision in a large human cadaver series. Surg Endosc 2013;27:74-80. [Crossref] [PubMed]
- Koedam TWA, Veltcamp Helbach M, van de Ven PM, et al. Transanal total mesorectal excision for rectal cancer: evaluation of the learning curve. Tech Coloproctol 2018;22:279-87. [Crossref] [PubMed]
- Veltcamp Helbach M, van Oostendorp SE, Koedam TWA, et al. Structured training pathway and proctoring; multicenter results of the implementation of transanal total mesorectal excision (TaTME) in the Netherlands. Surg Endosc 2020;34:192-201. [Crossref] [PubMed]
- Atallah S, Albert M, Monson JR. Critical concepts and important anatomic landmarks encountered during transanal total mesorectal excision (taTME): toward the mastery of a new operation for rectal cancer surgery. Tech Coloproctol 2016;20:483-94. [Crossref] [PubMed]
- Veltcamp H, van Oostendorp SE, Koedam TWA, et al. Structured training pathway and 7 proctoring; multicenter results of the implementation of transanal total mesorectal excision (TaTME) in the Netherlands. In: van Oostendorp SE. Minimally invasive treatment strategies for rectal cancer. Surgical Endoscopy 1st edition; 2021:110-27.
- Lee L, Kelly J, Nassif GJ, et al. Defining the learning curve for transanal total mesorectal excision for rectal adenocarcinoma. Surg Endosc 2020;34:1534-42. [Crossref] [PubMed]
- Keller DS, Delaney CP, Senagore AJ, et al. Uptake of enhanced recovery practices by SAGES members: a survey. Surg Endosc 2017;31:3519-26. [Crossref] [PubMed]
- Abbott SC, Stevenson ARL, Bell SW, et al. An assessment of an Australasian pathway for the introduction of transanal total mesorectal excision (taTME). Colorectal Dis 2018;20:O1-6. [Crossref] [PubMed]
- Francis N, Penna M, Mackenzie H, et al. Consensus on structured training curriculum for transanal total mesorectal excision (TaTME). Surg Endosc 2017;31:2711-9. [Crossref] [PubMed]
- TaTME Guidance Group representing the ESCP (European Society of Coloproctology), in collaboration with the ASCRS (American Society of Colon and Rectal Surgeons), ACPGBI (Association of Coloproctology of Great Britain and Ireland), et al. International expert consensus guidance on indications, implementation and quality measures for transanal total mesorectal excision. Colorectal Dis 2020;22:749-55.
- Veltcamp Helbach M, van Oostendorp SE, Koedam TWA, et al. Structured training pathway and proctoring; multicenter results of the implementation of transanal total mesorectal excision (TaTME) in the Netherlands. Surgical Endoscopy 2020;34:192-201. [Crossref] [PubMed]
- Simo V, Tejedor P, Jimenez LM, et al. Oncological safety of transanal total mesorectal excision (TaTME) for rectal cancer: mid-term results of a prospective multicentre study. Surg Endosc 2021;35:1808-19. [Crossref] [PubMed]
- Rullier E, Denost Q, Vendrely V, et al. Low rectal cancer: classification and standardization of surgery. Dis Colon Rectum 2013;56:560-7. [Crossref] [PubMed]
- Bordeianou L, Maguire LH, Alavi K, et al. Sphincter-sparing surgery in patients with low-lying rectal cancer: techniques, oncologic outcomes, and functional results. J Gastrointest Surg 2014;18:1358-72. [Crossref] [PubMed]
- Koch MJ, Tanis PJ, Bemelman WA, et al. Purse-string reinforcement in transanal total mesorectal excision: a further essential step to increase oncological safety - a video vignette. Colorectal Dis 2020;22:219-20. [Crossref] [PubMed]
- Vannijvel M, Wolthuis AM. Limitations and Concerns with Transanal Total Mesorectal Excision for Rectal Cancer. Clin Colon Rectal Surg 2022;35:141-5. [Crossref] [PubMed]
- Atallah S. Transanal Minimally Invasive Surgery (TAMIS) and Transanal Total Mesorectal Excision (taTME): Springer; 2019.
- Aka AA, Wright JP, Monson JR. Transanal Total Mesorectal Excision. Techniques in Minimally Invasive Surgery: Springer; 2021:413-27.
- Atallah S, Rullier E. Strategies for Ultralow-Lying Rectal Cancer. Transanal Minimally Invasive Surgery (TAMIS) and Transanal Total Mesorectal Excision (taTME): Springer; 2019:285-97.
- Robertson RL, Brown CJ. Indications for Malignant Neoplasia of the Rectum. Transanal Minimally Invasive Surgery (TAMIS) and Transanal Total Mesorectal Excision (taTME): Springer; 2019:187-96.
- Penna M, Cunningham C, Hompes R. Transanal Total Mesorectal Excision: Why, When, and How. Clin Colon Rectal Surg 2017;30:339-45. [Crossref] [PubMed]
- Westwood DA, Cuda TJ, Hamilton AER, et al. Transanal total mesorectal excision for rectal cancer: state of the art. Tech Coloproctol 2018;22:649-55. [Crossref] [PubMed]
- Biondo S, Trenti L, Espin E, et al. Two-Stage Turnbull-Cutait Pull-Through Coloanal Anastomosis for Low Rectal Cancer: A Randomized Clinical Trial. JAMA Surg 2020;155:e201625. [Crossref] [PubMed]
- Guillem JG, Cutait R, Remzi F. Turnbull-Cutait Pull-Through-An Old Procedure With a New Indication? JAMA Surg 2020;155:e201756. [Crossref] [PubMed]
- Jiang HP, Li YS, Wang B, et al. Pathological outcomes of transanal versus laparoscopic total mesorectal excision for rectal cancer: a systematic review with meta-analysis. Surg Endosc 2018;32:2632-42. [Crossref] [PubMed]
- Guan X, Liu Z, Longo A, et al. International consensus on natural orifice specimen extraction surgery (NOSES) for colorectal cancer. Gastroenterol Rep (Oxf) 2019;7:24-31. [Crossref] [PubMed]
- Izquierdo KM, Unal E, Marks JH. Natural orifice specimen extraction in colorectal surgery: patient selection and perspectives. Clin Exp Gastroenterol 2018;11:265-79. [Crossref] [PubMed]
- Roodbeen SX, Penna M, van Dieren S, et al. Local Recurrence and Disease-Free Survival After Transanal Total Mesorectal Excision: Results From the International TaTME Registry. J Natl Compr Canc Netw 2021;jnccn20505. Epub ahead of print. [Crossref] [PubMed]
- Yao H, An Y, Zhang H, et al. Transanal Total Mesorectal Excision: Short-term Outcomes of 1283 Cases from a Nationwide Registry in China. Dis Colon Rectum 2021;64:190-9. [Crossref] [PubMed]
- Zeng Z, Luo S, Chen J, et al. Comparison of pathological outcomes after transanal versus laparoscopic total mesorectal excision: a prospective study using data from randomized control trial. Surg Endosc 2020;34:3956-62. [Crossref] [PubMed]
- Fleshman J, Branda M, Sargent DJ, et al. Effect of Laparoscopic-Assisted Resection vs Open Resection of Stage II or III Rectal Cancer on Pathologic Outcomes: The ACOSOG Z6051 Randomized Clinical Trial. JAMA 2015;314:1346-55. [Crossref] [PubMed]
- Stevenson AR, Solomon MJ, Lumley JW, et al. Effect of Laparoscopic-Assisted Resection vs Open Resection on Pathological Outcomes in Rectal Cancer: The ALaCaRT Randomized Clinical Trial. JAMA 2015;314:1356-63. [Crossref] [PubMed]
- Chen TY, Wiltink LM, Nout RA, et al. Bowel function 14 years after preoperative short-course radiotherapy and total mesorectal excision for rectal cancer: report of a multicenter randomized trial. Clin Colorectal Cancer 2015;14:106-14. [Crossref] [PubMed]
- Li Y, Bai X, Niu B, et al. A prospective study of health related quality of life, bowel and sexual function after TaTME and conventional laparoscopic TME for mid and low rectal cancer. Tech Coloproctol 2021;25:449-59. [Crossref] [PubMed]
- de Lacy FB, Turrado-Rodriguez V, Torroella A, et al. Functional Outcomes and Quality of Life After Transanal Total Mesorectal Excision for Rectal Cancer: A Prospective Observational Study. Dis Colon Rectum 2022;65:46-54. [Crossref] [PubMed]
- Deijen CL, Velthuis S, Tsai A, et al. COLOR III: a multicentre randomised clinical trial comparing transanal TME versus laparoscopic TME for mid and low rectal cancer. Surg Endosc 2016;30:3210-5. [Crossref] [PubMed]
- Lelong B, de Chaisemartin C, Meillat H, et al. A multicentre randomised controlled trial to evaluate the efficacy, morbidity and functional outcome of endoscopic transanal proctectomy versus laparoscopic proctectomy for low-lying rectal cancer (ETAP-GRECCAR 11 TRIAL): rationale and design. BMC Cancer 2017;17:253. [Crossref] [PubMed]
- de Lacy FB, Roodbeen SX, Ríos J, et al. Three-year outcome after transanal versus laparoscopic total mesorectal excision in locally advanced rectal cancer: a multicenter comparative analysis. BMC Cancer 2020;20:677. [Crossref] [PubMed]
Cite this article as: Yuksel BC, Er S. Transanal total mesorectal excision as a natural orifice specimen extraction technique. Ann Laparosc Endosc Surg 2022;7:33.