Hand-assisted laparoscopic nephrectomy for a kidney transplant: surgical technique—tips and tricks—how we do it?
Introduction
The laparoscopic donor nephrectomy was first introduced in 1995 by Ratner. It is now carried out by three different approaches: laparoscopic transperitoneal donor nephrectomy (LDN), laparoscopic hand-assisted transperitoneal donor nephrectomy (HALDN), and laparoscopic retroperitoneal donor nephrectomy (LRDN). At present, there is no substantial evidence to support the use of one laparoscopic approach in preference to the other; however, there is some evidence suggesting that HALDN represents the most cost-effective method of donor surgery achieving similar clinical benefits of pure laparoscopic approaches with less operative time (1).
In the potential living donor nephrectomy for transplant, the safety must remain as maximal priority because it is performed on a healthy patient who accepts the risks of major surgery for the benefit of the recipient. The process of donation should be as least traumatic, painful, and uncomfortable as possible to the donor (2,3).
It is essential to maintain the mortality and morbidity of live donors as low as possible. A study shows that the mortality rate must be almost 0%, at the time that we need to preserve the kidney in optimal condition for transplantation (4).
HALDN encompasses the benefits of the minimally invasive approach reducing the operative time and learning curve in comparison with the totally laparoscopic procedure.
Hand assisted laparoscopic surgeries are at least as safe as totally laparoscopic procedures, in terms of time for return to normal activity are also comparable and require fewer trocars (5).
Hand-assisted laparoscopic surgery tends to decrease operation time and conversion rate compared to conventional laparoscopy for colorectal surgery (6).
We have noticed that being able to insert a hand into the surgical field resembles an open approach, which facilitates traction and makes the procedure easier for residents with less experience, taking into account that the kidney has to be taken out through a small incision anyway.
This technique has shown excellent results, both for the donor and the recipient.
Compared to classic laparoscopic nephrectomy, HALDN reduces the operative time (2.02 vs. 3.12 h) and warm ischemic time (1.23 vs. 3.9 min) (7,8). HALDN has a relatively short learning curve reflected by the rapid decrease in difficulty scores and operative times by fourth surgery (9), nevertheless there is a need for more trials to compare this specific results.
Patient selection and workup
Almost all donors eligible for an open surgical procedure can undergo hand-assisted laparoscopic surgery. All patients must undergo a complete history and physical examination, as well as an assessment by the nephrologist and the complete protocol for the potential living donor (10).
Radiological evaluation is necessary to characterize vascular anatomy and detect anomalies. We prefer a CT scan with arterial, venous, and excretory phases (11). Due to the anatomical and surgical advantages, the left kidney would be the first option for the transplant.
The final decision is made based on renal function and the number of renal arteries that should be taken into account for the reconstruction during bench surgery and implant to the recipient.
Preoperative preparation
The patient must have blood typed and screened before the procedure. Before surgery, a prophylactic antibiotic is administered, usually second-generation cephalosporin. All the materials needed for surgery are listed in (Table 1).
Table 1
| 30-degree 10 mm laparoscope lens |
| 5 mm Maryland dissector |
| 5 mm Grasper |
| 5 mm scissors |
| 5 mm suction irrigation system |
| 5 mm harmonic scalpel or Ligasure |
| Hand-access device (Gelport) |
| Large or medium Hem-o-Lok |
| Linear endoscopic stapler with vascular loads |
| Trocars: two 12 mm and one 5 mm |
| Sutures: long absorbable suture for fascial closure and 3-0 absorbable suture for skin closure |
Surgical technique
Regarding ethical considerations, no formal ethics approval was required by the research committee: “Comité de Investigacion del Hospital Central Sur de Alta Especialidad PEMEX”, due to the absence of experimental treatments and the adequate protection of patient’s personal data. Written informed consent was obtained for the publication of the video presented in this manuscript. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
We will describe in the first place the surgical technique for the left HALDN; later, we will talk about the adjustments that should be considered for the right HALDN.
Position
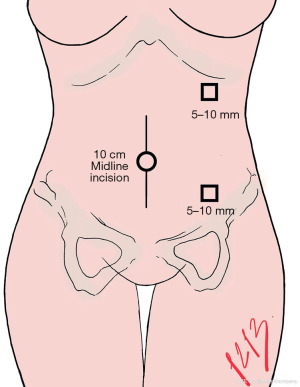
All patients must undergo general anesthesia with endotracheal intubation, and a Foley catheter is inserted. While in a supine position, the patient skin is marked for the trocar incision access. The anatomical landmarks are left midclavicular line, 2 cm below the last costal arch for the camera port, and 2 cm above the anterior superior iliac crest for the working port (Figure 1).
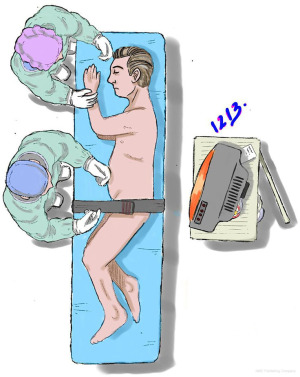
The patient is placed in a lateral decubitus position, with the operative side upwards. The kidney rest is positioned beneath the contralateral iliac crest and raised, then the table is flexed. Appropriate measures should be taken to avoid the patient’s injury, the pressure sites must be cradled on bean bags.
Additional security may be obtained by using padded straps (Figure 2).
Trocar and Hand access device placement
The initial midline incision should be 10 cm long, at the umbilicus, in order to introduce the hand access device (Gelport). It is also possible to place the access device through a Pfannenstiel incision. Through the hand access device (Gelport), a 12 mm trocar is inserted, and pneumoperitoneum is created by insufflation of CO2 up to 12 mmHg of intraabdominal pressure.
The laparoscope is inserted through this port, and a diagnostic laparoscopy is performed, looking for adhesions and adequate position of abdominal content. After that, the rest of the ports are inserted under direct vision. Two 12 mm ports are placed using the already made skin marks however, the position should be reassessed under direct vision because gravity and reposition changes.
Hand retraction
At this point, the surgeon introduces his left-hand without losing pneumoperitoneum and, with the right hand, handle a laparoscopic instrument. The intra-abdominal hand is used to retract the left colon medially; this retraction allows a clear view of the avascular dissection plane of Toldt. Using the harmonic scalpel, an incision must be done in this plane from the iliac vessels to the splenic flexure; dissection must be continued until releasing the splenic angle. This maneuver exposes the retroperitoneal space from the psoas caudally to the Gerota’s fascia cranially.
A plane of dissection is developed between the tail of the pancreas and Gerota’s fascia; this will allow medial traction of the pancreas, together with the descending colon, obtaining a better view of the Gerota’s fascia.
Following the edge of the upper pole, the dissection between the Gerota’s fascia and the tail of the pancreas must be carried out with caution to avoid injuring the pancreas.
To continue the caudal dissection, the ureter should be identified medially to the psoas muscle alongside the gonadal vein; both structures are carefully dissected from the psoas and surrounding fat. The gonadal vein is followed up to the renal vein and the ureter until the lower pole of the kidney. All attachments on the medial side need to be released from the level of the renal hilum.
The lower pole of the kidney should be freed from lateral to medial.
The next step is to incise bottom-up the Gerota’s fascia and release the renal capsule from it.
Safety triangle
There is no consensus regarding its anatomical landmarks but it has previously been described by various authors as the “golden triangle”, which contains the proximal irrigation of the ureter this is why dissection at this level should be protected and avoided reducing urological complications (12,13).
In our technique we describe the “safety triangle” which is between the lower pole of the kidney, tracing a horizontal line until crossing the gonadal vein and following the gonadal vein on its way to the confluence with the renal vein. As can be seen in Figure 3.
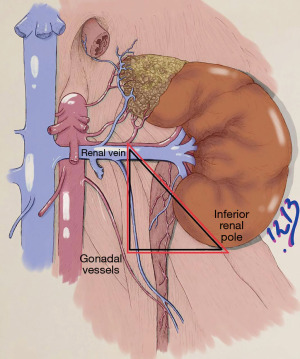
Care must be taken to leave perihilar fat intact due to its relevance for irrigation. A plane between the upper pole of the kidney and adrenal must be developed to maintain the gland intact.
At this moment, the kidney can be mobilized anteriorly and medially to allow posterior dissection of the hilum, which is completed when both the renal vein and artery are adequately identified and circumferentially freed. The artery runs posterior to the vein and must be dissected up to its aortic origin; if there are more than one artery, they must be dissected and respected, because there is no intrarenal anastomosis.
Once the ureter, gonadal vein, renal artery, and renal vein are adequately dissected. The ureter and gonadal vein are secured each one with one Hem-o-Lok, as proximal to the psoas muscle as possible, and cut with laparoscopic scissors. The renal artery is clipped with two Hem-o-Lok proximal to the aorta and cut with laparoscopic scissors. The last structure must be the renal vein, some authors prefer to secure this vessel with a laparoscopic linear stapler. We prefer the use of Hem-o-Lok in most left nephrectomies, after skeletonization of the renal vein.
The entire specimen is then removed through the hand port and handed to the reception team for cold perfusion with preservation solution; we use Organ preservation solution 1,000 cc with 5,000 IU heparin. The pressure of pneumoperitoneum is decreased for evaluation of adequate hemostasis. Then trocars are removed under direct vision, and finally, the hand-assisted device is taken out. An example of this technique is shown in Video 1 so you can follow these steps visually.
Closure
The midline hand port fascia is closed with a long-term absorbable suture in a continuous fashion with small bites technique (4:1). The rest of the ports are closed with long term absorbable suture in a figure of eight fashion (14). Skin is closed with subdermal stitches of a 3-0 absorbable suture.
Right nephrectomy
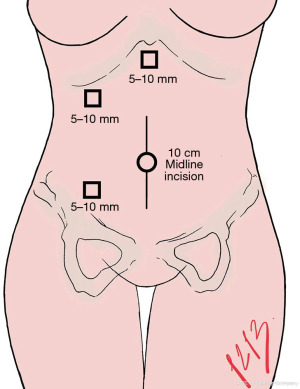
In case of right nephrectomy, the marks for the trocar incision points are the right midclavicular line, 2 cm below the costal arch, for the working port (10 mm). We place the camera port (10 mm) 2 cm above and medial to the anterior superior iliac crest, and a third port (5 mm) is marked in the subxiphoid region to place a hepatic retractor (Figure 4).
The approach begins with the dissection of Toldt’s line, which is performed from cephalic to caudal. In a similar way that in the left nephrectomy, the left hand is the intra-abdominal hand that helps with medial retraction of the colon. An adequate release of the hepatic flexure must be performed, being careful not to apply too much traction avoiding bleeding from the subhepatic adhesions. The section of the hepatic triangular ligament must be done to free the right hepatic lobe from the abdominal wall. This facilitates the cephalic traction of the liver with a hepatic retractor introduced through the subxiphoid port, improving the hepatic’s angle of the colon exposure, as well as the superior renal pole and the inferior vena cava (IVC) exposures.
We continue the dissection from lateral to medial in the avascular plane between the Gerota’s fascia and the mesentery of the colon, which can be distinguished by a pale yellow color of the fascia and a darker yellow color of the mesentery. This dissection will continue until the medial portion of the kidney is exposed. Unlike left nephrectomy, in a right nephrectomy, medial mobilization of the duodenum must be performed with the Kocher maneuver. This allows adequate exposure of the renal vein and IVC, facilitating dissection of the vascular structures.
The right ureter may be located in the same position as on the left side, medially to the psoas muscle. The renal vein is identified by dissecting its emergence from the infrahepatic vena cava; an adequate dissection will offer a correct exposure of the ureter. As said before it is essential to preserve the periureteral tissue during its dissection, mainly between the lower pole of the kidney and the ureter (safety triangle), to prevent devascularization. The path is followed to the bifurcation of the iliac vessels, the site where the section will be made, similar to the contralateral side.
Renal dissection can be continued from lateral to medial, circumferentially, medializing the kidney with the intrabdominal hand, releasing the posterior adhesions with an energy device.
When the renal hilum is completely dissected circumferentially and prepared for cutting, the artery can be clipped with Hem-o-Locks. In addition, we prefer to secure the renal vein with Hem-o-Locks similar to the left nephrectomy. In case of using a laparoscopic stapler to secure the vein, the camera and the working port must be exchanged, allowing an adequate position of the stapler through the port of the right lower quadrant, obtaining a better orientation towards the renal vein—IVC junction. A 30 mm endo-stapler with a vascular cartridge must be fired as close as possible to the IVC, trying to preserve as much length as possible of the renal vein. Sometimes it is necessary to take a little bite of the IVC; for this, it is crucial to maintain a traction of the renal specimen laterally with the dominant hand. With this maneuver, better exposure and length of the renal vein is obtained during stapling.
Comments
Post-operative management
Most patients begin ambulation in the first 24 hours. On postoperative day 1, clear liquids are started, then progress to a soft diet. Finally, the patient is discharged after a 2-day in-hospital stay. The follow up as an outpatient must be on days 15, 30 and then 3–6 months, with complete blood count (CBC) and serum creatinine, a normalization of the glomerulation filtration rate is expected in the first six months. However, this is achieved in rare cases. Finally, annual monitoring is carried out if possible by the 1rst care provider.
Tips, tricks, and pitfalls
- The patient’s position must be optimal. Measures should be taken to avoid the patient’s injury and additional security may be obtained by using padded straps;
- Prior to trocar placement, we recommend assessing the site once the patient is already in the final position, to ensure surgeon ergonomics;
- Always respect the safety triangle formed between the lower pole of the kidney, Gonadal vein, and Renal vein this step will maintain perihilar fat intact. This is important due to its relevance for irrigation;
- Try to preserve as much length as possible of the renal vein, this is relevant for the future renal transplant;
- Consider anatomical variants such as retro-aortic or posterior renal veins;
- Carefully dissect and ligate accessory veins that may drain into the renal vein such as accessory lumbar or gonadal veins;
- Ensure that gonadal vein dissection is complete and does not include the gonadal artery, avoiding compromising gonadal irrigation.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://ales.amegroups.com/article/view/10.21037/ales-22-31/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Regarding ethical considerations, no formal ethics approval was required by the research committee: “Comité de Investigacion del Hospital Central Sur de Alta Especialidad PEMEX”, due to the absence of experimental treatments and the adequate protection of patient’s personal data. Written informed consent was obtained for the publication of the video presented in this manuscript. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wolf JS Jr, Moon TD, Nakada SY. Hand assisted laparoscopic nephrectomy: comparison to standard laparoscopic nephrectomy. J Urol 1998;160:22-7. [Crossref] [PubMed]
- Ratner LE, Ciseck LJ, Moore RG, et al. Laparoscopic live donor nephrectomy. Transplantation 1995;60:1047-9. [PubMed]
- Mohan Rao M, Lionel G, Hensman C, et al. Living kidney donation made easier. Transplant Proc 2000;32:1570-1. [Crossref] [PubMed]
- Khauli RB. Laparoscopic donor nephrectomy is the future. Transplant Proc 2003;35:41-2. [Crossref] [PubMed]
- Meijer DW, Bannenberg JJ, Jakimowicz JJ. Hand-assisted laparoscopic surgery: an overview. Surg Endosc 2000;14:891-5. [Crossref] [PubMed]
- Moloo H, Haggar F, Coyle D, et al. Hand assisted laparoscopic surgery versus conventional laparoscopy for colorectal surgery. Cochrane Database Syst Rev 2010;CD006585. [Crossref] [PubMed]
- Slakey DP, Wood JC, Hender D, et al. Laparoscopic living donor nephrectomy: advantages of the hand-assisted method. Transplantation 1999;68:581-3. [Crossref] [PubMed]
- Shockcor NM, Sultan S, Alvarez-Casas J, et al. Minimally invasive donor nephrectomy: current state of the art. Langenbecks Arch Surg 2018;403:681-91. [Crossref] [PubMed]
- Gaston KE, Moore DT, Pruthi RS. Hand-assisted laparoscopic nephrectomy: prospective evaluation of the learning curve. J Urol 2004;171:63-7. [Crossref] [PubMed]
- Nogueira M, Kavoussi LR, Bhayani SB. Laparoscopic live donor nephrectomy: current status. BJU Int 2005;95:59-64. [Crossref] [PubMed]
- Piros L, Langer RM. Laparoscopic donor nephrectomy techniques. Curr Opin Organ Transplant 2012;17:401-5. [Crossref] [PubMed]
- Streeter EH, Little DM, Cranston DW, et al. The urological complications of renal transplantation: a series of 1535 patients. BJU Int 2002;90:627-34. [Crossref] [PubMed]
- Buttigieg J, Agius-Anastasi A, Sharma A, et al. Early urological complications after kidney transplantation: An overview. World J Transplant 2018;8:142-9. [Crossref] [PubMed]
- Deerenberg EB, Harlaar JJ, Steyerberg EW, et al. Small bites versus large bites for closure of abdominal midline incisions (STITCH): a double-blind, multicentre, randomised controlled trial. Lancet 2015;386:1254-60. [Crossref] [PubMed]
Cite this article as: Romero JA, Meza J, Marmolejo A, Bathory R, Ruiz-Funes AP, Farell J, Bandin A. Hand-assisted laparoscopic nephrectomy for a kidney transplant: surgical technique—tips and tricks—how we do it? Ann Laparosc Endosc Surg 2022;7:38.