Lack of diagnostic efficacy of endoscopy for paraoesophageal hiatus hernia
Introduction
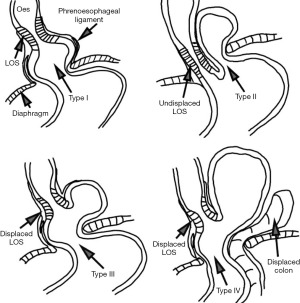
Hiatus hernia (HH), defined as herniation of the stomach and the gastroesophageal junction (GOJ) above the diaphragm through the oesophageal hiatus, is prevalent in 10–50% of the population (1) (Figure 1). There are four different types of HH. Current recommendations for the diagnosis of HH are similar to that of the gastroesophageal reflux disease (GORD) work-up as recommended by the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) guidelines, and includes imaging studies such as computerised tomography (CT), barium meal [upper gastrointestinal (GI) study] and gastroscopy (2). The accuracy of such investigations is important, as differing anatomical abnormalities have different prognoses (1). Medical gastroenterology has had a nihilistic approach to HH, not fully appreciating the risk profiles and outcomes of different types of HH (3).
The surgical literature divides hiatal hernias into three or four types (Figure 1). The type I HH (sliding hernia) accounts for 95% of all hernias and the paraoesophageal HH (intrathoracic stomach) for 5% of all type II to IV hernias. This has been well summarised in the literature and is well illustrated pictorially by Abbara et al. (4).
Giant paraoesophageal hiatus hernia (PEH), or large type III/IV HH, is defined as HH with more than 30% of the stomach in the thoracic cavity when the stomach rises above the GOJ with or without another organ present in the sac (i.e., colon). This type of HH is allegedly quite rare with reported incidence of less than 0.1% (5), although there are series with large numbers reported, suggesting some variance (6). Acute gastric volvulus (AGV) is an uncommon complication of PEH with high mortality rates of up to 50% (7-9). Strangulated gastric volvulus (GV) can cause bleeding, gastric necrosis and subsequent perforation of the stomach leading to septic peritonitis and shock. AGV can clinically present as the Borchadt’s triad in 70% of cases being the inability to pass a nasogastric tube, pain in the upper abdomen with severe retching and the inability to vomit (10). Patients with chronic GV (CGV) frequently have intermittent symptoms of dyspnoea, post prandial epigastric or chest pain associated with early satiety, dysphagia, anaemia, heartburn and bloating (11). The symptomology is not necessarily recognised as suggestive of gastric disease, imposing a challenge in correct diagnosis (Table 1) (12).
Table 1
| Symptoms | % |
|---|---|
| Shortness of breath (dyspnoea) | 70.1 |
| Chest pain | 55.5 |
| Heartburn | 51.2 |
| Dysphagia | 44.5 |
| Regurgitation | 43.9 |
| Anaemia | 20.1 |
| Aspiration | 11.0 |
PEH, paraoesophageal hiatus hernia.
Failure to recognise the nature of herniation of PEH exposes the patient to considerable risk. Mortality from PEH (type 2, 3 and 4) in the Finnish population was shown to reach 16.4% if conservatively managed (13). Skinner found a mortality rate of 29% when PEH were managed conservatively without surgery (14,15). A recent population-based American study confirmed the cost efficacy and reduction in mortality for patients undergoing elective PEH repair before AGV occurred (16). Mortality reached 5.1% for emergency fundoplication for AGV compared to 1.1% in elective cases similar to others (7,12,14). A large study has shown laparoscopic repair of PEH is associated with a lower mortality compared to an open approach (17).
The current SAGES guideline for the diagnosis of HH includes the barium meal contrast study (BM) and gastroscopy. However, the accuracy of these tests relative to the operative findings remains uncertain, especially in the presence of CGV. Endoscopic assessment of PEH and GV remain somewhat unreliable due to the subjective nature of the report, and perhaps, the lack of anatomical reference points at endoscopy. There are no firm guidelines available for clinicians regarding diagnostic criteria of GV and PEH, although it has been recommended in the literature (18). PEH alone, in truly asymptomatic patients, is not an indication for surgery according to SAGES recommendation (18).
We assessed the performance criteria of pre-operative diagnostic modalities against the operative findings in order to establish the accuracy and optimal combination to establish the correct diagnosis for PEH in a well-documented large series of 231 cases. We present the following article in accordance to the STARD reporting checklist (available at https://ales.amegroups.com/article/view/10.21037/ales-22-11/rc). Legends and flowsheets can be found in Table S1 and Figures S1-S4.
Methods
Large PEHs had been diagnosed in 231 consecutive patients identified from database in a single practice during the period 1/1/2008 to 1/1/2017 and retrospectively analysed. Patients who underwent PEH repair for clinical and various diagnostic modalities with reported preoperative endoscopy by community specialists and/or BM conducted at community facilities were included in the study. Informed consent was obtained at the time of initial consultation. Exclusion criteria included re-operation and inability to obtain an adequate report of endoscopy or BM. All data were prospectively stored in a password-protected computer database with the approval of the Ethics Committee at Concord Hospital (CH62/6/2011-092) and study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Operative anatomy was determined as the ‘reference standard’ of PEH diagnosis and the presence of CGV to determine the sensitivity and specificity of endoscopy and BM. A single surgeon recorded operative findings for all patients in this study and assessed patient preoperatively with access to all index test results.
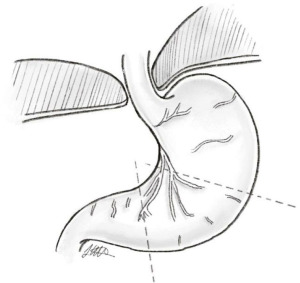
PEH was diagnosed at laparoscopy by presence of stomach above the level of the GOJ. The size, appearance of hernial sac, presence of PEH and GV according to defined criteria were documented prospectively at the time of operation by a single surgeon. The size of HH was defined by the percentage of intrathoracic stomach on laparoscopic hiatal view. The anatomical landmark of the “crows foot” of descending left gastric vessels that marks the location of the nerves of Latarjet was used to define 75% of the stomach at the level of the hiatal arch (Figure 2: dotted lines mark 50% and 75% respectively). Presence of other organs in the hernia sac were recorded.
Reports of BM prior to surgery were obtained and patients were excluded from analysis if unavailable. The usual protocol undertaken at suburban facilities for BM include anteroposterior and oblique views in upright and supine positions, with double contrast administration and stomach distention (19,20). The size and type of PEH, the presence of GV were recorded from the radiology reports for each patient. The endoscopic size of PEH was categorised into small (<2 cm), moderate (2–4 cm) or large (>4 cm or >30%). Description of HH were recorded (sliding, rolling, mixed, giant hiatus hernia (GHH), PEH, undescribed, or unclear such as “fixed”).
Endoscopy reports from up to 3 years prior to the referral of patients for surgical assessment were evaluated where possible. Patients were referred to this single subspecialised surgeon’s practice for opinion of HH by different specialists and therefore endoscopy was conducted in general gastroenterology or surgery practice. Variables recorded were: description of HH (rolling, mixed, sliding, PEH, unclear or undescribed), presence of GV, physician or surgeon, presence of Barrett’s or Cameron’s ulcer. Type II–IV HH were categorised as PEH. ‘Unclear’ description of HH included a possible dictation error and uncertainty expressed in the report.
Statistical analysis
Statistical analysis was performed using SPSS Statistical Analysis Software V24 (SPSS Inc., Chicago, IL, USA). The Shapiro-Wilk test was used to test for normality of data. T-test, chi-square test, Fisher’s exact test, McNemar’s test for discordance, Pearson correlation test and Point-Biserial correlation tests were used to analyse data. Data was presented with standard deviation (SD) or mean with range and confidence interval (CI) as appropriate. Statistical significance was assumed with a P value of less than 0.05. The sensitivity and specificity of gastroscopy and BM in diagnosing PEH and GV were calculated by a conventional two-by-two table with results being recorded as dichotomous outcomes, with the reference standard being intraoperative findings at the time of surgery.
Results
Large PEHs were diagnosed in 231 patients between January 2008 to January 2017 and 172 underwent surgical repair. One hundred and thirty patients were eligible for this study: both endoscopy and BM results were available in 60 patients; BM alone was available in 46 patients and endoscopy alone in 24 patients. There were 36 males (27.7%) and 94 females (72.3%), and the mean age was 70 years (SD ±8.734).
Intraoperative findings
The mean intraoperative size of the HH was 66.88% with a range of 10–100% (SD ±19.66). Type IV PEH was present in 19 patients (14.6%) and type III PEH in 85.4%. CGV was present intraoperatively in 48 patients (36.9%). The presence of CGV was positively correlated with the intraoperative size of the PEH (P≤0.001).
Endoscopic findings
Endoscopies were done by gastroenterologists in 66 patients (78.6%) and by general surgeons in 18 patients (21.4%). HH was not diagnosed or mentioned in 2 patients. In the 84 patients who had endoscopy, 82 (97.6%) were reported to have “HH”. Barrett’s oesophagus was evident in 8 patients (9.5%) and Cameron’s ulcer within the HH was seen in 7 (8.3%) patients. Hill grade was not mentioned in reports.
Endoscopy: type of HH
PEH was diagnosed in 7 patients only (8%) on endoscopy; identification or suggestion of the type of HH was present in 18 (21.4%) endoscopy reports (Table 2). The content of HH was described in terms of anatomical landmarks of the stomach (i.e., body, fundus, stomach) in 32 reports (38%).
Table 2
| Type of HH | HH (type 1) | PEH (type 2–4) | No mention of type | Unclear | Sensitivity |
|---|---|---|---|---|---|
| Endoscopic diagnosis (n=84) | 9 (11%) | 7 (8%) | 66 (79%) | 2 (2%) | 8.33% |
| Barium meal diagnosis (n=106) | 31 (29%) | 41 (39%) | 30 (28%) | 4 (3%) | 38.68% |
HH, hiatus hernia; PEH, paraoesophageal hiatus hernia.
Endoscopy finding: size of HH
The size of HH was recorded in only 48 out of the 84 patients (57%): 15 used descriptive words (huge, large, massive, most, narrow, very large) whereas 33 used numerical values (Figure 3). There was no mention of the size of HH in the remaining 36 (42.9%). HH size was reported by percentage in 11 reports with a range of 30–100% (mean: 60.5%) and by centimetres in 22 reports with a range of 3–10 cm (mean: 7.0 cm). It was evident numerical ‘size’ was a measure simply of the distance of the GOJ from the crural diaphragm (CD), which signifies the superior dislocation of the GOJ and not a volumetric measure of the amount of HH. The content of HH was described in terms of anatomical landmarks of the stomach (i.e., body, fundus, stomach) in 32 reports (38%).
In our analysis, the size of the HH on endoscopy was classified into four categories: not documented (NA), small (<2 cm), moderate (2–5 cm) and large (>5 cm or >30%). This was correlated with operative findings of small, moderate and large HH (all of which were PEH). There was no statistically significant correlation between the endoscopic and the operative findings (P=0.719).
Endoscopy: presence of GV
CGV was diagnosed or suggested by endoscopy in total of 8 patients (9.5%) in this group. AGV was diagnosed in 2 patients.
Barium findings
All patients who underwent BM prior to surgery were reported to have a HH.
BM: size of HH
The size of the HH was described as either quantitative or qualitative values in 100 out of 106 patients. Descriptive words such as small, moderate or large were used to describe the size of HH in 48, whereas 52 used percentage or centimetre to measure the size of HH; what axis the numerical reading referred to was unclear. Size of the HH was not mentioned in 6 (5.7%) reports. The mean percentage of herniated stomach reported was 31.6% (SD ±19.7) compared with the mean intraoperative finding of 68.0% (SD ±19.7).
BM: type of HH & GV
The type of HH was reported in 76 out of 106 patients (71.7%) on BM in comparison to endoscopy (21.4%) (Table 2). PEH was correctly identified in 41 patients (39%). CGV was observed in 18 (16.9%) patients on BM report, of which 8 (44.4%) had GV intraoperatively.
Comparison of endoscopy & BM
The sensitivity of BM and endoscopy in terms of HH type identified were 38.68% (95% CI, 29.38–48.63%) and 8.33% (95% CI, 3.42–16.42%) respectively. Overall sensitivity of BM for GV was 20.51% (95% CI, 9.3–36.46%) and specificity was 85.07% (95% CI, 74.26–92.60%). Endoscopy for GV had sensitivity of 10.71% (95% CI, 2.27–28.23%) and 91.07% specificity (95% CI, 80.38–97.04%) as shown on Table 3.
Table 3
| Test | Barium meal (n=106) | Endoscopy (n=84) | |||
|---|---|---|---|---|---|
| Value | 95% CI | Value | 95% CI | ||
| Sensitivity | 20.51% | 9.3–36.46 | 10.71% | 2.27–28.23 | |
| Specificity | 85.07% | 74.26–92.60 | 91.07% | 80.38–97.04 | |
| Predictive value of positive test | 44.44% | 25.64–64.99 | 37.50% | 18.89–73.32 | |
| Predictive value of negative test | 64.77% | 60.37–68.94 | 67.11% | 64.15–71.62 | |
| Likelihood ratio of positive test | 1.37 | 0.59–3.19 | 1.2 | 0.47–5.50 | |
| Likelihood ratio of negative test | 0.93 | 0.77–1.13 | 0.98 | 0.79–1.12 | |
GV, gastric volvulus; CI, confidence interval.
The ‘presence’ of HH regardless of its type or size, was correctly diagnosed by either BM or endoscopy in all patients. The sensitivity for diagnosing HH with endoscopy and BM was 97.61% (95% CI, 88–100%) and 100% (95% CI, 94–100%) respectively.
McNemar’s test was used for comparison of discordance between BM and endoscopy for identification of GV. There was a statistically significant discordance in BM finding compared with intraoperative finding of GV (P=0.049) and endoscopy compared with intraoperative finding of GV (P=0.001).
There was a statistically significant positive correlation between BM size of HH when measured in proportion of stomach in the hernia with intraoperatively measured size of HH (r=0.668; n=55; P=0.001). From Table 4, it can be seen that size of HH at endoscopy, when reported, does correspond to the operative findings (P=0.719).
Table 4
| Size at operation (reference standard) | Size at endoscopy (based on %/cm, qualitative category) | ||||
|---|---|---|---|---|---|
| Small | Moderate | Large | No description | Total | |
| Moderate | 0 | 0 | 1 | 3 | 4 |
| Large | 1 | 5 | 37 | 37 | 80 |
| Total | 1 | 5 | 38 | 40 | 84 |
HH, hiatus hernia.
Surgeons commented more frequently on size and type of HH (14/18, 78%) than physicians (36/66, 55%) with no statistical significance (P=0.105).
Discussion
Multiple studies have examined endoscopy and BM as diagnostic tools for HH with conflicting conclusions (5,21,22). A retrospective analysis of 100 patients undergoing anti-reflux surgery has shown that there was no correlation between intraoperative measurement of HH size and preoperative BM, indicating that BM was a poor test to characterize HH (21). Another study found no correlation between BM, endoscopy and high-resolution manometry regarding HH characterisation, concluding that all three tests were necessary (22). This is likely because each test evaluates different parameters of anatomy, and HRM only measures vertical distance between GOJ and diaphragm (22). BM and endoscopy diagnosed HH with a high sensitivity of 100% and 98% respectively in our study, but rarely characterised these as PEH (18).
There are no generally-accepted guidelines on endoscopic assessment of HH (18,23). The technical diagnosis of HH may be different when the GOJ position cannot be readily appreciated due to Barrett’s metaplasia and when there is poor distention of the stomach. The retroflexed view is recommended in recent endoscopic guidelines (24). This method is subjective and relies heavily on personal experience of endoscopist. Bytzer et al. (25) showed the sensitivity of endoscopic evaluation was highly dependent upon clinical history, undermining objectivity. Considering the subjective nature of endoscopic assessment, a position statement was released by the British Society of Gastroenterology (BSG) to standardize diagnostic quality of upper endoscopy. Specific statements regarding HH were made: “the presence of HH should be documented and measured’ with a preference to assessing the hiatal integrity with the retroflexion view as opposed to measuring the distance between the squamocolumnar junction and diaphragmatic impression” (24). The appearance of gastoesophageal junction ‘gastroeosphageal flap valve’ on retroflexion can be described using the Hill classification with significance to reflux and presence of HH. The grade IV gastroesophageal valve is always associated with the presence of HH (26). In our view, the retroflexed position is most likely to diagnose PEH/GV but recognize that in large HH or in the presence of GV, retroflexion may not be possible.
A striking feature in this study was the discordance in reporting of the HH amongst endoscopists. Many (40.5%) did not adequately comment on the HH size. Size description was arbitrary in 19% using non-definitive terminology such as small, moderate, large and massive. The size of HH in terms of quantitative measurement (either percentage of stomach in the hernial sac or as length in centimetres of the defect) was reported in less than half the patients. Length in centimetre invariably only described the degree of dislocation of GOJ above the CD and did little to differentiate a sliding HH from PEH. Increasing awareness among endoscopists on the importance of the type of HH in determining surgical consultation has been reported (23).
A discrepancy in reporting was evident in BM, similarly to endoscopy. The type of HH was not specified in many (32% of all reports), however the size of HH with specific details (i.e., percentage of herniated stomach or subjective size description) was documented adequately (94.3%) compared with endoscopy. Presence of GV was commented on by the majority of reporting radiologists (92.5%). Despite the subjective nature of reporting, the presence of the X-ray film can allow for re-examination in ways that endoscopy cannot. BM also shows relativity to chest landmarks. Endoscopy may be best for luminal detail rather than anatomical position, or gastric orientation.
The sensitivity of BM and endoscopic diagnosis of PEH was not adequate for appropriate clinical management (Table 2). The importance of the distinction between the simple sliding HH and PEH is the potential for entrapment and incarceration. Results in this series show that endoscopy is inferior to BM in terms of sensitivity and specificity for the diagnosis of PEH, partly due to operator-dependent factors in the recognition of anatomical pathology and lack of standardized reporting criteria. Similar reporting phenomena were observed in the BM reports which lacked detail and systematic description and categorization. Linke et al. (27) have also found subjective variations in both barium meal and endoscopy results when reporting a HH. It is apparent that in order to correctly diagnose a PEH, there needs to be standardization in the reporting of both BM and endoscopy, and probably standardised training/education in the anatomical types of herniation for endoscopists and radiologists. The authors have frequently viewed radiographs that clearly contained information at significant and surgically important variance with the radiology reports.
It is well published in the literature that AGV and CGV can be challenging to diagnose (10,16), with previous studies showing BM to have the greatest yield compared to endoscopy and plain chest X-ray (16,28). AGV can be intermittent and self-resolving in nature, which adds to the diagnostic difficulty (5). CT with 3D reformatted images also serve as a sensitive tool in detecting HH and GV. Diagnostic reports will enable appropriate risk stratification of patients who require prompt referral to upper GI surgery for management. This is especially relevant when the size of HH exceeds 30% of the stomach and becomes more likely to develop PEH, GV and its sequelae.
The recommendation is that BM reports include description of the oesophagus, position and nature of the GOJ, position and rotation of the stomach. HH should be described in terms of: type, size, presence of GV and presence of other structures within the chest. This allows adequate clinical diagnosis and risk stratification. Single contrast BM may not achieve these results, due to inadequate distension of the stomach and lack of head down positioning (29).
When there are mechanical symptoms in the HH such as dyspnoea or chest pain, minimal investigations should include both endoscopy and double contrast BM. The clinician must be aware that reporting may not be adequate. Retroflection of the endoscope should be mandatory, with adequate examination of the CD. Both BM and endoscopy should report the nature of the oesophagus, any axis deviation, the level of the GOJ, and the level of the CD. The attitude of the stomach, percentage involvement of stomach in the herniation should be reported numerically rather than ambiguous descriptions. Should diagnostic confusion persist, CT scan of the chest and upper abdomen with adequate stomach distension would be advised.
Limitations of this study
This study was conducted in a single specialised academic practice and therefore are not generalisable. However, the reports of the diagnostic tests used for analysis in this study reflect community practice as tests were conducted at non-specific diagnostic centres. Intraoperative findings were reported by a single surgeon with more than 900 personal cases of PEH, and therefore the estimation of HH size will be accurate and consistent.
Endoscopy was non-uniform with multiple endoscopists. The time frame for inclusion criteria was set at 3 years from the date of the operation and this may contribute to a lack of sensitivity due to the progression of disease. There may be selection bias related to patient referral in a community setting and the location of testing centres. In future studies, an endoscopy immediately prior to surgery may be more useful in determining the sensitivity and specificity of endoscopy for diagnosis of PEH and GV but does not contribute to clinical decision making.
Conclusions
The anatomical description lacked consistency with both endoscopy and radiology reporting; standardisation of reporting may increase the reliability of these tests. Identification of the type of HH is important to accurately risk stratify the disease together with patient symptoms. Both barium meal and endoscopy had poor sensitivity in detecting the type of HH but highly sensitive for diagnosing the presence of HH.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://ales.amegroups.com/article/view/10.21037/ales-22-11/rc
Data Sharing Statement: Available at https://ales.amegroups.com/article/view/10.21037/ales-22-11/dss
Peer Review File: Available at https://ales.amegroups.com/article/view/10.21037/ales-22-11/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://ales.amegroups.com/article/view/10.21037/ales-22-11/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Informed consent was obtained at the time of initial consultation. All data were prospectively stored in a password-protected computer database with the approval of the Ethics Committee at Concord Hospital (CH62/6/2011-092) and study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hill LD, Tobias JA. Paraesophageal hernia. Arch Surg 1968;96:735-44. [Crossref] [PubMed]
- Kohn GP, Price RR, DeMeester SR, et al. Guidelines for the management of hiatal hernia. Surg Endosc 2013;27:4409-28. [Crossref] [PubMed]
- Mittal RK. Hiatal hernia: myth or reality? Am J Med 1997;103:33S-9S. [Crossref] [PubMed]
- Abbara S, Kalan MM, Lewicki AM. Intrathoracic stomach revisited. AJR Am J Roentgenol 2003;181:403-14. [Crossref] [PubMed]
- Rashid F, Thangarajah T, Mulvey D, et al. A review article on gastric volvulus: a challenge to diagnosis and management. Int J Surg 2010;8:18-24. [Crossref] [PubMed]
- Luketich JD, Nason KS, Christie NA, et al. Outcomes after a decade of laparoscopic giant paraesophageal hernia repair. J Thorac Cardiovasc Surg 2010;139:395-404, 404.e1.
- Le Page PA, Furtado R, Hayward M, et al. Durability of giant hiatus hernia repair in 455 patients over 20 years. Ann R Coll Surg Engl 2015;97:188-93. [Crossref] [PubMed]
- Shivanand G, Seema S, Srivastava DN, et al. Gastric volvulus: acute and chronic presentation. Clin Imaging 2003;27:265-8. [Crossref] [PubMed]
- Smith CD, McClusky DA, Rajad MA, et al. When fundoplication fails: redo? Ann Surg 2005;241:861-9; discussion 869-71. [Crossref] [PubMed]
- Chau B, Dufel S. Gastric volvulus. Emerg Med J 2007;24:446-7. [Crossref] [PubMed]
- McElreath DP, Olden KW, Aduli F. Hiccups: a subtle sign in the clinical diagnosis of gastric volvulus and a review of the literature. Dig Dis Sci 2008;53:3033-6. [Crossref] [PubMed]
- Lee F, Khoma O, Mendu M, et al. Does composite repair of giant paraoesophageal hernia improve patient outcomes? ANZ J Surg 2021;91:310-5. [Crossref] [PubMed]
- Sihvo EI, Salo JA, Räsänen JV, et al. Fatal complications of adult paraesophageal hernia: a population-based study. J Thorac Cardiovasc Surg 2009;137:419-24. [Crossref] [PubMed]
- Polomsky M, Hu R, Sepesi B, et al. A population-based analysis of emergent vs. elective hospital admissions for an intrathoracic stomach. Surg Endosc 2010;24:1250-5. [Crossref] [PubMed]
- Skinner DB, Belsey RH. Surgical management of esophageal reflux and hiatus hernia. Long-term results with 1,030 patients. J Thorac Cardiovasc Surg 1967;53:33-54. [Crossref] [PubMed]
- Gourgiotis S, Vougas V, Germanos S, et al. Acute gastric volvulus: diagnosis and management over 10 years. Dig Surg 2006;23:169-72. [Crossref] [PubMed]
- Fullum TM, Oyetunji TA, Ortega G, et al. Open versus laparoscopic hiatal hernia repair. JSLS 2013;17:23-9. [Crossref] [PubMed]
- Kahrilas PJ. High-resolution manometry findings with hiatus hernia. Ann Laparosc Endosc Surg 2021;6:5. [Crossref]
- Kreel L, Herlinger H, Glanville J. Technique of the double contrast barium meal with examples of correlation with endoscopy. Clin Radiol 1973;24:307-14. [Crossref] [PubMed]
- Nolan D. The double-contrast barium meal. A radiological atlas. Aylesbury: Aylesbury Publishing, 1980.
- Koch OO, Schurich M, Antoniou SA, et al. Predictability of hiatal hernia/defect size: is there a correlation between pre- and intraoperative findings? Hernia 2014;18:883-8. [Crossref] [PubMed]
- Weitzendorfer M, Köhler G, Antoniou SA, et al. Preoperative diagnosis of hiatal hernia: barium swallow X-ray, high-resolution manometry, or endoscopy? Eur Surg 2017;49:210-7. [Crossref] [PubMed]
- Sfara A, Dumitrascu DL. The management of hiatal hernia: an update on diagnosis and treatment. Med Pharm Rep 2019;92:321-5. [Crossref] [PubMed]
- Beg S, Ragunath K, Wyman A, et al. Quality standards in upper gastrointestinal endoscopy: a position statement of the British Society of Gastroenterology (BSG) and Association of Upper Gastrointestinal Surgeons of Great Britain and Ireland (AUGIS). Gut 2017;66:1886-99. [Crossref] [PubMed]
- Bytzer P. Information bias in endoscopic assessment. Am J Gastroenterol 2007;102:1585-7. [Crossref] [PubMed]
- Hennig A, Kurian AA. Flexible endoscopy and hiatal hernias. Ann Laparosc Endosc Surg 2021;6:45. [Crossref]
- Linke GR, Borovicka J, Schneider P, et al. Is a barium swallow complementary to endoscopy essential in the preoperative assessment of laparoscopic antireflux and hiatal hernia surgery? Surg Endosc 2008;22:96-100. [Crossref] [PubMed]
- Teague WJ, Ackroyd R, Watson DI, et al. Changing patterns in the management of gastric volvulus over 14 years. Br J Surg 2000;87:358-61. [Crossref] [PubMed]
- Levine MS, Carucci LR, DiSantis DJ, et al. Consensus Statement of Society of Abdominal Radiology Disease-Focused Panel on Barium Esophagography in Gastroesophageal Reflux Disease. AJR Am J Roentgenol 2016;207:1009-15. [Crossref] [PubMed]
Cite this article as: Mugino M, Little SC, Weerasinghe DP, Falk GL, Van der Wall H. Lack of diagnostic efficacy of endoscopy for paraoesophageal hiatus hernia. Ann Laparosc Endosc Surg 2022;7:21.