
Current management of large bowel obstruction: a narrative review
Introduction
Large bowel obstruction (LBO) is a serious but common event. There are many causes of this condition and patients may present with a variety of symptoms and across all age groups (1). LBO is one of the most common reasons for a specialist referral to a colorectal surgeon. Advances in surgical techniques and technology have seen the management of this condition undergo a paradigm change (2,3). Rapid evaluation and diagnosis are pivotal for good patient outcomes (4). This article provides an up to date summary of the management of the most common causes of LBO and has been conducted in accordance with the Narrative Review reporting checklist (available at https://ales.amegroups.com/article/view/10.21037/ales-21-45/rc).
Methods
A literature search was conducted compiling previously published data on the management of LBO including current international guidelines. English language articles on PubMed/Medline, Embase and Google Scholar were searched using the following terms: “Large Bowel Obstruction”, “Malignant Large Bowel Obstruction”, “Volvulus”, “Diverticular Stricture”, “Self Expanding Metal Stent”, “Colonic Stent” with a date range of January 1980–March 2022. English language articles relevant to the aetiology, pathophysiology and management mechanical LBO were included. Case series, retrospective studies, randomised trials and society guidelines were included. Articles on patients under 18 years, acute colonic pseudo-obstruction and slow colonic transit were not included. Preference was given to society guidelines, systematic reviews, and other narrative reviews. Articles were then selected by relevance and appropriateness at the discretion of the authors and this review is based on the synthesis of these data. The reference lists of these articles were then reviewed to expand the search (Table 1). An example of this search strategy has been provided in Figure S1.
Table 1
| Items | Specification |
|---|---|
| Date of search (specified to date, month and year) | 25 March 2022 |
| Databases and other sources searched | PubMed/Medline, Embase, Google Scholar |
| Search terms used (including MeSH and free text search terms and filters) | “Large Bowel Obstruction”, “Malignant Large Bowel Obstruction”, “Volvulus”, “Diverticular Stricture”, “Self Expanding Metal Stent”, “Colonic Stent” |
| Timeframe | January 1980–March 2022 |
| Inclusion and exclusion criteria (study type, language restrictions etc.) | Inclusion criteria: English language articles relevant to the aetiology, pathophysiology, management of patients with a mechanical LBO |
| Exclusion criteria: studies on patients under 18 years of age, paediatric guidelines, articles on acute colonic pseudo-obstruction, and slow colonic transit | |
| Selection process (who conducted the selection, whether it was conducted independently, how consensus was obtained, etc.) | Search conducted by first author. Both authors reviewed the retrieved articles and consensus reached based on discussion between authors on the relevance and impact on the narrative review |
LBO, large bowel obstruction.
Aetiology
Causes can be divided into mechanical and functional (Table 2). LBO caused by neoplasms are most common accounting for up to 50% of presentations (4,5) with the majority presenting with left sided obstruction. Colonic volvulus being the second most common cause is a result of axial rotation of the bowel on its mesentery and is most common in the sigmoid colon (76%) but can also occur in the caecum (22%) and transverse colon (2%) (6). The third most common cause is diverticular disease which can be caused by acute inflammation or chronic fibrosis leading to stricture formation (1).
Table 2
| Obstruction type | Disease |
|---|---|
| Mechanical | Neoplastic |
| Volvulus (sigmoid/caecal/transverse colon) | |
| Diverticular disease | |
| Intussusception | |
| Incarcerated hernia | |
| Abscess/inflammation/IBD | |
| Adhesions | |
| Foreign body | |
| Faecal impaction | |
| Functional | Toxic megacolon |
| Pseudo-obstruction |
LBO, large bowel obstruction; IBD, inflammatory bowel disease.
Functional presentations, that may mimic a mechanical LBO, include colonic pseudo-obstruction and this has a multifactorial aetiology. Causes include systemic illness, a narcotic-associated adynamic process or sepsis. Other functional presentations include toxic megacolon which can result from Clostridium difficile colitis or inflammatory bowel disease (IBD) (7).
Clinical presentation
Symptoms vary according to the underlying aetiology and can be subtle or profound. Most patients will describe a period of bloating and obstipation. Patients may also report a narrowing in the caliber of stools. Pain initially begins as colicky and “cramping” in nature but can progress to localised pain in the setting of threatened perforation (8). In the setting of right sided obstruction, pain can develop suddenly and can be a relatively early feature, however for left sided lesions, a protracted history of a change in bowel habit or rectal bleeding may precede. Vomiting is an uncommon presentation but can be a late feature associated with an incompetent ileocaecal valve or with right sided lesions (9). Alternatively, in the setting of volvulus, obstructive symptoms may progress rapidly (10).
Systemically, patients may have signs of hypovolaemia and electrolyte imbalance due to losses into the gastrointestinal system (4). This may progress to sepsis and shock in the eventuality of perforation. On examination, abdominal distension and tympany is commonly the only finding (8,11). Some may present with palpable masses in the setting of locally advanced or metastatic disease. Rectal examination may reveal blood or a rectal mass (12-14).
Diagnosis
Imaging
Plain abdominal X-rays, supine and erect, are simple and available diagnostic tools with an 84% sensitivity and 72% specificity (15). They are useful for diagnosing sigmoid or caecal volvulus. Contrast enema studies improve diagnostic sensitivity and specificity to 96% and 98% while providing information on the level of obstruction (16). While persisting as a recommendation in some guidelines, X-rays have been largely replaced by computed tomography (CT) which can be augmented using intravenous, oral and rectal contrast. Multiplanar CT can provide a sensitivity and specificity of 96% and 80% respectively (17). Caecal diameter of >12 cm has been traditionally used as a cut-off for impending perforation but it must be noted that perforation can occur at smaller diameters (11). A recent study concluded the presence of pericaecal fluid, caecal pneumatosis, caecal diameter >9 cm in the coronal plane and caecal volume >400 cm3 (although not routinely calculated) were more useful signs of impending caecal perforation (18).
In the setting of volvulus, CT has a 100% sensitivity and >90% specificity (19). Characteristic features include a “coffee bean” sign which can also be seen on abdominal radiographs and the ‘birds-beak’ appearance characterised by two limbs of the volvulized colonic segment meeting at the point of torsion. On CT imaging, mesenteric swirling has also been described which corresponds to rotation about the mesenteric vessels. Most importantly accurate assessment of colonic distension, hypoperfusion, pneumatosis, pericolonic fluid, pneumoperitoneum and portal venous gas are concerning features for ischaemia or perforation (20).
Endoscopy
Endoscopic evaluation is valuable to assess patients who are stable without impending perforation. Risk of perforation is low at 1–2% with the use of C02 insufflation (21). Useful information can be obtained such as the nature and level of obstruction. Tissue biopsies of the lesion can be taken, and the quality of mucosa can be assessed. Traversability of the obstructing lesion can also be assessed to facilitate planning for subsequent stenting. For patients with a sigmoid volvulus not successfully decompressed with bedside rigid sigmoidoscopy, flexible sigmoidoscopy can facilitate decompression and more reliably identify a characteristic two points of twisted or converging mucosa with dilated intervening colon in 85–90% of patients (22). Decompression will be therapeutic in a functional aetiology.
Management
Initial resuscitation
Patients presenting with acute LBO have an exaggerated metabolic response to surgery which is multifactorial. The Association of Coloproctology of Great Britain and Ireland (ACGBI) report on colorectal cancer outcomes, 30-day mortality increases with increasing American Society of Anesthesiologists (ASA) grading. Pre-operative patient optimization is therefore pivotal for improved outcomes which include earlier return to gut function, reduced stress response, reduced complications, and accelerated recovery (23).
Early gastrointestinal decompression followed by adequate fluid and electrolyte resuscitation should be prioritized to make-up for fluid imbalances and, if possible, preoperative stoma marking undertaken by a specialist stoma nurse given the high likelihood of stoma formation in these patients. Early nutritional assessment for post-operative enteral or parenteral support should be performed to mitigate the metabolic response to surgery and address pre-existing malnutrition (4).
Malignant LBO
Seven to twenty-nine percent of patients with colorectal cancer present with an acute LBO of which >75% are distal to the splenic flexure (24). Twelve to nineteen percent of these patients will present with a perforation either of the tumour or the caecum (25). Decision making for the management of malignant LBO often depends on the location of the tumour, patient factors, surgeon’s expertise, and the available resources. Regardless, the three tenants of management remain (I) damage control—in the form of decompression of the obstruction, (II) primary resection observing oncologic principles, and (III) restoration of intestinal continuity (26).
End/loop colostomy
Proximal stoma, followed by a segmental resection and subsequent stoma reversal or ‘3-stage-procedure’ has historically been employed for obstructing cancers to reduce morbidity (13). The benefits include a shorter operative time and a reduced risk of contamination (13). Several studies have since shown no benefit of this approach for colonic obstruction (27,28). Accordingly, its application is limited to the setting of obstructing mid to low rectal cancers or initially unresectable colonic tumours, largely to facilitate neoadjuvant treatment. In the palliative setting, a decompressive stoma as a definitive procedure facilitates the commencement of chemotherapy and alleviation of symptoms (29). A laparoscopic loop ileostomy, transverse colostomy, end colostomy or end-loop (Abcarian type) colostomy can be performed expeditiously (30). Loop colostomies can be associated with prolapse and bypass into the distal limb, especially the loop transverse colostomy (31). Loop ileostomies are associated with less prolapse and odor however, in the setting of an incompetent ileocaecal valve, may not relieve the obstruction (26). Ileostomies are however associated with higher rates of skin irritation, dehydration and renal failure (32). A recent systematic review attempting to compare morbidity between loop ileostomy, loop colostomy and end colostomy found a high degree of heterogeneity between studies and largely observational data making objective comparison of the morbidity of these procedures difficult (32).
Resection without primary anastomosis (Hartmann’s procedure)
For distal colonic obstruction, the Hartmann’s procedure which involves a rectosigmoidectomy, closure of the rectal stump and formation of an end stoma, is the most frequently chosen surgical option (33). It allows resection of the pathologic segment and avoids the morbidity of anastomotic leak. It is relatively straightforward to perform and remains the procedure of choice for high-risk patients or the clinically unstable. Only 30–40% of patients ultimately undergo a reversal procedure (34). This low level of reversal may be more related to the morbidity profile of patients selected for this procedure.
For proximal and especially right sided colonic obstructions, a primary anastomosis is favoured, given the lower morbidity profile of ileocolic anastomoses with leak rates at 2–5% (35,36). It must be considered that in the emergency setting, anastomotic leakage has been reported to be up to 9% (37). Careful patient selection must therefore be observed. A diverting loop ileostomy or end ileostomy with colonic mucous fistula are safe alternatives.
A primary anastomosis should be avoided in the setting of faecal peritonitis, shock, sepsis, ASA IV patient or widespread peritoneal malignancy (4,38).
Resection with primary anastomosis
This approach achieves decompression, resection and reconstruction in a one-stage procedure. It is also associated with an overall lower hospital stay and avoids the morbidity of a stoma. Until recently, primary anastomosis in the setting of emergency surgery has been avoided, however recent evidence has allowed more accurate identification of high-risk patients to allow for better patient selection. These parameters include pre-operative renal failure, ASA III–IV, malnutrition, and immunosuppression (39). Further evidence reports low haemoglobin, operative field contamination, hyperglycaemia, duration of surgery >3 hours, vasopressors, delayed timing of antibiotic prophylaxis and epidural analgesia as additional risk factors that can guide decision making (40).
There are several options for resection and primary anastomosis.
Subtotal colectomy
Originally described by Klatt for slow colonic transit, this technique is the preferred method in the presence of localized caecal perforation or if synchronous colonic lesions are suspected (41). Earlier studies have shown a low leak rate with ileocolic anastomoses of <10% after subtotal colectomy in the setting of obstruction (42-44). Many of these reports were small, single centre studies.
From a functional perspective, subtotal colectomy confers increased bowel frequency particularly in the short term with many patients relying on anti-diarrhoeal agents. Some authors report that bowel frequency improves over a period of 2–6 months (45,46). A more recent meta-analysis of functional outcomes following oncological bowel resections suggest this is not the case. Furthermore, some included studies of this meta-analysis indicate increased dysfunction following right sided resections leading authors to hypothesize the possibility of dysfunction relating to the loss of the ileocaecal valve (47). A retrospective Mayo Clinic series found an overall reduced quality of life in these patients relating to increased stool frequency, need for dietary alterations and restrictions in life activities. Although not directly affecting continence, this series highlighted that an increase in stool frequency and less formed consistency may worsen quality of life in patients with pre-existing marginal defaecatory disorders and should therefore be a consideration in this cohort (48).
Segmental colectomy
For right sided obstructing lesions, segmental colectomy in the form of a right hemicolectomy is the preferred approach; outcomes are generally favourable, with a relatively low risk of anastomotic leak (49). Decision making for left sided lesions is more controversial. While previously quoted anastomotic leak rates of up to 50% have deterred surgeons from this approach, more recent data suggest that this risk is lower in appropriately selected patients (50,51). The SCOTIA trial, which was an randomized controlled trial (RCT) of 91 patients, comparing subtotal colectomy and segmental colectomy with on-table colonic lavage (OTL) in obstructed patients reported no significant difference in anastomotic leakage (9% subtotal colectomy vs. 5% segmental colectomy, P=0.68), a significantly higher bowel frequency in the subtotal colectomy group (three or more bowel motions a day, P=0.01) and a substantially higher rate of eventual stoma formation in the subtotal colectomy group: 7 of 47 patients (14.9%) vs. 1 of 44 (2.3%) in the segmental resection group (52). Given these quality-of-life findings and as no significant difference was found in anastomotic leak, segmental colectomy is considered safe and the preferred option in appropriately selected patients.
OTL
Historically, primary anastomosis has been avoided due to the belief that bowel preparation was necessary for a safe anastomosis (53). OTL, originally described as antegrade lavage via an ileotomy or appendicostomy, was developed for this reason (54). More recently, a closed irrigation and collection system (Retrowash; Intermark Medical Interventions Ltd., Bromley, Kent, UK) has simplified this procedure. With the increasing trend to avoid bowel preparation, the need for colonic lavage has been challenged. There is limited data on primary anastomosis after segmental resection without colonic lavage. Randomised data from the SCOTIA trial has been applied to justify a primary anastomosis and utilizes either segmental colectomy with lavage, or subtotal colectomy (52). Retrospective comparative trials with small sample sizes have shown no difference in anastomotic leak rate but noted longer operative duration (55,56). A randomized prospective trial of 60 patients comparing on-table lavage and manual decompression concluded, similarly, that there was no difference in anastomotic leak, wound infection rate and length of stay (57). It must be noted that exclusion criteria included any patient needing a subtotal colectomy or any patient deemed to be at high risk for an anastomotic leak, and this information highlights the importance of patient selection regardless of technique employed. Ultimately both techniques are safe and the decision to perform an on-table lavage currently is an individual choice.
Self-expanding metal stent (SEMS)
SEMS can be utilized as an alternative to a colostomy in the palliative setting and as a bridge to surgery. Technically SEMS can be deployed endoscopically with fluoroscopic guidance or radiologically alone. Clinical success rates are comparable in retrospective studies, however endoscopic guidance confers a slight advantage for technical success 100% vs. 92.1%, P=0.038 (3,58). Absolute contraindications to SEMS are colonic ischaemia, perforation and the intraversability of the obstructing lesion with a guidewire (3). Relative contraindications include coagulopathy, lesions at flexures, which can be technically difficult to stent, and low rectal lesions which can lead to tenesmus and bleeding (3). SEMS can be deployed through a therapeutic endoscope via a working channel. This technique allows for direct visualisation of the obstructing lesion. Alternatively, the ‘over the wire’ technique can be employed when angulation of the bowel limits visibility (59).
SEMS are associated with a complication rate of 25% (60,61). Perforation occurs in 9.5% of cases and can result in peritoneal dissemination of malignancy and sepsis (62). Stent migration occurs in 1–10% of cases and can be caused by incorrect stent selection, reduction in size of the obstructing lesion due to adjuvant therapy, and is sometimes seen in benign or extracolonic disease (63). Stent occlusion can also occur due to tumour ingrowth and is seen in 11.1%. This can be amenable to further endoscopic treatment such as ablation using laser or argon plasma coagulation or a repeat stenting procedure (59).
Palliative setting
SEMS is the preferred and well established treatment for palliation as it avoids surgery and more importantly the morbidity of a stoma. A recent meta-analysis of 4 RCTS comparing emergency surgery and palliative stenting and analysing a total of 125 patients, reported that there were no significant differences in mortality, mean survival, length of intensive care unit (ICU) admission and length of stay (62). Their use has also been associated with a shorter time to induction of chemotherapy (64). There is however no difference in long term survival (29,65). When bevacizumab therapy is planned, caution must be maintained due to the risk of perforation. Many international guidelines, recommend against SEMS in this setting (3,66). This recommendation has recently been challenged. A study of 199 patients where 104 received stents, 1 patient in the bevacizumab and 3 in the non-bevacizumab group (P=0.549) experienced stent related perforations (67). Median patency of SEMS in the palliative setting is 3–12 months with 50% remaining patent at 12 months (68). These patients are amenable to repeat endoscopic intervention reported in up to 100% of patients with a maintained patency of 80% (3).
In the setting of extracolonic malignant obstruction, data is limited. Although reported success rates are lower owing to the often-multifocal nature of these obstructions, short term complications are fewer in the reported literature (3,58).
Bridge to surgery
The use of stents as a bridge to surgery is more controversial. The reported benefits include allowing time for decompression and correction of the patient’s physiology. It also facilitates tumour staging, bowel preparation, minimally invasive surgery and may result in the avoidance of a stoma (3,69). There is, however, no consensus among international guidelines regarding SEMS as a bridge to surgery. This is largely due to conflicting results from several retrospective studies. Kim et al. raised concerns regarding the oncological safety of stenting curable disease citing a reduction in the overall survival (OS) and disease-free survival (DFS) (70). The 2006 Dutch Stent-in-2 Trial was suspended due to a high number of adverse events and reported a rate of colonic perforation approaching 20% (71). A further assessment of the oncological results reported a potential for negative oncological outcomes (72). A subsequent French trial was also suspended for similar safety concerns and a high technical failure rate (73). The more recent multicenter ESCO Trial of 144 patients however found no statistical difference in OS and DFS and lower stoma rates associated with SEMS. Five out of 56 (8.9%) patients in the SEMS group, however, had procedure related perforations (74). This information, including the previously reported safety concerns, highlights the importance of an experienced proceduralist.
Nonetheless, SEMS as a bridge to surgery are associated with shorter hospital stays, less blood loss and a lower permanent and temporary stoma rate when compared to emergency surgery (72,74). As such, several society guidelines now advocate the use of SEMS as a bridge to surgery. With opinion still divided, outcomes of the larger Phase III UK ColoRectal Endoscopic Stenting Trial (CREST) may provide more robust guidance.
Summary
Given the complexity of presentation in patients with malignant LBO, Figure 1 is a suggested treatment algorithm to aid with decision making. Where stenting as a bridge to surgery is considered, the authors suggest careful consideration of the local expertise and patient’s disease given the presented evidence.

Volvulus
Colonic volvulus is particularly prevalent in young men in the endemic “volvulus-belt” which includes Africa, South America, Russia, Eastern Europe, India, Brazil, and the Middle East. In North America, Western Europe and Australia colonic volvulus represents 5% of all LBOs with sigmoid volvulus affecting older men >70 years old and caecal volvulus affecting younger women <60 years old (6).
Sigmoid volvulus
Sigmoid volvulus is caused by an anti-clockwise rotation of the sigmoid colon about its mesocolon. Most sources cite a narrow-based mesentery and a long, redundant sigmoid colon to be pre-disposing factors (20). It is occasionally linked to Chagas and Hirschprung’s diseases but is more commonly a result of a chronic, non-specific motility disorder. Torsion beyond 180˚ leads to obstruction and can subsequently lead to ischaemia (75).
Decompression
Several techniques have been described for decompression which include flexible sigmoidoscopy, rigid sigmoidoscopy, blind passage of a flatus tube and barium enema (76). The use of enemas has fallen largely out of vogue and endoscopic decompression, with a flexible sigmoidoscope, is the preferred approach for uncomplicated sigmoid volvulus (76). Findings are typified by two points of twisted converging mucosa with intervening dilated bowel which are pathognomonic to the disease (22). Success rates of flexible sigmoidoscopic decompression range from 55–94% (19,77). Flexible sigmoidoscopy is often cited as superior to rigid sigmoidoscopy as the latter can miss colonic ischaemia in 24% of cases (10). Cost and logistics however dictate that some institutions would attempt rigid sigmoidoscopic decompression as a first-line procedure. A rectal tube can be placed proximal to the obstruction for 24–72 hours with concurrent oral aperients (78). Predictive factors for successful decompression are the absence of abdominal tenderness, laxative use and a history of open abdominal surgery (79). The effect of the latter factor is postulated to be secondary to post-operative adhesions reducing mobility of the colon. Recurrence has been reported in 48–61% of patients (10). Therefore, in patients who are surgical candidates, an operation should be planned either electively or during the same admission (10).
Surgery
In the presence of peritonism or haemodynamic instability, ischaemia should be suspected. Resection of the ischaemic bowel should be performed without detorsion to prevent endotoxin and potassium release and avoid inadvertent perforation. Unlike malignant LBO, there is a paucity of high-quality data to support decision making in the setting of an emergency resection. Decision to perform a primary anastomosis in the emergency setting should be guided by the patient’s risk factors, systemic state and the condition of the colon. A retrospective study of 106 cases comparing primary anastomosis and Hartmann’s procedure; where anastomosis was performed at the surgeons discretion, reported an anastomotic leak rate of 7% with a mortality rate of 6.6% across both groups (80).
The role of laparoscopic surgery in this setting is yet to be defined. The redundancy of large intestine, distension and its lack of fixation make laparoscopic exposure challenging. Comparative data suggest no difference in outcomes between the two approaches (81). Nevertheless, laparoscopy can be a useful adjunct to plan surgical incisions. Left sided McBurney incisions, Pfannenstiel incisions and lower midline laparotomies have been described approaches in the elective setting (20,82).
In the past, surgeons have investigated several non-resectional techniques. Surgical detorsion is associated with a recurrence rate of 40–60% and is therefore discouraged (6). Extraperitoneal fixation has had similar results apart from one group who had no recurrences over 6 years (83). Some groups have reported on mesosigmoidoplasty which is associated with recurrence rates of 21% (84). Given the paucity of high-quality data, none of these procedures have become mainstream.
Caecal volvulus
Incomplete rotation of the midgut can lead to inadequate fixation of the caecum to the retroperitoneum. Pregnancy and previous surgery where normal anatomy can be altered can also predispose to this condition. Clockwise rotation of the caecum on the ileocolic pedicle can lead to obstruction and ischaemia (75). In rarer situations an ‘upward-folding’ of the caecum creates a transition point and is referred to as a caecal bascule (85).
The role of endoscopy is limited here due to a low efficacy (10) and high recurrence rate. Surgery is therefore the gold standard for management (78). This condition carries a mortality rate of 12% which is elevated to 33% in the presence of caecal gangrene (86). Data on outcomes following resection are derived from older retrospective studies and consistently report better outcomes than non-resectional techniques (22). Outcomes of primary anastomosis in this setting are not well reported, however, it is generally advocated (78). In the setting of faecal peritonitis or haemodynamic instability, a resection with ileostomy and mucous fistula is a safe practice (87).
Caecostomy is associated with a recurrence rate of 14% and mortality of up to 52% and has largely been abandoned (88). Similarly caecopexy has a reported recurrence, complication and mortality rate of 13, 15 and 10% respectively (89).
Summary
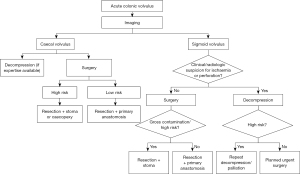
The treatment strategies for colonic volvulus are unique to other forms of LBO because of the more prominent role of endoscopic treatment. While non-resectional surgery has been advocated by many authors, the morbidity and recurrence rates associated with this approach, make these techniques less attractive. Figure 2 summarizes a suggested treatment algorithm for the management of colonic volvulus.
Diverticular disease
In Western populations, complicated diverticular disease represents the second most common cause of LBO (90). As a result of micro- or macroscopic perforation of diverticula, chronic inflammation can develop. These patients can present with either acute or chronic obstructive symptoms (91,92). Differentiating these patients from those with malignant LBO can be difficult clinically and even radiologically. Endoscopic evaluation where possible is informative (90). Presentation with LBO accounts for approximately 10% of patients with diverticular disease (93).
SEMS
SEMS have been explored by interested groups as a bridge to surgery or for palliation of high-risk patients. Technical success rates of >75% have been reported by dedicated units (94). These diverticular strictures are relatively rigid, translating to significant radial force on the stents, which may limit full expansion. Furthermore, the lack of mucosal pathology for the stent to grip onto can result in migration (95). A study of the outcomes of SEMS in benign colonic obstruction reported stenting in diverticular disease to be high risk for late and early perforation raising the important issue of safety (94).
Surgery
Surgical resection followed by a temporary colostomy (Hartmann’s procedure) or primary anastomosis is largely accepted to be the preferred treatment for stenosing diverticular disease (33). Regarding the technical aspects of surgery, there is currently inconclusive evidence to support high or low ligation of the inferior mesenteric artery with regards to its effects on bowel function and anastomotic leak, however it may be pertinent to perform a high ligation if there is diagnostic uncertainty about the nature of obstruction (96). The role of laparoscopic surgery in complicated diverticular disease is well established. The use of a hand-port can aid in blunt dissection of a phlegmon and has been shown to reduce operative time (97). The use of ureteric catheters or stents, while commonly employed in surgery for complex diverticular disease, has limited evidence for the avoidance of ureteric injuries (98). A recent systematic review on prophylactic ureteric stent placement prior to colonic surgery, found no change in the incidence of ureteric injury with no increase in morbidity. Their use was however associated with longer operating times (99). More recently, lighted ureteric stents or ureteric catheters instilled with indocyanine green have been utilised to provide both visual and tactile cues (100,101).
Summary
Surgery remains the mainstay of treatment for LBO caused by diverticular strictures. Given the chronic inflammatory nature, these operations can be technically challenging. Prophylactic ureteric catheters can act as a useful adjunct. In selected patients, who can be confidently diagnosed with a benign diverticular stricture, there may be a role for a non-oncologic, close mesocolic dissection. SEMS currently cannot be recommended in this setting due to the high complication rate.
Conclusions
LBO may result from a spectrum of conditions with various complexities. Patients can present in extremis or with subacute symptoms. The ability to manage these conditions requires a broad skillset of surgical and endoscopic techniques. There is an abundance of literature to guide decision making but prospective studies are limited. Ultimately decision making requires a combination of evidence and clinical acumen to provide the best outcomes for patients.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://ales.amegroups.com/article/view/10.21037/ales-21-45/rc
Peer Review File: Available at https://ales.amegroups.com/article/view/10.21037/ales-21-45/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://ales.amegroups.com/article/view/10.21037/ales-21-45/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yeo HL, Lee SW. Colorectal emergencies: review and controversies in the management of large bowel obstruction. J Gastrointest Surg 2013;17:2007-12. [Crossref] [PubMed]
- Franke AJ, Iqbal A, Starr JS, et al. Management of Malignant Bowel Obstruction Associated With GI Cancers. J Oncol Pract 2017;13:426-34. [Crossref] [PubMed]
- van Hooft JE, Veld JV, Arnold D, et al. Self-expandable metal stents for obstructing colonic and extracolonic cancer: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Update 2020. Endoscopy 2020;52:389-407. [Crossref] [PubMed]
- Finan PJ, Campbell S, Verma R, et al. The management of malignant large bowel obstruction: ACPGBI position statement. Colorectal Dis 2007;9:1-17. [Crossref] [PubMed]
- Webster PJ, Aldoori J, Burke DA. Optimal management of malignant left-sided large bowel obstruction: do international guidelines agree? World J Emerg Surg 2019;14:23. [Crossref] [PubMed]
- Halabi WJ, Jafari MD, Kang CY, et al. Colonic volvulus in the United States: trends, outcomes, and predictors of mortality. Ann Surg 2014;259:293-301. [Crossref] [PubMed]
- Pereira P, Djeudji F, Leduc P, et al. Ogilvie's syndrome-acute colonic pseudo-obstruction. J Visc Surg 2015;152:99-105. [Crossref] [PubMed]
- Catena F, De Simone B, Coccolini F, et al. Bowel obstruction: a narrative review for all physicians. World J Emerg Surg 2019;14:20. [Crossref] [PubMed]
- Aslar AK, Ozdemir S, Mahmoudi H, et al. Analysis of 230 cases of emergent surgery for obstructing colon cancer--lessons learned. J Gastrointest Surg 2011;15:110-9. [Crossref] [PubMed]
- Swenson BR, Kwaan MR, Burkart NE, et al. Colonic volvulus: presentation and management in metropolitan Minnesota, United States. Dis Colon Rectum 2012;55:444-9. [Crossref] [PubMed]
- Jaffe T, Thompson WM. Large-Bowel Obstruction in the Adult: Classic Radiographic and CT Findings, Etiology, and Mimics. Radiology 2015;275:651-63. [Crossref] [PubMed]
- Wilkinson N. Management of Rectal Cancer. Surg Clin North Am 2020;100:615-28. [Crossref] [PubMed]
- Ansaloni L, Andersson RE, Bazzoli F, et al. Guidelenines in the management of obstructing cancer of the left colon: consensus conference of the world society of emergency surgery (WSES) and peritoneum and surgery (PnS) society. World J Emerg Surg 2010;5:29. [Crossref] [PubMed]
- Lee K, Oh HK, Cho JR, et al. Surgical Management of Sigmoid Volvulus: A Multicenter Observational Study. Ann Coloproctol 2020;36:403-8. [Crossref] [PubMed]
- Ramanathan S, Ojili V, Vassa R, et al. Large Bowel Obstruction in the Emergency Department: Imaging Spectrum of Common and Uncommon Causes. J Clin Imaging Sci 2017;7:15. [Crossref] [PubMed]
- Chapman AH, McNamara M, Porter G. The acute contrast enema in suspected large bowel obstruction: value and technique. Clin Radiol 1992;46:273-8. [Crossref] [PubMed]
- Jacob SE, Lee SH, Hill J. The demise of the instant/unprepared contrast enema in large bowel obstruction. Colorectal Dis 2008;10:729-31. [Crossref] [PubMed]
- Sabbagh C, Siembida N, Yzet T, et al. What are the predictive factors of caecal perforation in patients with obstructing distal colon cancer? Colorectal Dis 2018;20:688-95. [Crossref] [PubMed]
- Atamanalp SS. Treatment of sigmoid volvulus: a single-center experience of 952 patients over 46.5 years. Tech Coloproctol 2013;17:561-9. [Crossref] [PubMed]
- Perrot L, Fohlen A, Alves A, et al. Management of the colonic volvulus in 2016. J Visc Surg 2016;153:183-92. [Crossref] [PubMed]
- Kahi CJ, Rex DK. Bowel obstruction and pseudo-obstruction. Gastroenterol Clin North Am 2003;32:1229-47. [Crossref] [PubMed]
- Naveed M, Jamil LH, Fujii-Lau LL, et al. American Society for Gastrointestinal Endoscopy guideline on the role of endoscopy in the management of acute colonic pseudo-obstruction and colonic volvulus. Gastrointest Endosc 2020;91:228-35. [Crossref] [PubMed]
- Anderson AD, McNaught CE, MacFie J, et al. Randomized clinical trial of multimodal optimization and standard perioperative surgical care. Br J Surg 2003;90:1497-504. [Crossref] [PubMed]
- Dauphine CE, Tan P, Beart RW Jr, et al. Placement of self-expanding metal stents for acute malignant large-bowel obstruction: a collective review. Ann Surg Oncol 2002;9:574-9. [Crossref] [PubMed]
- Runkel NS, Schlag P, Schwarz V, et al. Outcome after emergency surgery for cancer of the large intestine. Br J Surg 1991;78:183-8. [Crossref] [PubMed]
- Hsu J, Sevak S. Management of Malignant Large-Bowel Obstruction. Dis Colon Rectum 2019;62:1028-30. [Crossref] [PubMed]
- Kronborg O. Acute obstruction from tumour in the left colon without spread. A randomized trial of emergency colostomy versus resection. Int J Colorectal Dis 1995;10:1-5. [Crossref] [PubMed]
- De Salvo GL, Gava C, Pucciarelli S, et al. Curative surgery for obstruction from primary left colorectal carcinoma: primary or staged resection? Cochrane Database Syst Rev 2004;2004:CD002101. [Crossref] [PubMed]
- Takahashi H, Okabayashi K, Tsuruta M, et al. Self-Expanding Metallic Stents Versus Surgical Intervention as Palliative Therapy for Obstructive Colorectal Cancer: A Meta-analysis. World J Surg 2015;39:2037-44. [Crossref] [PubMed]
- Prasad ML, Pearl RK, Abcarian H. End-loop colostomy. Surg Gynecol Obstet 1984;158:380-2. [PubMed]
- Du R, Zhou J, Tong G, et al. Postoperative morbidity and mortality after anterior resection with preventive diverting loop ileostomy versus loop colostomy for rectal cancer: A updated systematic review and meta-analysis. Eur J Surg Oncol 2021;47:1514-25. [Crossref] [PubMed]
- Malik T, Lee MJ, Harikrishnan AB. The incidence of stoma related morbidity - a systematic review of randomised controlled trials. Ann R Coll Surg Engl 2018;100:501-8. [Crossref] [PubMed]
- Sartelli M, Weber DG, Kluger Y, et al. 2020 update of the WSES guidelines for the management of acute colonic diverticulitis in the emergency setting. World J Emerg Surg 2020;15:32. [Crossref] [PubMed]
- Frago R, Ramirez E, Millan M, et al. Current management of acute malignant large bowel obstruction: a systematic review. Am J Surg 2014;207:127-38. [Crossref] [PubMed]
- Jessen M, Nerstrøm M, Wilbek TE, et al. Risk factors for clinical anastomotic leakage after right hemicolectomy. Int J Colorectal Dis 2016;31:1619-24. [Crossref] [PubMed]
- Jurowich C, Lichthardt S, Matthes N, et al. Effects of anastomotic technique on early postoperative outcome in open right-sided hemicolectomy. BJS Open 2018;3:203-9. [Crossref] [PubMed]
- Teixeira Farinha H, Melloul E, Hahnloser D, et al. Emergency right colectomy: which strategy when primary anastomosis is not feasible? World J Emerg Surg 2016;11:19. [Crossref] [PubMed]
- Arezzo A, Migliore M, Chiaro P, et al. The REAL (REctal Anastomotic Leak) score for prediction of anastomotic leak after rectal cancer surgery. Tech Coloproctol 2019;23:649-63. [Crossref] [PubMed]
- Biondo S, Parés D, Frago R, et al. Large bowel obstruction: predictive factors for postoperative mortality. Dis Colon Rectum 2004;47:1889-97. [Crossref] [PubMed]
- Huisman DE, Reudink M, van Rooijen SJ, et al. LekCheck: A Prospective Study to Identify Perioperative Modifiable Risk Factors for Anastomotic Leakage in Colorectal Surgery. Ann Surg 2022;275:e189-97. [Crossref] [PubMed]
- Klatt GR. Role of subtotal colectomy in the treatment of incapacitating constipation. Am J Surg 1983;145:623-5. [Crossref] [PubMed]
- Hennekinne-Mucci S, Tuech JJ, Bréhant O, et al. Emergency subtotal/total colectomy in the management of obstructed left colon carcinoma. Int J Colorectal Dis 2006;21:538-41. [Crossref] [PubMed]
- Morgan WP, Jenkins N, Lewis P, et al. Management of obstructing carcinoma of the left colon by extended right hemicolectomy. Am J Surg 1985;149:327-9. [Crossref] [PubMed]
- Torralba JA, Robles R, Parrilla P, et al. Subtotal colectomy vs. intraoperative colonic irrigation in the management of obstructed left colon carcinoma. Dis Colon Rectum 1998;41:18-22. [Crossref] [PubMed]
- Brief DK, Brener BJ, Goldenkranz R, et al. Defining the role of subtotal colectomy in the treatment of carcinoma of the colon. Ann Surg 1991;213:248-52. [Crossref] [PubMed]
- Arnaud JP, Bergamaschi R. Emergency subtotal/total colectomy with anastomosis for acutely obstructed carcinoma of the left colon. Dis Colon Rectum 1994;37:685-8. [Crossref] [PubMed]
- Verkuijl SJ, Jonker JE, Trzpis M, et al. Functional outcomes of surgery for colon cancer: A systematic review and meta-analysis. Eur J Surg Oncol 2021;47:960-9. [Crossref] [PubMed]
- You YN, Chua HK, Nelson H, et al. Segmental vs. extended colectomy: measurable differences in morbidity, function, and quality of life. Dis Colon Rectum 2008;51:1036-43. [Crossref] [PubMed]
- Hsu TC. Comparison of one-stage resection and anastomosis of acute complete obstruction of left and right colon. Am J Surg 2005;189:384-7. [Crossref] [PubMed]
- Kozman DR, Engledow AH, Keck JO, et al. Treatment of left-sided colonic emergencies: a comparison of US, UK and Australian surgeons. Tech Coloproctol 2009;13:127-33. [Crossref] [PubMed]
- Engledow AH, Bond-Smith G, Motson RW, et al. Treatment of left-sided colonic emergencies: a comparison of US and UK surgical practices. Colorectal Dis 2009;11:642-7. [Crossref] [PubMed]
- Single-stage treatment for malignant left-sided colonic obstruction: a prospective randomized clinical trial comparing subtotal colectomy with segmental resection following intraoperative irrigation. The SCOTIA Study Group. Subtotal Colectomy versus On-table Irrigation and Anastomosis. Br J Surg 1995;82:1622-7. [PubMed]
- Ortiz H, Biondo S, Ciga MA, et al. Comparative study to determine the need for intraoperative colonic irrigation for primary anastomosis in left-sided colonic emergencies. Colorectal Dis 2009;11:648-52. [Crossref] [PubMed]
- Dudley HA, Racliffe AG, McGeehan D. Intraoperative irrigation of the colon to permit primary anastomosis. Br J Surg 1980;67:80-1. [Crossref] [PubMed]
- Naraynsingh V, Rampaul R, Maharaj D, et al. Prospective study of primary anastomosis without colonic lavage for patients with an obstructed left colon. Br J Surg 1999;86:1341-3. [Crossref] [PubMed]
- Cross KL, Rees JR, Soulsby RH, et al. Primary anastomosis without colonic lavage for the obstructed left colon. Ann R Coll Surg Engl 2008;90:302-4. [Crossref] [PubMed]
- Lim JF, Tang CL, Seow-Choen F, et al. Prospective, randomized trial comparing intraoperative colonic irrigation with manual decompression only for obstructed left-sided colorectal cancer. Dis Colon Rectum 2005;48:205-9. [Crossref] [PubMed]
- Kim JY, Kim SG, Im JP, et al. Comparison of treatment outcomes of endoscopic stenting for colonic and extracolonic malignant obstruction. Surg Endosc 2013;27:272-7. [Crossref] [PubMed]
- Ribeiro IB, de Moura DTH, Thompson CC, et al. Acute abdominal obstruction: Colon stent or emergency surgery? An evidence-based review. World J Gastrointest Endosc 2019;11:193-208. [Crossref] [PubMed]
- Kim C, Park JJ, Seo YS, et al. Complications of self-expandable colorectal stenting for the treatment of acute large bowel obstruction. Gastrointestinal Endoscopy 2005;61:AB262. [Crossref]
- Morino M, Arezzo A, Farnesi F, et al. Colonic Stenting in the Emergency Setting. Medicina (Kaunas) 2021;57:328. [Crossref] [PubMed]
- Ribeiro IB, Bernardo WM, Martins BDC, et al. Colonic stent versus emergency surgery as treatment of malignant colonic obstruction in the palliative setting: a systematic review and meta-analysis. Endosc Int Open 2018;6:E558-67. Erratum in: Endosc Int Open 2018;6:C1. [Crossref] [PubMed]
- Khot UP, Lang AW, Murali K, et al. Systematic review of the efficacy and safety of colorectal stents. Br J Surg 2002;89:1096-102. [Crossref] [PubMed]
- Zhao XD, Cai BB, Cao RS, et al. Palliative treatment for incurable malignant colorectal obstructions: a meta-analysis. World J Gastroenterol 2013;19:5565-74. [Crossref] [PubMed]
- Finlayson A, Hulme-Moir M. Palliative colonic stenting: a safe alternative to surgery in stage IV colorectal cancer. ANZ J Surg 2016;86:773-7. [Crossref] [PubMed]
- Young G; Cancer Council Australia Colorectal Cancer Guidelines Working Party. Clinical practice guidelines for the prevention, early detection and management of colorectal cancer. Sydney: Cancer Council Australia, 2018.
- Lee JH, Emelogu I, Kukreja K, et al. Safety and efficacy of metal stents for malignant colonic obstruction in patients treated with bevacizumab. Gastrointest Endosc 2019;90:116-24. [Crossref] [PubMed]
- van den Berg MW, Ledeboer M, Dijkgraaf MG, et al. Long-term results of palliative stent placement for acute malignant colonic obstruction. Surg Endosc 2015;29:1580-5. [Crossref] [PubMed]
- Cheung HY, Chung CC, Tsang WW, et al. Endolaparoscopic approach vs conventional open surgery in the treatment of obstructing left-sided colon cancer: a randomized controlled trial. Arch Surg 2009;144:1127-32. [Crossref] [PubMed]
- Kim JS, Hur H, Min BS, et al. Oncologic outcomes of self-expanding metallic stent insertion as a bridge to surgery in the management of left-sided colon cancer obstruction: comparison with nonobstructing elective surgery. World J Surg 2009;33:1281-6. [Crossref] [PubMed]
- van Hooft JE, Bemelman WA, Oldenburg B, et al. Colonic stenting versus emergency surgery for acute left-sided malignant colonic obstruction: a multicentre randomised trial. Lancet Oncol 2011;12:344-52. [Crossref] [PubMed]
- Sloothaak DA, van den Berg MW, Dijkgraaf MG, et al. Oncological outcome of malignant colonic obstruction in the Dutch Stent-In 2 trial. Br J Surg 2014;101:1751-7. [Crossref] [PubMed]
- Pirlet IA, Slim K, Kwiatkowski F, et al. Emergency preoperative stenting versus surgery for acute left-sided malignant colonic obstruction: a multicenter randomized controlled trial. Surg Endosc 2011;25:1814-21. [Crossref] [PubMed]
- Arezzo A, Passera R, Lo Secco G, et al. Stent as bridge to surgery for left-sided malignant colonic obstruction reduces adverse events and stoma rate compared with emergency surgery: results of a systematic review and meta-analysis of randomized controlled trials. Gastrointest Endosc 2017;86:416-26. [Crossref] [PubMed]
- Bauman ZM, Evans CH. Volvulus. Surg Clin North Am 2018;98:973-93. [Crossref] [PubMed]
- Atamanalp SS. Sigmoid volvulus. Eurasian J Med 2010;42:142-7. [Crossref] [PubMed]
- Quénéhervé L, Dagouat C, Le Rhun M, et al. Outcomes of first-line endoscopic management for patients with sigmoid volvulus. Dig Liver Dis 2019;51:386-90. [Crossref] [PubMed]
- Vogel JD, Feingold DL, Stewart DB, et al. Clinical Practice Guidelines for Colon Volvulus and Acute Colonic Pseudo-Obstruction. Dis Colon Rectum 2016;59:589-600. [Crossref] [PubMed]
- Iida T, Nakagaki S, Satoh S, et al. Clinical outcomes of sigmoid colon volvulus: identification of the factors associated with successful endoscopic detorsion. Intest Res 2017;15:215-20. [Crossref] [PubMed]
- Kuzu MA, Aşlar AK, Soran A, et al. Emergent resection for acute sigmoid volvulus: results of 106 consecutive cases. Dis Colon Rectum 2002;45:1085-90. [Crossref] [PubMed]
- Basato S, Lin Sun Fui S, Pautrat K, et al. Comparison of two surgical techniques for resection of uncomplicated sigmoid volvulus: laparoscopy or open surgical approach? J Visc Surg 2014;151:431-4. [Crossref] [PubMed]
- Al Dhaheri M, Nada MA, El Ansari W, et al. Left iliac fossa mini-incision sigmoidectomy for treatment of sigmoid volvulus. Case series of six patients from Qatar. Int J Surg Case Rep 2020;75:534-8. [Crossref] [PubMed]
- Bhatnagar BN, Sharma CL. Nonresective alternative for the cure of nongangrenous sigmoid volvulus. Dis Colon Rectum 1998;41:381-8. [Crossref] [PubMed]
- Oren D, Atamanalp SS, Aydinli B, et al. An algorithm for the management of sigmoid colon volvulus and the safety of primary resection: experience with 827 cases. Dis Colon Rectum 2007;50:489-97. [Crossref] [PubMed]
- Lung BE, Yelika SB, Murthy AS, et al. Cecal bascule: a systematic review of the literature. Tech Coloproctol 2018;22:75-80. [Crossref] [PubMed]
- Ballantyne GH, Brandner MD, Beart RW Jr, et al. Volvulus of the colon. Incidence and mortality. Ann Surg 1985;202:83-92. [Crossref] [PubMed]
- O'Mara CS, Wilson TH Jr, Stonesifer GL, et al. Cecal volvulus: analysis of 50 patients with long-term follow-up. Curr Surg 1980;37:132-6. [PubMed]
- Madiba TE, Thomson SR. The management of cecal volvulus. Dis Colon Rectum 2002;45:264-7. [Crossref] [PubMed]
- Rabinovici R, Simansky DA, Kaplan O, et al. Cecal volvulus. Dis Colon Rectum 1990;33:765-9. [Crossref] [PubMed]
- Hawkins AT, Wise PE, Chan T, et al. Diverticulitis: An Update From the Age Old Paradigm. Curr Probl Surg 2020;57:100862. [Crossref] [PubMed]
- Boostrom SY, Wolff BG, Cima RR, et al. Uncomplicated diverticulitis, more complicated than we thought. J Gastrointest Surg 2012;16:1744-9. [Crossref] [PubMed]
- Wolff BG, Boostrom SY. Prophylactic resection, uncomplicated diverticulitis, and recurrent diverticulitis. Dig Dis 2012;30:108-13. [Crossref] [PubMed]
- Onur MR, Akpinar E, Karaosmanoglu AD, et al. Diverticulitis: a comprehensive review with usual and unusual complications. Insights Imaging 2017;8:19-27. [Crossref] [PubMed]
- Keränen I, Lepistö A, Udd M, et al. Outcome of patients after endoluminal stent placement for benign colorectal obstruction. Scand J Gastroenterol 2010;45:725-31. [Crossref] [PubMed]
- Stefanidis D, Richardson W, Farrell TM, et al. SAGES guidelines for the surgical treatment of esophageal achalasia. Surg Endosc 2012;26:296-311. [Crossref] [PubMed]
- Schultz JK, Azhar N, Binda GA, et al. European Society of Coloproctology: guidelines for the management of diverticular disease of the colon. Colorectal Dis 2020;22:5-28. [Crossref] [PubMed]
- McCafferty MH, Roth L, Jorden J. Current management of diverticulitis. Am Surg 2008;74:1041-9. [Crossref] [PubMed]
- Croghan SM, Zaborowski A, Mohan HM, et al. The sentinel stent? A systematic review of the role of prophylactic ureteric stenting prior to colorectal resections. Int J Colorectal Dis 2019;34:1161-78. [Crossref] [PubMed]
- Hird AE, Nica A, Coburn NG, et al. Does prophylactic ureteric stenting at the time of colorectal surgery reduce the risk of ureteric injury? A systematic review and meta-analysis. Colorectal Dis 2021;23:1060-70. [Crossref] [PubMed]
- Boyan WP Jr, Lavy D, Dinallo A, et al. Lighted ureteral stents in laparoscopic colorectal surgery; a five-year experience. Ann Transl Med 2017;5:44. [Crossref] [PubMed]
- White LA, Joseph JP, Yang DY, et al. Intraureteral indocyanine green augments ureteral identification and avoidance during complex robotic-assisted colorectal surgery. Colorectal Dis 2021;23:718-23. [Crossref] [PubMed]
Cite this article as: Rajan R, Clark DA. Current management of large bowel obstruction: a narrative review. Ann Laparosc Endosc Surg 2022;7:23.


