Update on neuromodulation for fecal incontinence
Introduction
Since its introduction in 1994, sacral neuromodulation (SNM), also termed sacral nerve stimulation (SNS), has established its central role in the surgical treatment algorithm of fecal incontinence (FI) (1) and has gained broad acceptance. A population-based study of 621 patients in New York state treated for FI from 2011 to 2014 reflects a clear decline in the numbers of sphincteroplasties after SNM became available in the US in 2011 (2).
The technique’s minimal invasiveness, low risk and low comorbidity contributed to its increased acceptance, as did the option of a therapeutic test phase on which to base the decision for chronic stimulation, its sustained clinical efficacy, and reproducible outcomes. The following update on SNM will address new indications and recent developments in technique, such as standardized electrode implantation, and recent guidance for programming and trouble shooting.
Update on spectrum of indications
The high predictive value of a positive test stimulation with very limited risk caused a pragmatic, trial-and-error approach to patient selection to evolve, with new indications explored. This led to the successful application across a broad spectrum of etiologies: e.g., neurological dysfunction including spinal disc prolapse (3), unilateral traumatic pudendal neuropathy (4), spina bifida (5), muscular dystrophy (6); conditions arising from resective colorectal surgery such as proctocolectomy with ileoanal J-Pouch reconstruction for colitis (7); rectal prolapse repair (8,9); rectal resection for cancer (10) with or without neoadjuvant chemoradiation (11-14); proctectomy with colorectal or coloanal anastomosis for rectal cancer with or without neoadjuvant radio chemotherapy (15); neoadjuvant and adjuvant chemoradiation/radiotherapy for endometrial and rectal cancer (16-18); congenital FI (19) including that from anorectal malformations (20); and FI related to external anal sphincter atrophy (21). Although these reports include only a small number of patients for the most part, they outline the therapy’s potential. In all cases, the selection process for permanent SNM was based on the findings of test stimulation.
The changing paradigm in the surgical treatment of FI is also highlighted in a recent retrospective study of 461 patients from five European and one US center (22). The comparison of two 4-year periods (2000–2003 and 2007–2010) demonstrated that the use of SNM as the primary intervention increased from 29% to 89%, while sphincter repair or sphincteroplasty as the primary intervention for a sphincter lesion <90 degrees decreased from 68% to 46%.
With a growing interest in a multidisciplinary approach to pelvic floor and pelvic organ disorders, the combined effect of SNM on both urinary disorders and FI has gained wider attention. In 30 patients with obstetric anal sphincter injury (OASIS) with 3° or 4°perineal tears (23), SNM as the primary treatment not only resulted in a reduction of FI measured with St Mark’s score of 11.2±5.3 (baseline 19.0±2.5 vs. 6 months 7.7±5.5) accompanied by a significant improvement in quality of life and patient satisfaction, but also in a reduction in concomitant urinary incontinence measured with International Consultation on Incontinence Questionnaire Urinary Incontinence Short Form by 5.3±5.8 (baseline 11.3±6.45 vs. 6 months’ follow-up 6.1±6.0). In a different study, SNM percutaneous nerve evaluation (PNE) as a first-line treatment demonstrated a reduction in weekly FI episodes of 94.5%, from a median of 4.8 (2.0–11.0) to 0.5 (0–2.0) (P<0.001), giving further support to the idea of SNM as a first-line treatment for patients presenting with FI and a sphincter gap (24).
However, the spectrum of patients successfully treated with SNM has reached beyond those with FI to include patients presenting with other complex anorectal dysfunctions. With the increased understanding of low anterior resection syndrome (LARS), the frequency of its occurrence (25) and its clinical implications, an interest in the efficacy of SNM developed. Early studies, most with only a few patients, indicated that all symptom components contributing to LARS (e.g., clustering, urgency, frequency, and incontinence to flatus and liquid) were significantly improved (26).
In a review of ten studies with a total of 75 patients, a clear clinical benefit (Cleveland Clinic Incontinence score, Altomare Score, Williams classification, LARS score) was found (27). Although outcome measures varied among the studies (in particular, the LARS score was used in only three), all demonstrated significant improvement. A further recent metanalysis of 13 studies with a total of 114 patients indicated a success rate of SNM in LARS of 83% (28). Single-center studies demonstrated a sustained improvement of CCIS and LARS scores, the therapy being successful in 86% at 5-year follow-up (29). Within the population of patients with LARS treated with SNM, the outcome appears to be less favorable in those with very low anastomosis and who have had radio/chemotherapy (30). Overall, the use of SNM for LARS is still in its infancy. However, given that therapeutic options for this condition are limited, a treatment algorithm proposed by the Bordeaux group (31) may point in the future direction (i.e., positioning SNM after failure of medical management and pelvic floor rehabilitation, biofeedback and trans anal irrigation, but before more invasive options such as antegrade enema or definitive stoma).
Another field of growing interest, which remains controversial, addresses whether and to which extent a concomitant internal rectal prolapse negatively affects clinical outcome. In a retrospective study of 84 patients the presence of an underlying high-grade internal rectal prolapse (HIRP Oxford Grade III and IV) negatively impacted functional outcome seen at one-year follow-up (FISI: 37 to 23; P<0.01 vs. 38 to 34 P=0.16). Multivariate analysis indicated that HIRP was predictive, but coexisting recto- or enterocele was not (32). However, these findings are challenged by a recent publication in which preoperative defecography demonstrated that the only predictive factor for a positive clinical outcome was not the presence of an intussusception, but an increased anorectal angle at rest (33).
The recent introduction of magnetic resonance imaging (MRI) safe and smaller, rechargeable neurostimulation devices will allow the field of application to expand further to those in whom regular MRI is needed (e.g., patients with progressive neurological conditions or oncological interventions requiring surveillance or in whom the size of the device posed a problem). Additionally, MRI safety will reduce the need for device removal if MRI is indicated—currently accounting for up to 23% of SNM explantations (34).
Revisiting the technique
Despite the high success rate of SNM in FI, the failure rate is also considerable during both the test and chronic treatment phases ranging between 20–25% for each. This can be attributed to various factors such as suboptimal electrode placement and programming and inadequate reprogramming.
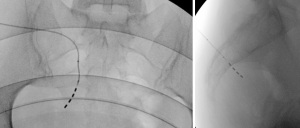
In the effort to improve the treatment outcome, foramen electrode implantation and programming have been revisited and standardized by practitioners with a high-volume implant population (35-37). Ten procedural steps were identified and described in detail to optimize the key aspect of the procedure—i.e., tined lead electrode placement: patient position and preparation, use of X-ray and marking, foramen needle placement, introducer placement, tined lead electrode placement, removal of the introducer, tunneling, implanted pulse generator (IPG) pocket and tunnelling, device insertion, antibiotic prophylaxis. The essence of the standardized electrode placement technique is the regular use a less rigid, curved stylet for placement and the use of radiologic imaging and marking to define the upper medial quadrant of the foramen, the preferred entry point. Imaging will guide the progression of the electrode through the foramen and placement (see Figure 1 for typical appearance) can be monitored by a combination of specific motor or sensory responses to stimulation. The objective is to place as many of the four electrode contacts in proximity to the target nerve. This can be assumed when low stimulation intensity—preferably below 2.0 mA—results in the expected motor or sensory response.
The standardization should lead to fewer side effects, more programming options, less battery consumption with consequent battery longevity, and improved clinical outcome. Findings of an anatomical study confirm that the new imaging-guided implantation technique results in close contact between the electrode and target nerve (38). Recent findings indicate that electromyography (EMG) recordings of the pelvic floor musculature during electrode placement can be a further aid to better stimulation response (39). This has confirmed the medial upper quadrant as the best location for electrode insertion (40).
Findings after the implementation of the standardized technique in both colorectal and urologic surgery (where the identical technique is used) confirm its superiority (41): the number of electrode contacts close to the target nerve is increased (42); the stimulation intensities to achieve the desired motor/sensory response are decreased (43) [also evident in a comparison of historical (36) and recent data (42)]; the risk of negative side effects is lowered if all four contact are close to the target nerve (44), a higher rate of therapeutic effective test stimulation phases results in more full implant (45); and the clinical outcome is improved (41,42). Operative time appears not to be increased (46).
Programming after electrode implantation is also key to a successful outcome and becomes relevant at the initial setting of the stimulation parameters, at adjustments during the course of therapeutic stimulation, and when troubleshooting is needed. There are differences in programming between the test phase with an external pulse generator (which allows only bipolar stimulation if a tined lead is used) and the permanently implanted IPG (which allows both uni- and bipolar stimulation). As yet, there is little evidence of the best (clinically most efficient) program setting after implantation, and programming is often arbitrary. It should be systematic and follow certain principles (36) (Figure 2).
Even if multiple electrode contacts are properly placed close to the target nerve, one may be sufficient to achieve the therapeutic benefit. Only a few programming settings seem to be beneficial in reducing symptoms. To define the active electrode—the cathode (negative pole)—only four electrode configurations can be identified. A defined muscular or sensory response with the lowest stimulation intensity will determine cathode selection. If unipolar stimulation is established, the IPG will become the anode (positive pole); if bipolar stimulation, one other electrode contact on the tined lead will be programmed as the anode. The closer the anode to the cathode, the smaller the created electrical field. Continuous stimulation is most common, although cyclic or intermittent stimulation can be used to extend battery life without reducing the clinical benefit. The value of changing other stimulation parameters, such as pulse frequency (Hz) and width, is not clear in the context of initial programming; for trouble shooting, adjustment of these parameters may be beneficial. Regular follow-up is advisable to assess outcome and adjust stimulation parameters if clinical need arises.
In the context of trouble shooting, data on reprogramming remain scarce (43). Information is based on the practical recommendations of practitioners with a high-volume implant population and derive primarily from personal experience accumulated over time (37). Reprogramming follows the principles of initial programming: to maintain the lowest possible amplitude while achieving the best possible sensory response to stimulation without adverse effects. The indications for reprogramming are loss of stimulation, loss of efficacy, and negative side effects such as pain. For loss of stimulation and efficacy, one first confirms that the pulse generator has not been accidently switched off, lead migration has not occurred, and the measurement of impedance indicates the unlikelihood of a technical cause or hardware failure. An increase in stimulation amplitude should then be tried. If this does not result in the desired effect, different electrode configurations need to be tested--following the algorithm of the initial programming. Switching from unipolar to bipolar stimulation (or vice versa) offers additional options. Changes in pulse frequency can be tried (47,48). Usually only one parameter setting should be changed, with sufficient time allowed for evaluation. Thorough documentation of the change in parameter setting may help if future adjustments become necessary. For the treatment of pain, device infection must be ruled out. If other causes of pain (such as back pain) are excluded, the next step will be to distinguish between the presence of the device or the stimulation as the origin. For the latter, the stimulation should be switched off for a period of time. If relief occurs, the amplitude should be decreased up to 50% of the habituated sensory threshold after re-activation. If the pure presence of the device is the cause, revisional surgery should be considered.
Summary
SNM has evolved to become a key therapy for FI. The current indications include a wide variety of pathophysiological and pathomorphological causes, but have also reached beyond FI to include complex conditions such as concomitant evacuation disorders and LARS. To enhance the outcome of SNM further, crucial elements of the therapy such as electrode placement, programming, and reprogramming have been revisited and standardized.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Lucia Oliveira, Steven D. Wexner and Sarah A. Vogler) for the series “The Pelvic Floor and Anorectal Disorders” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://ales.amegroups.com/article/view/10.21037/ales-2022-03/coif). The series “The Pelvic Floor and Anorectal Disorders” was commissioned by the editorial office without any funding or sponsorship. The author is a medical advisor to Medtronic, receiving consulting fees from Medtronic and payment for lectures and educational events. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Matzel KE. Fecal Incontinence in: Herold A, Lehur PA, Matzel KE, et al. editors. EMM Coloproctology. 2nd, Springer 2017:87-102.
- Xu Z, Fleming FJ, Justiniano CF, et al. Trends in Surgeon-Level Utilization of Sacral Nerve Stimulator Implantation for Fecal Incontinence in New York State. Dis Colon Rectum 2018;61:107-14. [Crossref] [PubMed]
- Jarrett ME, Matzel KE, Christiansen J, et al. Sacral nerve stimulation for faecal incontinence in patients with previous partial spinal injury including disc prolapse. Br J Surg 2005;92:734-9. [Crossref] [PubMed]
- Leblanc D, McFadden N, Lebel M, et al. Fecal continence can be restored by sacral neurostimulation after traumatic unilateral pudendal neuropathy: a case report. Int J Colorectal Dis 2015;30:569-70. [Crossref] [PubMed]
- Lansen-Koch SM, Govaert B, Oerlemans D, et al. Sacral nerve modulation for defaecation and micturition disorders in patients with spina bifida. Colorectal Dis 2012;14:508-14. [Crossref] [PubMed]
- Buntzen S, Rasmussen OO, Ryhammer AM, et al. Sacral nerve stimulation for treatment of fecal incontinence in a patient with muscular dystrophy: report of a case. Dis Colon Rectum 2004;47:1409-11. [Crossref] [PubMed]
- Meurette G, Wong M, Paye F, et al. Sacral nerve stimulation for the treatment of faecal incontinence after ileal pouch anal anastomosis. Colorectal Dis 2011;13:e182-3. [Crossref] [PubMed]
- Jarrett ME, Matzel KE, Stösser M, et al. Sacral nerve stimulation for fecal incontinence following surgery for rectal prolapse repair: a multicenter study. Dis Colon Rectum 2005;48:1243-8. [Crossref] [PubMed]
- Robert-Yap J, Zufferey G, Rosen H, et al. Sacral nerve modulation in the treatment of fecal incontinence following repair of rectal prolapse. Dis Colon Rectum 2010;53:428-31. [Crossref] [PubMed]
- Jarrett ME, Matzel KE, Stösser M, et al. Sacral nerve stimulation for faecal incontinence following a rectosigmoid resection for colorectal cancer. Int J Colorectal Dis 2005;20:446-51. [Crossref] [PubMed]
- Ratto C, Grillo E, Parello A, et al. Sacral neuromodulation in treatment of fecal incontinence following anterior resection and chemoradiation for rectal cancer. Dis Colon Rectum 2005;48:1027-36. [Crossref] [PubMed]
- de Miguel M, Oteiza F, Ciga MA, et al. Sacral nerve stimulation for the treatment of faecal incontinence following low anterior resection for rectal cancer. Colorectal Dis 2011;13:72-7. [Crossref] [PubMed]
- Holzer B, Rosen HR, Zaglmaier W, et al. Sacral nerve stimulation in patients after rectal resection--preliminary report. J Gastrointest Surg 2008;12:921-5. [Crossref] [PubMed]
- Thomas GP, Bradshaw E, Vaizey CJ. A review of sacral nerve stimulation for faecal incontinence following rectal surgery and radiotherapy. Colorectal Dis 2015;17:939-42. [Crossref] [PubMed]
- Mizrahi I, Chadi SA, Haim N, et al. Sacral neuromodulation for the treatment of faecal incontinence following proctectomy. Colorectal Dis 2017;19:O145-52. [Crossref] [PubMed]
- Schiano di Visconte M, Munegato G. The value of sacral nerve stimulation in the treatment of faecal incontinence after pelvic radiotherapy. Int J Colorectal Dis 2009;24:1111-2. [Crossref] [PubMed]
- Maeda Y, Høyer M, Lundby L, et al. Temporary sacral nerve stimulation for faecal incontinence following pelvic radiotherapy. Radiother Oncol 2010;97:108-12. [Crossref] [PubMed]
- Thin NN, Carrington EV, Grimmer K, et al. Advancement anoplasty and sacral nerve stimulation: an effective combination for radiation-induced anal stenosis. Int J Colorectal Dis 2011;26:211-3. [Crossref] [PubMed]
- Lagares-Tena L, Millán-Paredes L, Lázaro-García L, et al. Sacral neuromodulation in patients with congenital faecal incontinence. Special issues and review of the literature. Tech Coloproctol 2018;22:89-95. [Crossref] [PubMed]
- Grossi U, Carrington EV, Scott SM, et al. Sacral neuromodulation for anorectal dysfunction secondary to congenital imperforate anus: report of two cases. Int J Colorectal Dis 2014;29:889-90. [Crossref] [PubMed]
- Santoro GA, Infantino A, Cancian L, et al. Sacral nerve stimulation for fecal incontinence related to external sphincter atrophy. Dis Colon Rectum 2012;55:797-805. [Crossref] [PubMed]
- Ong K, Bordeianou L, Brunner M, et al. Changing paradigm of sacral neuromodulation and external anal sphincter repair for faecal incontinence in specialist centres. Colorectal Dis 2021;23:710-5. [Crossref] [PubMed]
- Rydningen M, Dehli T, Wilsgaard T, et al. Sacral neuromodulation compared with injection of bulking agents for faecal incontinence following obstetric anal sphincter injury - a randomized controlled trial. Colorectal Dis 2017;19:O134-44. [Crossref] [PubMed]
- Rydningen MB, Dehli T, Wilsgaard T, et al. Sacral neuromodulation for faecal incontinence following obstetric sphincter injury - outcome of percutaneous nerve evaluation. Colorectal Dis 2017;19:274-82. [Crossref] [PubMed]
- Sun R, Dai Z, Zhang Y, et al. The incidence and risk factors of low anterior resection syndrome (LARS) after sphincter-preserving surgery of rectal cancer: a systematic review and meta-analysis. Support Care Cancer 2021;29:7249-58. [Crossref] [PubMed]
- Eftaiha SM, Balachandran B, Marecik SJ, et al. Sacral nerve stimulation can be an effective treatment for low anterior resection syndrome. Colorectal Dis 2017;19:927-33. [Crossref] [PubMed]
- Huang Y, Koh CE. Sacral nerve stimulation for bowel dysfunction following low anterior resection: a systematic review and meta-analysis. Colorectal Dis 2019;21:1240-8. [Crossref] [PubMed]
- Ram E, Meyer R, Carter D, et al. The efficacy of sacral neuromodulation in the treatment of low anterior resection syndrome: a systematic review and meta-analysis. Tech Coloproctol 2020;24:803-15. [Crossref] [PubMed]
- De Meyere C, Nuytens F, Parmentier I, et al. Five-year single center experience of sacral neuromodulation for isolated fecal incontinence or fecal incontinence combined with low anterior resection syndrome. Tech Coloproctol 2020;24:947-58. [Crossref] [PubMed]
- Rubio-Perez I, Saavedra J, Marijuan JL, et al. Optimizing sacral neuromodulation for low anterior resection syndrome: learning from our experience. Colorectal Dis 2020;22:2146-54. [Crossref] [PubMed]
- Harji D, Fernandez B, Boissieras L, et al. A novel bowel rehabilitation programme after total mesorectal excision for rectal cancer: the BOREAL pilot study. Colorectal Dis 2021;23:2619-26. [Crossref] [PubMed]
- Prapasrivorakul S, Gosselink MP, Gorissen KJ, et al. Sacral neuromodulation for faecal incontinence: is the outcome compromised in patients with high-grade internal rectal prolapse? Int J Colorectal Dis 2015;30:229-34. [Crossref] [PubMed]
- Kollmann CT, Pretzsch EB, Kunz A, et al. Anorectal angle at rest predicting successful sacral nerve stimulation in idiopathic fecal incontinence-a cohort analysis. Int J Colorectal Dis 2020;35:2293-9. [Crossref] [PubMed]
- Guzman-Negron JM, Pizarro-Berdichevsky J, Gill BC, et al. Can Lumbosacral Magnetic Resonance Imaging be Performed Safely in Patients with a Sacral Neuromodulation Device? An In Vivo Prospective Study. J Urol 2018;200:1088-92. [Crossref] [PubMed]
- Matzel KE, Chartier-Kastler E, Knowles CH, et al. Sacral Neuromodulation: Standardized Electrode Placement Technique. Neuromodulation 2017;20:816-24. [Crossref] [PubMed]
- Lehur PA, Sørensen M, Dudding TC, et al. Programming Algorithms for Sacral Neuromodulation: Clinical Practice and Evidence-Recommendations for Day-to-Day Practice. Neuromodulation 2020;23:1121-9. [Crossref] [PubMed]
- Dudding TC, Lehur PA, Sørensen M, et al. Reprogramming Sacral Neuromodulation for Sub-Optimal Outcomes: Evidence and Recommendations for Clinical Practice. Neuromodulation 2021;24:1247-57. [Crossref] [PubMed]
- Müller C, Reissig LF, Argeny S, et al. Standardized fluoroscopy-guided implantation technique enables optimal electrode placement in sacral neuromodulation: a cadaver study. Tech Coloproctol 2021;25:215-21. [Crossref] [PubMed]
- Vaganée D, Van de Borne S, Voorham-van der Zalm P, et al. Pelvic Floor Muscle Electromyography as a Guiding Tool During Lead Placement and (Re)Programming in Sacral Neuromodulation Patients: Validity, Reliability, and Feasibility of the Technique. Neuromodulation 2020;23:1172-9. [Crossref] [PubMed]
- Vaganée D, Voorham J, Voorham-van der Zalm P, et al. Needle Placement and Position of Electrical Stimulation Inside Sacral Foramen Determines Pelvic Floor Electromyographic Response-Implications for Sacral Neuromodulation. Neuromodulation 2019;22:709-15. [Crossref] [PubMed]
- Vaganée D, Kessler TM, Van de Borne S, et al. Sacral neuromodulation using the standardized tined lead implantation technique with a curved vs a straight stylet: 2-year clinical outcomes and sensory responses to lead stimulation. BJU Int 2019;123:E7-E13. [Crossref] [PubMed]
- Duelund-Jakobsen J, Laurberg S, Lundby L. The functional outcome of sacral nerve stimulation for faecal incontinence can be improved by using lead model 3889 and a standardized implantation technique. Colorectal Dis 2018;20:O152-7. [Crossref] [PubMed]
- Govaert B, Rietveld MP, van Gemert WG, et al. The role of reprogramming in sacral nerve modulation for faecal incontinence. Colorectal Dis 2011;13:78-81. [Crossref] [PubMed]
- McChesney SL, Jorden J, Galandiuk S, et al. Number of electrode motor responses and outcomes after sacral neuromodulation for fecal incontinence. Dis Col Rectum 2020;63:e185.
- Connor J, Long A, Goudelocke C. Optimized sacral neuromodulation lead placement is feasible and does not increase operative times. Neurourol Urodyn 2018;37:S653.
- Adelstein SA, Lee W, Gioia K, et al. Outcomes in a contemporary cohort undergoing sacral neuromodulation using optimized lead placement technique. Neurourol Urodyn 2019;38:1595-601. [Crossref] [PubMed]
- Dudding TC, Vaizey CJ, Gibbs A, et al. Improving the efficacy of sacral nerve stimulation for faecal incontinence by alteration of stimulation parameters. Br J Surg 2009;96:778-84. [Crossref] [PubMed]
- Duelund-Jakobsen J, Dudding T, Bradshaw E, et al. Randomized double-blind crossover study of alternative stimulator settings in sacral nerve stimulation for faecal incontinence. Br J Surg 2012;99:1445-52. [Crossref] [PubMed]
Cite this article as: Matzel KE. Update on neuromodulation for fecal incontinence. Ann Laparosc Endosc Surg 2022;7:16.