
New concepts in the pathophysiology of fecal incontinence
Introduction
Fecal incontinence (FI) defined as the recurrent uncontrolled passage of feces (1) is a relatively common condition with considerable unmet needs in terms of effective treatments. FI may consist of feces of normal consistency, only liquid form, or mostly of flatus. It is not necessarily accompanied by urinary leakage, although the two disorders often occur together. While most new developments in medicine now arise from targeting defined disease mechanisms, progress for FI has been limited by poor understanding of pathophysiology and a dependence on looking for, and trying to treat, the obvious (2). This review questions current dogma and proposes a new approach.
Risk factors for FI
FI can result from a single catastrophic event such as major anorectal injury, but more commonly develops from accumulated risks (Table 1). The majority of patients present in their late 50s when combined exposures to risks, e.g., serial pregnancy, childbirth, and age are sufficient to compromise normal function. What is vital to appreciate is that prevalence of FI (based at least on community survey data) is similar in males and females, and hence risk factors other than obstetric trauma, which is so frequently cited as the primary cause of FI in women, must play a pivotal role. Data for some risks are conflicting.
Table 1
| Risk factor | Mechanisms | Notes |
|---|---|---|
| Advancing age | Reductions in coordinated muscle function, cognition and mobility. Changes in stool consistency | Demonstrated by almost all epidemiological studies (3,4). Major determinant of nursing home referral (5) |
| Female sex | Vulnerability due to loss of vaginal support and elasticity after childbirth, hormonal | Majority of patients seeking care are female (6,7) but community surveys (3,4) suggest unexplained similar prevalence between sexes |
| Parity with uncomplicated vaginal delivery [and Caesarean section (8)] | Stretch/compression injury leading to pelvic floor laxity (muscular, ligamentous and fascial) and neuropathy leading to muscular weakness, pelvic floor descent on straining or pelvic organ prolapse | Controversial: considered major risk factor in clinical populations with FI (7,9,10) and a risk based on obstetric cohorts (11,12); however weak or no risk in population surveys, even with prolonged 2nd stage of labour (4,13,14) |
| OASIS (3rd and 4th degree tears) | Difficult delivery with direct injury to sphincter musculature; increased risk of fascial/ligamentous stretching and nerve injury (as above) | Significant risk factor in isolation (9,15) although time to symptom onset is dichotomous (immediate or in older age) (8). Majority of women with OASIS do not develop FI in short-term (16) |
| Iatrogenic and traumatic anal sphincter injuries | Direct injury to sphincter musculature (IAS and/or EAS). Major trauma may covertly damage pelvic floor and innervation | Textbook causation, especially where internal anal sphincter is injured at fissure (sphincterotomy and anal stretch), haemorrhoidal or fistula surgery |
| Menopause | Low oestrogen levels alter neuromuscular functions of anorectum and pelvic floor leading to reduced muscle contractile force | Difficult to distinguish from age effects. Oestrogen replacement may not be effective (17) |
| CNS diseases, e.g., dementia, multiple sclerosis and stroke | Decreased cognition, immobility; loss of higher control of spinobulbar reflexes | Dementia leads to 4x risk in cohort studies (18). Identified as an independent risk factor for FI in several epidemiological studies (19-21) FI major problem in nursing homes (21) |
| Spinal cord and peripheral nerve injury | Loss of reflex control of anorectal functions. Loss of volitional pelvic floor contraction; anal sphincter not controlled by higher centers | Textbook causation in spinal cord injury (including occult) and cauda equina injury; severe diabetic motor-sensory and autonomic neuropath |
| Loose stool/diarrhea | Liquid stool more easily overcomes barrier. Some specific conditions may have effects on rectal urge and contractility, e.g., irritable bowel syndrome and post-cholecystectomy diarrhea | Cross-sectional (1,22-24) cohort studies (25) and case-control studies (26) uniformly support loose stool or chronic diarrhea as risks |
| Colonic resection and LARS | Factors include bowel shortening and rapid transit. LARS may have direct effects on anorectal sensory and motor functions | Observational data including prospective cohort studies (27) |
| Constipation | Occurs with or without impaction/overflow: failure to keep rectum empty risks FI if other risks present; chronic retention may affect afferent functions, e.g., to CNS | Incomplete evacuation a risk factor in some studies (24,25); cross-sectional data show substantive overlap of FI and functional constipation (7) |
| Rectal inflammation (proctitis) by any cause | Increased rectal afferent (urgency) and motor (hypercontractility) functions. Crohn’s disease may also affect anus | Textbook causation supported by epidemiology, e.g., IBD (28) and pelvic radiation (25,29) |
| Diabetes | Autonomic (30) and enteric neuropathy (31), with effects on colonic motility (32), pelvic floor (33), anorectal (34) and smooth muscle function as well as on mucus secretion and blood flow/turgor in anal valves | Textbook association supported by observational data |
| Obesity | Increased pressure on pelvic floor; possible alterations in sphincter functions (but also other secondary effects of metabolic syndrome) | Conflicting data but most large population surveys support (3,25,35,36) |
| Chronic illness (disease burden) | Poly-mechanistic including constipation | Comorbidity count, especially conditions like depression |
FI, fecal incontinence; OASIS, obstetric anal sphincter injuries; IAS, internal anal sphincter; EAS, external anal sphincter; LARS, low anterior resection syndrome; CNS, central nervous system; IBD, inflammatory bowel disease.
The classical barrier theory
In medical school, students are often taught the paradigm of ‘passage and passenger’ to describe the two main contributing risk factors for FI: a dysfunctional barrier (with a focus mainly on the anal sphincters), and liquid stool consistency placing the barrier at increased risk. This paradigm is helpful but not wholly correct. It underpins common treatment approaches such as pads and plugs, stool hardeners, e.g., loperamide, and bulking agents, e.g., ispaghula. As referenced in Table 1, there is good epidemiological data that overt injury to the barrier, e.g., through 3rd and 4th degree tears at childbirth and the diagnosis of states characterized by chronic diarrhea, e.g., diarrhea-predominant irritable bowel syndrome (D-IBS) confer a significant risk of developing FI.
Textbook descriptions of the continence barrier include: the resting tone of the anal sphincter, which is conferred by the internal anal sphincter (IAS) ~55–75%, the external anal sphincter (EAS) ~25%, and anal canal vascular columns ~15% (37,38), which interdigitate to provide a hermetic seal with turgor maintained by hydrostatic pressure within the vascular spaces (39). Further factors include resting tone of the pelvic floor musculature that maintains the anorectal angle conferred by tonic puborectalis contraction, and the ability [in response to anorectal sensory function such as sampling (40)] to volitionally contract the EAS (41). However, voluntary contraction of the anal sphincter can be normally maintained only for 15 seconds (42), so this is a ‘last resort’ reaction. While significant direct injury to the barrier may be sufficient alone to cause a physical gutter (or fistula) through which feces may bypass the continence mechanism, the majority of patients, even those women with obstetric anal sphincter injuries (OASIS) (after primary sphincter repair at the time of childbirth) have no overt ongoing defect.
In the late 1970s and 1980s, it became evident that overt injury to the barrier was insufficient to explain the association of FI with a number of other risk factors including pregnancy (especially with instrumental or protracted labour), chronic straining and pelvic organ prolapse (43,44) and various neurological injuries, e.g., cauda-equina syndrome. A series of seminal studies (45-49) utilized technological developments to directly measure intra-anal pressure (anorectal manometry) (50), or neurophysiological techniques, including electromyography (EMG) and nerve conduction studies (50,51). These demonstrated that poor contractile function of the sphincters, especially of the puborectalis, which like the EAS is in state of tonic contraction, were major factors in FI development with or without direct injury to sphincter integrity (52).
Electrophysiological recordings showed that such changes were at least in part due to pudendal nerve injury, occurring as a consequence of direct sphincter injury, pelvic floor muscle and ligamentous stretching or compression during pregnancy or chronic straining (53) with perineal descent (54). All these factors tend to progress with time after an initial injury (49,52), and all are inter-related. Weak muscles perform even less well when handicapped by floppy, non-elastic ligaments, and ‘ballooning’ descent of the pelvic floor on coughing and straining. A further factor in coordinated pelvic floor functioning is the central location of the vagina, which is itself an elastic structure. Hence, vaginal prolapse, associated with ligamentous stretch injury and damage to muscles and their innervation, is especially likely to lead to organ prolapse (55) and also urinary and/or FI. Several minimally invasive surgical techniques utilising tape insertions, to generate fibrous tissue support for damaged fascia and ligaments have been developed on this basis for selected patients (56) (with the caveat of the current embargo on use of tapes and mesh in any form in many countries). Together these concepts provide a ‘barrier-centric’ paradigm for the pathophysiology of FI (Figure 1).
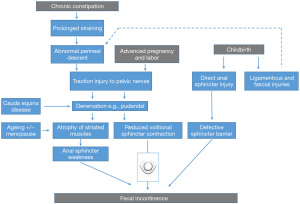
However, this schema is not the whole story. Table 2 lists some of the main limitations of this model. The major factor missing from this conceptualisation is recognition of the sensation of urge that is described by the majority of patients before they become aware of incontinence. Urge-related episodes of incontinence were recently reported by 75% of 2,452 patients presenting with FI to a tertiary center (7) While, it is acknowledged that reduced anal tone may lead to transgression of fecal content into the highly sensitive upper anal canal causing urge (just as trigone stimulation and upper urethral entry of urine causes bladder urge), this explanation is incomplete as a link between urgency and EAS injuries (see below). Tied to this is the recognition that no motor system, such as the pelvic floor musculature and its sphincter systems, can function without modulation by sensory input. Information about rectal content and readiness to defaecate is thus sent to the spinal cord, brainstem and brain. Disturbed anal sensation is a feature of many anorectal disorders, including incontinence, prolapse, haemorrhoids and slow transit constipation (65). There is therefore a need for a better model. A starting point is the rectum.
Table 2
| 3rd and 4th degree tears only occur in 3% deliveries (16,57) and only a minority of parous women with FI have major (overt) sphincter injuries. Epidemiological data show that the majority of women even with 3rd and 4th degree tears do not develop FI (16), and those that do present only when other risk factors such as age (8) have accumulated |
| FI is almost as common in men as women in surveys (3,4). Only a small proportion of men (~25%) (58) have evidence of sphincter injury (usually from anal surgery or trauma) (58) |
| Changes over the life-course: infants develop continence when unmodulated spinal reflex activity becomes controlled by mature central connections with assimilation of acquired behavioural patterns of cognition. In senility, continence may deteriorate with decreasing cognitive capacity. Whether this is always a frontally-mediated deficit, or relates to basal brain white matter small vessel disease (far more likely) is a matter for debate and research. Either way, it is well accepted that the control of social defecation and continence is centrally mediated by cortical influences on spinal and bulbar reflexes (equally for micturition and the bladder) |
| Most FI is classified as urge incontinence (7) defined as “incontinence occurring with a strong, sudden need to defaecate that is difficult to delay”. Urgency is a conscious perceived, i.e., cortical, event. Urge incontinence is considered a marker of external sphincter dysfunction (59-61). However, while a disrupted barrier may explain incontinence, it does not fully explain urgency |
| FI has a strong link with functional conditions such as IBS (62). This link is not confined to patients with loose stool. Urgency of normal stool is also common (63) |
| Surgical attempts at augmenting the continence barrier are almost all now obsolete (2). Some of these suffered from infection and erosion of foreign bodies but use of native muscle, e.g., posterior repair and graciloplasty also led to unacceptable functional consequences especially in causing obstructed defecation. Even sphincteroplasty, while still has outcomes that deteriorate significantly with time (2) and the role of injectable biomaterials (bulking agents) is not established (2) |
| In contrast, the dominant therapy, SNM does not augment sphincter structure and has no obvious effects on anal motor function (64) |
FI, fecal incontinence; SNM, sacral neuromodulation; IBS, irritable bowel syndrome.
The role of the rectum in the pathophysiology of FI
It is surprising that the apparent main storage organ for feces receives relatively little attention compared to the anal sphincters. This is not so for urinary incontinence and the bladder. For instance, a quick PubMed search reveals that 37.2% of studies of urinary incontinence (UI) incorporate bladder in the title compared to only 24.4% of those on FI incorporating the rectum. While clearly a crude approach, this is interesting because, on basic physical principles alone, the rectum should have a greater role in incontinence than the bladder. Feces, unlike urine, is a non-Newtonian fluid, even in liquid form (66,67). Its flow behaviour does not obey the law of viscosity (68) but is something akin therefore to a bottle of tomato ketchup, i.e., it does not come out with basic actions such as shaking or sudden moderate squeezing, with such forces producing a shear effect through the fluid rather than flow (69).
This may account for the very low incidence of stress FI with only ~2% of patients having isolated classic stress symptoms on coughing, sneezing, movement (7) [compared to 50% of patients with UI having isolated stress UI (70)]. When stress FI does occur it is frequently for flatus (7), explained by the gas phase shearing through the fluid as it changes viscosity in response to stress (69). Much as the rest of the colon requires forceful contractions to move feces in an abroad direction, the corollary is that for feces to leak, there must be an organized contraction of the rectum (+/− conscious compression by Valsalva).
The urethra and the bladder as a model for the anorectum
In the field of UI, 11% of patients have pure urge UI (UUI) and a further 36% have mixed urge and stress UI (70). The pathophysiology of UUI, and its main sub-diagnosis: overactive bladder (OAB), is almost exclusively concerned with the bladder and its reflexes, and treatments, e.g., drugs, intra-vesical botulinum toxin A injection and sacral neuromodulation (SNM) are focussed on stabilising the detrusor muscle to prevent contraction and unwanted micturition. Considering the general scientific principle of parsimony, it is odd that two very similar muscular organs sharing embryology (both have two systems derived from the cloaca), basic functions (storage and emptying) and single ‘exit pipes’ passing through a common sheet of muscle (pelvic floor) would come to have very different mechanisms of failure leading to incontinence. Of course they do not.
Both the bladder and rectum utilise smooth muscle contractile forces (peristalsis and bladder contraction) to expel contents. Both have smooth muscle sphincters controlling ‘sampling’ via upper anal canal and upper urethra respectively, and striated sphincters as minor players. Both use tension on the efflux pipe (urethra and puborectalis/rectum/anal canal) to oppose anterior and posterior walls (kinking) to maintain continence. Finally, the nervous system controls of both systems are very similar and this may account for the shared utility of SNM, which is currently considered the gold-standard procedure for both OAB and FI using an identical procedure (electrode placement and stimulation parameters) (71).
The rectum should therefore not be considered a passive reservoir. Rather, it is a contractile and sensate organ, that like the bladder, is subject to fine reflex control. Understanding the fine balance between filling and expulsion phases is key to understanding FI.
Abnormal anorectal afferent function
Active defecation can be considered to commence with the urge to pass feces. Continence is ultimately dependent upon an awareness of lower bowel content and warning of impending defecation. Hence, intact sensory functioning and integrity of anorectal afferent mechanisms are fundamental to both processes.
Firstly, it is important to consider where the urge to defaecate originates. Most evidence (principally involving distension studies, which mimic filling) points to the rectum as the primary organ responsible for this sensation (72-74). However, in the seminal 1951 study by Goligher and Hughes (72), although the majority of patients likened the sensation of balloon distension to “that of wind or motion in the rectum requiring evacuation”, other patients stated that they had “never experienced anything quite comparable before”, suggesting either simple rectal distension may be incapable of recreating the normal urge or that other organs are involved. This is supported by observations that those without a rectum [e.g., following surgery (75-77)] still perceive an urge to defaecate, though the quality of that sensation may be altered (77). Lane and Parks, for example, reported that the majority of 12 patients who had undergone rectal resection with colo-anal anastomosis described a normal sense of “perineal fullness” to distension, indicating that receptors responsible for the appreciation of impending evacuation may also lie outside the bowel (76). Indeed, Broens et al. using a combined manometry and proctographic study showed that the exquisitely sensitive proximal anal canal and/or its surrounding structures (e.g., muscle spindle and tendon organ stretch-sensitive receptors in the levator ani/puborectalis) played an important role in the desire-to-defecate sensation (78).
Taking available evidence together, the ‘call to stool’ is likely a complex, multifactorial process with perception of urge regulated by distal colonic, rectal and extra-rectal sensory mechanisms (the ‘early warning system’), with the rectum principally responsible for graded sensations of filling, and the anal canal and pelvic floor musculature providing discriminatory function and sensory ‘fine tuning’ crucial to continence (including through the ‘sampling’ reflex) (40). Distension of the anus is not associated with an urge-sensation (72).
Rectal distension results in deformations of the rectal wall, which induce alterations in tension encoded by mechanoreceptors. Specialized intraganglionic laminar endings located in the myenteric plexus (79) are the mechanotransduction sites of extrinsic sacral spinal afferent neurons in the guinea pig rectum and these have recently been characterized in human tissue (80). When a sufficient volume of stool distends the rectum, the perception of rectal fullness is communicated to the cortex via such afferent pathways. Rectal mucosal chemoreceptors (to substances like capsaicin) likely also involve afferent signalling via spinal afferents. Urge sensation is abolished with bilateral loss of sacral nerves (81).
Rectal sensation is inextricably linked to rectal biomechanical factors (e.g., capacity and compliance). In a normally functioning rectum (like the bladder) the urge to defaecate will dissipate, in the absence of further distension, due to the mechanism of ‘receptive relaxation’ [i.e., its ‘reservoir’ function (72,82)]. The degree of this relaxation is considered to be an important independent factor in the pathogenesis of fecal urgency and FI. A non-compliant (i.e., ‘stiff’) or small capacity rectum [as classically seen in inflammatory bowel disease (IBD) (73,83,84), post-pelvic irradiation (85), or following rectal surgery (27,86), but also in idiopathic FI (87)] is less able to adapt to filling, with urgency occurring at an earlier stage. Several studies have shown that reduced rectal compliance is commonly associated with rectal hypersensitivity (reduced thresholds to sensory stimuli) in patients with urge FI (88-93). In such patients, the rectum may also be hypercontractile, or hyper-reactive (87,90,94). Clinically, these patients have increased urgency and frequency of defecation, and greater lifestyle restrictions than those FI patients without rectal hypersensitivity (87,88).
Conversely, a large capacity, ‘lax’ (or hyper-compliant) rectum is frequently allied to rectal hyposensitivity (elevated thresholds to sensory stimuli) (95). Such patients invariably present with constipation and evacuatory difficulties associated with an impaired or absent urge to defaecate (96,97), though a sizeable proportion also have coexistent FI (presumed to be ‘overflow’) (53,95,97), manifest primarily as fecal seepage (10,98). It has been postulated that in patients with impaired rectal sensation, the normal compensatory EAS contraction to filling (excitatory reflex) is delayed or absent, allowing FI to ensue in the presence of low anal tone due to relaxation of the IAS (inhibitory reflex) which occurs at lower distending volumes than that of first perception of rectal distension (90,99-101).
Overall, rectal sensory disturbances in FI are common (~20% overall) with hypersensitivity more frequently found in females, and hyposensitivity in males (102). Neurophysiological studies have shown that impaired conduction through anorectal afferent pathways and reduced cortical activation are very common in FI (103). In patients with rectal hyposensitivity, latencies from rectal stimulation to cortical response are prolonged (104).
Rectal reflexes and the control of rectal contractility
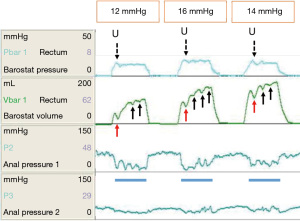
The above discourse implicates the rectum as a sensate organ, and disturbances of rectal afferent function as important biomarkers of FI in a variety of conditions. However, as already noted, the rectum must contract or be compressed to evacuate fecal contents. The control of rectal contractility, like the bladder, is subject to intrinsic reflexes. It is well established that the rectum provides afferent information for the rectoanal inhibitory reflex (RAIR). The IAS has an important role in ‘guarding the anal canal’. It relaxes briefly several times per hour (105,106) to allow sampling of rectal contents. It is also known, although widely neglected, that rectal distension elicits a (reflex) rectal contraction to generate a conscious defecatory urge (101,107). Sun et al. showed that in healthy subjects, the onset of a rectal contraction always occurred at the same time that a rectal sensation was perceived, and that the duration of rectal sensation correlated strongly with the duration of the rectal contractile response (RCR). Rectal sensation was always perceived at volumes well below those required to cause a sustained or deep anal relaxation [RAIR, conversely to the situation with rectal hyposensitivity (108)]. The RCR is probably a local reflex mediated by the rectal wall (enteric nervous system) however some historic data support a spinally-mediated reflex (107). It is best appreciated through rapid distension of an intra-rectal bag using the barostat (Figure 2). During both rapid phasic (109) and ramp inflation paradigms (93), a transient reduction in volume and concomitant increase in pressure within the bag is consistently observed in the early phase of distension, attributed to the RCR. Such contractions may be repetitive in health (90,93,109) and exaggerated in conditions such as IBS, characterized by urgency (94). Conversely, if perception of rectal distension (a sensory phenomenon) is indeed associated with the rectal contractile (motor) response, then it would be reasonable to speculate that the RCR may be attenuated in patients with rectal hyposensitivity. There is limited evidence to support this (110,111).
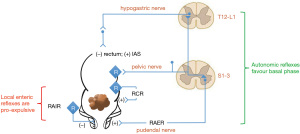
The RCR and RAIR, which both favour emptying, i.e., a pro-expulsive state, co-exist with reflexes that suppress defecation and promote a basal state. The rectoanal excitatory reflex (RAER) is mediated by parasympathetic pelvic afferents and the pudendal nerve (112,113) and is disturbed in patients with FI especially when pudendal neuropathy is present (112). Similarly, the sympathetic nervous system via the hypogastric nerves is inhibitory to rectal motor activity (114-116). In this way, the rectum is subject to a fine balance of local reflexes (pro-expulsion) and autonomic reflexes (pro-basal state) (Figure 3). In the bladder, the urethra and bladder are considered as a single functional unit for which a similar balance of local and autonomic reflexes are considered key to the switching between filling and voiding phases. The control of this switching is tightly regulated by the central nervous system (CNS) and this is discussed below.
The role of the colon in the pathophysiology of FI
It is a truism that FI cannot occur with an empty rectum (there may of course still be flatus incontinence or leakage of mucus) and some therapies focus on keeping the rectum empty on this basis, e.g., trans-anal irrigation. Although, the rectum has already been described as a storage organ in this monograph, it is actually questionable whether the rectum really is the main storage organ for feces. This is likely to be true only in the very late stages of continence, i.e., just prior to defecation; in health at least, the rectum is usually empty (117). There is evidence that that the main storage organ for feces is in fact the descending colon/sigmoid, where retrograde motor activity acts as a functional barrier by repelling feces.
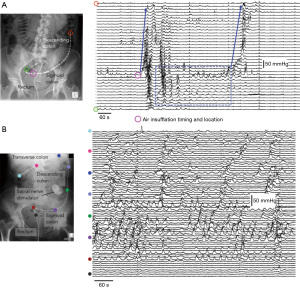
The colon fills the rectum with feces as a precursor to evacuation but may also have a role in keeping it empty. From the earliest human colonic manometric recordings, periodic, rhythmic colonic contractions have been described (118). Occurring at 2–8/min, with a primary site of origin at the rectosigmoid junction (119) this cyclic motor pattern has been shown to propagate over short regions of the colon in a predominately retrograde direction (120). While the physiological role of this cyclic motor pattern has not been clearly defined, it seems to play a role in normal colonic transit. Scintigraphic studies have linked these rhythmic contractions to subtle movement of colonic content (121-123), and radiologic studies have shown that content within the rectosigmoid junction can be moved back into the descending colon with rhythmic retrograde propulsion (124). More recently, studies utilizing high-resolution impedance manometry, have shown that a sub-sensory, low volume (60 mL) of air, infused into the sigmoid colon can trigger these cyclic motor patterns, which prevent the introduced air from reaching the rectum (125) (Figure 4A).
Manometric studies have shown that under normal circumstances the cyclic colonic motor pattern is inhibited by high-amplitude propagating contractions associated with defecation. This has been demonstrated in both healthy controls (126) and in patients with FI (22). However, general evidence linking the cyclic motor pattern with bowel continence/defecation remains mostly circumstantial. In the 1940s, diarrhea, induced with a subcutaneous injection of Mecholyl, a cholinergic drug, was seen to be associated with an increase in proximal colonic contractions with a concurrent inhibition of sigmoid contractions (127). Similarly in patients with ulcerative colitis, high stool frequency was associated with inhibited or absent contractions in the sigmoid colon (128). Studying the potential causes of diarrhea associated with alcohol ingestion, it was demonstrated that intravenously-infused alcohol caused significant inhibition of sigmoid contractility (129). In patients with low anterior resection syndrome (LARS), a significant reduction in the sigmoid cyclic motor pattern has been shown in comparison to healthy adults (130). In contrast, patients who underwent a low anterior resection but did not develop LARS, had a normal cyclic motor pattern in the sigmoid colon (131). Post-operative ileus has also been associated with sigmoid hyper-contractility (132).
Collectively, the studies discussed above suggest that motility in the sigmoid colon may act as a recto-sigmoid brake helping to control rectal filling and normal bowel continence (133). Importantly the cyclic motor pattern can be initiated by sacral nerve stimulation (134) (Figure 4B), which may also help to explain, in part, symptomatic improvement in patients with FI undergoing SNM treatment, especially in those with an anal sphincter defect (135-137).
The neural control of continence
Humans, like many other mammalian species (rats, cats and dogs), have a pattern of social defecation. This was teleologically advantageous: it has enabled us to live in groups, eat away from the bacterial hazards of feces and to wear warm clothes without soiling them. Key to this function is the ability to suppress defecation to a point of convenience and privacy (although defecation was historically communal). Such deferral is not, at least in health, conferred by the volitional continuous contraction of the anal sphincter against ongoing urge. As already noted, most people can maintain maximal squeeze, based on manometry, for only about 10–15 seconds (42). Rather, the sensation of urge dissipates after an initial stimulus (consider with-holding flatus in a crowded lift). The absence of ongoing distractive sensory input is common to the whole digestive system below the pharynx and also has a teleological benefit so that early humans could resume physical activity, e.g., hunting and protection without cognition being disturbed by visceral inputs.
In many ways, the lived experience of FI (at least of the most common urge FI) can be considered a failure of the deferral of defecation—an inability to switch off urge and expulsion when it is inconvenient and return the colon, rectum and anus to a physiological basal phase from a pre-expulsive phase (41). This is also true for the bladder which has two described phases: filling (99% time) and voiding (1% time). Bladder smooth muscle is continuously active during filling, but in an uncoordinated way, keeping the pressures very low. Isolated bladder experiments show the existence of micromotions (138)—small areas of muscle that contract while others relax without conferring a pressure increase. This constant motion is considered facilitatory for rapid adaptation to acute pressure changes, thus helping to maintain continence. However, the continuously active smooth muscle fibres also lead to continuous afferent firing which, in absence of protective mechanisms, could lead to continuous intrusions from desire to void. One implication of this spontaneous activity, however, is that central control must be mainly inhibitory.
The neural control of micturition (139-141) has been much better studied than defecation. During bladder filling, afferent information is conveyed through pelvic and hypogastric afferents up to the mesencephalic periaqueductal grey (PAG). Hypogastric efferents inhibit the bladder; pelvic efferents are silent during filling; and the pontine micturition center provides the switch between filling and voiding phases. The PAG connects to higher brain centers to provide the conscious perception of filling, desire or urge to void. If the situation is suitable for voiding, the pontine micturition center is activated leading to a synergic voiding (bladder contraction and at the same time urethral sphincter relaxation). If the time or place is inappropriate, the pontine center is inhibited by frontal brain activity and the desire to void is suppressed.
Switching in the pons between these phases occurs by differential afferent firing states in bladder sensory fibres. A low afferent firing state permits subconscious bladder filling, with inhibition of any sensory information to the cortex and suppression of detrusor activity. When the bladder fills toward capacity, a high afferent firing state ensues in which afferent information is no longer gated from the cortex (urge to void is experienced) and detrusor activity is promoted rather than suppressed by activation of detrusor pathways acting via suppression of Onuf’s nucleus in spinal cord and activation of parasympathetic efferents through the lumbosacral nuclei.
The plausible notion that the rectum behaves like the bladder in terms of differential firing states is not only based on parsimony. The bladder and rectum share the same peripheral nervous system organisation with similar inhibitory and excitatory reflexes. Furthermore, at least in animals, there is afferent convergence between both systems (142,143), and supraspinal control centers are located in the same brain regions (144-147). This appears also to hold for humans based on functional imaging studies that show common activation of the cortical insula during bladder filling and rectal distension (148).
The barrier in the neural control of continence
At the beginning of this review, the role of the sphincters and pelvic floor were described in respect of their contributions as a barrier to contain feces. This contribution was then set alongside the key role of the rectum and colon as arbiters of whether emptying could occur—this requiring active rectal emptying not just failure of the barrier.
The lived experience of urge incontinence implicates the volitional, although short-lived, contraction of the anal sphincter as a means of suppressing urge. It is possible, although unproven, that such contraction clears the upper anal canal after sampling thus reducing activation of mucosal receptors (78,149). A failure to clear the upper anal canal could therefore link EAS injury to ongoing stimulus-driven urge (59-61). However, this could not be the mechanism for the bladder where urine cannot be displaced and yet the urge to void also is suppressed. Abundant experimental and clinical urological literature show that stimulation of the urethra has marked effects on bladder physiology through some of the seven Barrington reflexes (those between urethra and bladder) [reviewed (150)]. Several of these are excitatory in nature and explain increased bladder activity originating from the urethra. Their normal role is to ensure complete bladder emptying as long as urine is flowing through the urethra. The downside of these reflexes is that they can be activated by sudden pressure impulses in the bladder, e.g., a cough (151) in the case of an insufficient internal sphincter causing bladder neck opening. However, others mediated by the pudendal nerves inhibit bladder contractile activity.
The critical role of the pudendal nerve in micturition has been well demonstrated in experimental animals since the 1930s. Since that time, a series of >100 high quality studies from Duke University and the University of Pittsburgh over a period of about 30 years have carefully unravelled the differential effects of pudendal nerve stimulation (PNS) on the bladder in alpha-chloralose-anesthetized cats. Such studies demonstrate that depending on stimulation frequency, electrical stimulation of pudendal afferents evokes spinal reflexes that either inhibit the bladder and promote continence or excite the bladder to promote micturition, the latter with the caveat that experiments were performed after spinal cord transection. Bladder inhibition by peripheral afferent PNS (experimentally via surface or needle stimulation of dorsal genital nerve) arises from activation of hypogastric efferents and subsequent synaptic and ganglionic inhibition of parasympathetic efferents (152,153) whereas the mechanisms of bladder excitation (above) are uncertain but may be due to convergence of pudendal and pelvic afferents (154) in the spinal cord. Increased urethral tone may have particular importance in gating urge sensation and inhibiting voiding in certain bladder emptying syndromes, e.g., Fowler’s syndrome (155) although the pathophysiology of this condition is far from assured (156).
The anal sphincter is also known to be richly innervated by afferent fibres (157) and these too signal anal tone. The pelvic floor muscles, and the EAS itself contain muscle spindles that have similar structures to those in skeletal muscles. These will have modulating effects on postural control of these muscles, by signalling muscle stretch and rate of change of stretch (45). In addition, there are Golgi tendon organs in the tendinous and fascial attachments of pelvic floor muscles, that signal muscle tension. The pelvic floor fascia, the perivaginal fascia and ligaments, and the peritoneum above the pelvis is liberally innervated by Pacinian corpuscles, that signal displacement and pressure. Pacinian corpuscles are also found in the fascial and peritoneal attachments of the anus and rectum respectively. These could play a role in detecting pressure changes due to filling of these organs.
Surface and needle stimulation of the dorsal genital nerve in humans has therapeutic effects for urge urinary (158) and urge FI (159,160) with demonstrable reductions in unwanted detrusor contractions (the motor correlate of urgency) in several studies of patients with UUI (158). Further, PNS using implanted electrodes on the pudendal nerve trunk, may have greater treatment effects in OAB than SNM (161,162) and has been trialled successfully for selected patients with FI (mainly those failing SNM or those with cauda equine syndrome) (163).
Summary
Integrated theory of FI pathophysiology
The important functions of the rectum, colon and CNS can be integrated with the classical barrier-centric model of FI pathophysiology. So doing treats the anorectum as a single functional unit in the same manner applied to study of the bladder and urethra. This unit requires contraction +/− external compression of the rectum for incontinence to occur but the control of rectal contractility is intimately dependent on reflexes from the anus, pelvic floor and probably other pelvic organs such as the vagina. This new version of the barrier is more than just the goalkeeper (it also controls play in the defence and midfield). The CNS controls switching this play between attack and defence. Together a new rectum-centric schema (Figure 5) provides a much better theory for explaining the pathophysiology of FI in terms of epidemiology and risk factors as well as the lived experience of urgency (Table 3).

Table 3
| Original criticism of barrier theory | New theory |
|---|---|
| Epidemiology of FI in parous women | FI can occur as a consequence of subclinical injury to sphincters and to pudendal/pelvic nerves. Subtle neuropathic or myopathic changes lead to change in reflex control of rectal contractility |
| Epidemiology of FI in men | The majority of FI in men is associated with problems of rectal sensation (generally hyposensation), and anorectal coordination (functional evacuation disorders) +/− IBS |
| Changes in continence over the life course | Key role of the CNS as controller of switching between basal and pro-expulsive phases. These require cortical, midbrain and spinal functions that develop in infancy and may be disturbed by disease or injury in later life |
| Lived experience of urgency | Central role of the rectum and particularly of rectal sensory functions in mediating both local reflex contractility and urge perception |
| Link to functional conditions such as IBS and functional evacuation disorders | These conditions are associated with demonstrable changes in rectal motor and sensory functions |
| Failure of direct repair and augmentation of the barrier | Static compression or constriction of the anal sphincters reduces dynamic variations in anal tone required for normal anorectal reflex activity leading especially to rectal evacuation problems |
| Success of SNM as therapy for FI and UUI | SNM, as studied to date, has effects that are predominantly on gating of spinal reflexes and central processing of urge perception |
FI, fecal incontinence; IBS, irritable bowel syndrome; CNS, central nervous system; SNM, sacral neuromodulation; UUI, urge urinary incontinence.
Areas for future research
- Revisiting work started in the 1980s, but discontinued, on fundamental anorectal excitatory and inhibitory reflex functions (the ‘Barrington’ reflexes of the anorectum) with concomitant but separate recordings of IAS, EAS and puborectalis contractile activities.
- Studies of the origins of anorectal sensation, especially of urgency.
- Functional brain imaging studies to further assess afferent functions of the anus, rectum, pelvic floor and distal colon.
- Continued studies of the relationship between the distal colon and the rectum as pertains to control of rectal filling, both in health and FI.
- Further integrated high-resolution studies of contractility and intra-luminal movement to better understand flow of solid, liquid and gas through the distal colon and anorectum in relation to perceived sensations.
- Experimental studies to better characterise the RCR and its relationship with cortical activation.
- Studies to determine the mechanism of action of SNM for therapy understanding but also as a means of better understanding the pathophysiology of incontinence.
- Mechanistic studies of PNS (nerve trunk and distal afferents) on anorectal functions.
- Pharmacological and physiological studies of the parasympathetic and sympathetic innervation of the distal colon, rectum and IAS.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Lucia Oliveira, Steven D. Wexner and Sarah A. Vogler) for the series “The Pelvic Floor and Anorectal Disorders” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://ales.amegroups.com/article/view/10.21037/ales-2022-02/coif). The series “The Pelvic Floor and Anorectal Disorders” was commissioned by the editorial office without any funding or sponsorship. CHK receives consulting fees from Medtronic and has a shareholding in Amber Therapeutics Ltd. The payments and support from Medtronic are related to sacral neuromodulation, which is relevant to this manuscript. The patents from Amber Therapeutics Ltd. are related to pudendal nerve stimulation, which is relevant to this manuscript. CHK receives research funding from Medtronic Inc for NIHR EME Subsonic study. CHK is the Chair of the International Continence Society ICI committee on surgery for faecal incontinence and the Chair of European Society of Coloproctology’s Research Committee. SMS has received honoraria from Laborie Medical Technologies Corp for teaching (providing lectures), which is irrelevant to this manuscript. MS received consulting fee from Cytogenetics – for international discussions on amyotrophic lateral sclerosis in 2021. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rao SS, Bharucha AE, Chiarioni G, et al. Functional Anorectal Disorders. Gastroenterology 2016; Epub ahead of print. [Crossref] [PubMed]
- O'Connell PR, Knowles CH, Maeda Y, et al. Surgery for Faecal Incontinence. Incontinence: 6th Edition; 2017:2087-142.
- Whitehead WE, Borrud L, Goode PS, et al. Fecal incontinence in US adults: epidemiology and risk factors. Gastroenterology 2009;137:512-7, 517.e1-2.
- Ditah I, Devaki P, Luma HN, et al. Prevalence, trends, and risk factors for fecal incontinence in United States adults, 2005-2010. Clin Gastroenterol Hepatol 2014;12:636-43.e1-2.
- Grover M, Busby-Whitehead J, Palmer MH, et al. Survey of geriatricians on the effect of fecal incontinence on nursing home referral. J Am Geriatr Soc 2010;58:1058-62. [Crossref] [PubMed]
- Nelson RL. Epidemiology of fecal incontinence. Gastroenterology 2004;126:S3-7. [Crossref] [PubMed]
- Vollebregt PF, Wiklendt L, Dinning PG, et al. Coexistent faecal incontinence and constipation: A cross-sectional study of 4027 adults undergoing specialist assessment. EClinicalMedicine 2020;27:100572. [Crossref] [PubMed]
- Larsson C, Hedberg CL, Lundgren E, et al. Anal incontinence after caesarean and vaginal delivery in Sweden: a national population-based study. Lancet 2019;393:1233-9. [Crossref] [PubMed]
- Lunniss PJ, Gladman MA, Hetzer FH, et al. Risk factors in acquired faecal incontinence. J R Soc Med 2004;97:111-6. [Crossref] [PubMed]
- Rao SS. Pathophysiology of adult fecal incontinence. Gastroenterology 2004;126:S14-22. [Crossref] [PubMed]
- Chaliha C, Sultan AH, Bland JM, et al. Anal function: effect of pregnancy and delivery. Am J Obstet Gynecol 2001;185:427-32. [Crossref] [PubMed]
- McKinnie V, Swift SE, Wang W, et al. The effect of pregnancy and mode of delivery on the prevalence of urinary and fecal incontinence. Am J Obstet Gynecol 2005;193:512-7; discussion 517-8. [Crossref] [PubMed]
- Rommen K, Schei B, Rydning A, et al. Prevalence of Anal Incontinence Among Norwegian Women, a Cross-Sectional Study (Hunt 3). Gastroenterology 2012;142:S568-9. [Crossref]
- van Meegdenburg MM, Trzpis M, Broens PM. Fecal incontinence and parity in the Dutch population: A cross-sectional analysis. United European Gastroenterol J 2018;6:781-90. [Crossref] [PubMed]
- Jorge JM, Wexner SD. Etiology and management of fecal incontinence. Dis Colon Rectum 1993;36:77-97. [Crossref] [PubMed]
- Abdel-Fattah M, Familusi A, Fielding S, et al. Primary and repeat surgical treatment for female pelvic organ prolapse and incontinence in parous women in the UK: a register linkage study. BMJ Open 2011;1:e000206. [Crossref] [PubMed]
- Staller K, Townsend MK, Khalili H, et al. Menopausal Hormone Therapy Is Associated With Increased Risk of Fecal Incontinence in Women After Menopause. Gastroenterology 2017;152:1915-1921.e1. [Crossref] [PubMed]
- Grant RL, Drennan VM, Rait G, et al. First diagnosis and management of incontinence in older people with and without dementia in primary care: a cohort study using The Health Improvement Network primary care database. PLoS Med 2013;10:e1001505. [Crossref] [PubMed]
- Chassagne P, Landrin I, Neveu C, et al. Fecal incontinence in the institutionalized elderly: incidence, risk factors, and prognosis. Am J Med 1999;106:185-90. [Crossref] [PubMed]
- Johanson JF, Irizarry F, Doughty A. Risk factors for fecal incontinence in a nursing home population. J Clin Gastroenterol 1997;24:156-60. [Crossref] [PubMed]
- Goodman C, Rycroft Malone J, Norton C, et al. Reducing and managing faecal incontinence in people with advanced dementia who are resident in care homes: protocol for a realist synthesis. BMJ Open 2015;5:e007728. [Crossref] [PubMed]
- Herbst F, Kamm MA, Morris GP, et al. Gastrointestinal transit and prolonged ambulatory colonic motility in health and faecal incontinence. Gut 1997;41:381-9. [Crossref] [PubMed]
- Rodger CJ, Nicol L, Anderson JH, et al. Abnormal colonic motility: a possible association with urge fecal incontinence. Dis Colon Rectum 2010;53:409-13. [Crossref] [PubMed]
- Menees SB, Almario CV, Spiegel BMR, et al. Prevalence of and Factors Associated With Fecal Incontinence: Results From a Population-Based Survey. Gastroenterology 2018;154:1672-1681.e3. [Crossref] [PubMed]
- Rey E, Choung RS, Schleck CD, et al. Onset and risk factors for fecal incontinence in a US community. Am J Gastroenterol 2010;105:412-9. [Crossref] [PubMed]
- Bharucha AE, Zinsmeister AR, Schleck CD, et al. Bowel disturbances are the most important risk factors for late onset fecal incontinence: a population-based case-control study in women. Gastroenterology 2010;139:1559-66. [Crossref] [PubMed]
- Battersby NJ, Bouliotis G, Emmertsen KJ, et al. Development and external validation of a nomogram and online tool to predict bowel dysfunction following restorative rectal cancer resection: the POLARS score. Gut 2018;67:688-96. [PubMed]
- Vollebregt PF, van Bodegraven AA, Markus-de Kwaadsteniet TML, et al. Impacts of perianal disease and faecal incontinence on quality of life and employment in 1092 patients with inflammatory bowel disease. Aliment Pharmacol Ther 2018;47:1253-60. [Crossref] [PubMed]
- Andreyev HJ, Benton BE, Lalji A, et al. Algorithm-based management of patients with gastrointestinal symptoms in patients after pelvic radiation treatment (ORBIT): a randomised controlled trial. Lancet 2013;382:2084-92. [Crossref] [PubMed]
- Low PA, Walsh JC, Huang CY, et al. The sympathetic nervous system in diabetic neuropathy. A clinical and pathological study. Brain 1975;98:341-56. [Crossref] [PubMed]
- Knowles CH, Scott SM, Wellmer A, et al. Sensory and autonomic neuropathy in patients with idiopathic slow-transit constipation. Br J Surg 1999;86:54-60. [Crossref] [PubMed]
- Battle WM, Snape WJ Jr, Alavi A, et al. Colonic dysfunction in diabetes mellitus. Gastroenterology 1980;79:1217-21. [Crossref] [PubMed]
- Rogers J, Levy DM, Henry MM, et al. Pelvic floor neuropathy: a comparative study of diabetes mellitus and idiopathic faecal incontinence. Gut 1988;29:756-61. [Crossref] [PubMed]
- Sun WM, Katsinelos P, Horowitz M, et al. Disturbances in anorectal function in patients with diabetes mellitus and faecal incontinence. Eur J Gastroenterol Hepatol 1996;8:1007-12. [Crossref] [PubMed]
- Uustal Fornell E, Wingren G, Kjølhede P. Factors associated with pelvic floor dysfunction with emphasis on urinary and fecal incontinence and genital prolapse: an epidemiological study. Acta Obstet Gynecol Scand 2004;83:383-9. [Crossref] [PubMed]
- Boreham MK, Richter HE, Kenton KS, et al. Anal incontinence in women presenting for gynecologic care: prevalence, risk factors, and impact upon quality of life. Am J Obstet Gynecol 2005;192:1637-42. [Crossref] [PubMed]
- Frenckner B, Euler CV. Influence of pudendal block on the function of the anal sphincters. Gut 1975;16:482-9. [Crossref] [PubMed]
- Lestar B, Penninckx F, Kerremans R. The composition of anal basal pressure. An in vivo and in vitro study in man. Int J Colorectal Dis 1989;4:118-22. [Crossref] [PubMed]
- Gibbons CP, Trowbridge EA, Bannister JJ, et al. Role of anal cushions in maintaining continence. Lancet 1986;1:886-8. [Crossref] [PubMed]
- Duthie HL, Bennett RC. The relation of sensation in the anal canal to the functional anal sphincter: a possible factor in anal continence. Gut 1963;4:179-82. [Crossref] [PubMed]
- Heitmann PT, Vollebregt PF, Knowles CH, et al. Understanding the physiology of human defaecation and disorders of continence and evacuation. Nat Rev Gastroenterol Hepatol 2021;18:751-69. [Crossref] [PubMed]
- Carrington EV, Brokjaer A, Craven H, et al. Traditional measures of normal anal sphincter function using high-resolution anorectal manometry (HRAM) in 115 healthy volunteers. Neurogastroenterol Motil 2014;26:625-35. [Crossref] [PubMed]
- Henry MM, Parks AG, Swash M. The pelvic floor musculature in the descending perineum syndrome. Br J Surg 1982;69:470-2. [Crossref] [PubMed]
- Kiff ES, Swash M. Slowed conduction in the pudendal nerves in idiopathic (neurogenic) faecal incontinence. Br J Surg 1984;71:614-6. [Crossref] [PubMed]
- Parks A, Swash M. Denervation of the anal sphincter causing idiopathic anorectal incontinence. J R Coll Surg Edinb 1979;24:94-6. [PubMed]
- Percy JP, Neill ME, Swash M, et al. Electrophysiological study of motor nerve supply of pelvic floor. Lancet 1981;1:16-7. [Crossref] [PubMed]
- Snooks SJ, Barnes PR, Swash M. Damage to the innervation of the voluntary anal and periurethral sphincter musculature in incontinence: an electrophysiological study. J Neurol Neurosurg Psychiatry 1984;47:1269-73. [Crossref] [PubMed]
- Snooks SJ, Henry MM, Swash M. Faecal incontinence due to external anal sphincter division in childbirth is associated with damage to the innervation of the pelvic floor musculature: a double pathology. Br J Obstet Gynaecol 1985;92:824-8. [Crossref] [PubMed]
- Snooks SJ, Swash M, Mathers SE, et al. Effect of vaginal delivery on the pelvic floor: a 5-year follow-up. Br J Surg 1990;77:1358-60. [Crossref] [PubMed]
- Neill ME, Parks AG, Swash M. Physiological studies of the anal sphincter musculature in faecal incontinence and rectal prolapse. Br J Surg 1981;68:531-6. [Crossref] [PubMed]
- Neill ME, Swash M. Increased motor unit fibre density in the external anal sphincter muscle in ano-rectal incontinence: a single fibre EMG study. J Neurol Neurosurg Psychiatry 1980;43:343-7. [Crossref] [PubMed]
- Swash M, Snooks SJ, Henry MM. Unifying concept of pelvic floor disorders and incontinence. J R Soc Med 1985;78:906-11. [Crossref] [PubMed]
- Lubowski DZ, Nicholls RJ. Faecal incontinence associated with reduced pelvic sensation. Br J Surg 1988;75:1086-8. [Crossref] [PubMed]
- Swash M. Anorectal incontinence: electrophysiological tests. Br J Surg 1985;72:S14-5. [Crossref] [PubMed]
- Rivington W. The Operation of Shortening the round Ligaments for Remedying Uterine Displacements. Br Med J 1885;1:425. [Crossref] [PubMed]
- Piñango-Luna S, Level-Córdova L, Petros PE, et al. A low cost artisan tension-free tape technique cures pelvic organ prolapse and stress urinary incontinence - proof of concept. Cent European J Urol 2020;73:490-7. [PubMed]
- Sultan AH, Kamm MA, Hudson CN, et al. Third degree obstetric anal sphincter tears: risk factors and outcome of primary repair. BMJ 1994;308:887-91. [Crossref] [PubMed]
- Burgell RE, Bhan C, Lunniss PJ, et al. Fecal incontinence in men: coexistent constipation and impact of rectal hyposensitivity. Dis Colon Rectum 2012;55:18-25. [Crossref] [PubMed]
- Gee AS, Durdey P. Urge incontinence of faeces is a marker of severe external anal sphincter dysfunction. Br J Surg 1995;82:1179-82. [Crossref] [PubMed]
- Hill J, Corson RJ, Brandon H, et al. History and examination in the assessment of patients with idiopathic fecal incontinence. Dis Colon Rectum 1994;37:473-7. [Crossref] [PubMed]
- Engel AF, Kamm MA, Bartram CI, et al. Relationship of symptoms in faecal incontinence to specific sphincter abnormalities. Int J Colorectal Dis 1995;10:152-5. [Crossref] [PubMed]
- Simrén M, Palsson OS, Heymen S, et al. Fecal incontinence in irritable bowel syndrome: Prevalence and associated factors in Swedish and American patients. Neurogastroenterol Motil 2017; [Crossref] [PubMed]
- Hunt MG, Wong C, Aajmain S, et al. Fecal incontinence in people with self-reported irritable bowel syndrome: Prevalence and quality of life. J Psychosom Res 2018;113:45-51. [Crossref] [PubMed]
- Carrington EV, Evers J, Grossi U, et al. A systematic review of sacral nerve stimulation mechanisms in the treatment of fecal incontinence and constipation. Neurogastroenterol Motil 2014;26:1222-37. [Crossref] [PubMed]
- Roe AM, Bartolo DC, Mortensen NJ. New method for assessment of anal sensation in various anorectal disorders. Br J Surg 1986;73:310-2. [Crossref] [PubMed]
- Woolley SM, Buckley CA, Pocock J, et al. Rheological modelling of fresh human faeces. J. Water, Sanit Hyg Dev 2014;4:484-9. [Crossref]
- de Loubens C, Dubreuil A, Lentle RG, et al. Rheology of human faeces and pathophysiology of defaecation. Tech Coloproctol 2020;24:323-9. [Crossref] [PubMed]
- Newton I. Philosophiæ Naturalis Principia Mathematica. London, Royal Society; 1687.
- Zamankhan P, Takayama S, Grotberg JB. Steady Displacement of Long Gas Bubbles in Channels and Tubes Filled by a Bingham Fluid. Phys Rev Fluids 2018;3:013302. [Crossref] [PubMed]
- Hannestad YS, Rortveit G, Sandvik H, et al. Epidemiology of Incontinence in the County of Nord-Trøndelag. A community-based epidemiological survey of female urinary incontinence: the Norwegian EPINCONT study. Epidemiology of Incontinence in the County of Nord-Trøndelag. J Clin Epidemiol 2000;53:1150-7. [Crossref] [PubMed]
- Matzel KE, Chartier-Kastler E, Knowles CH, et al. Sacral Neuromodulation: Standardized Electrode Placement Technique. Neuromodulation 2017;20:816-24. [Crossref] [PubMed]
- Goligher JC, Hughes ES. Sensibility of the rectum and colon. Its rôle in the mechanism of anal continence. Lancet 1951;1:543-7. [Crossref] [PubMed]
- Farthing MJ, Lennard-jones JE. Sensibility of the rectum to distension and the anorectal distension reflex in ulcerative colitis. Gut 1978;19:64-9. [Crossref] [PubMed]
- Kwan CL, Mikula K, Diamant NE, et al. The relationship between rectal pain, unpleasantness, and urge to defecate in normal subjects. Pain 2002;97:53-63. [Crossref] [PubMed]
- Simonsen OS, Stolf NA, Aun F, et al. Rectal sphincter reconstruction in perineal colostomies after abdominoperineal resection for cancer. Br J Surg 1976;63:389-91. [Crossref] [PubMed]
- Lane RH, Parks AG. Function of the anal sphincters following colo-anal anastomosis. Br J Surg 1977;64:596-9. [Crossref] [PubMed]
- Sun WM, Read NW, Katsinelos P, et al. Anorectal function after restorative proctocolectomy and low anterior resection with coloanal anastomosis. Br J Surg 1994;81:280-4. [Crossref] [PubMed]
- Broens P, Vanbeckevoort D, Bellon E, et al. Combined radiologic and manometric study of rectal filling sensation. Dis Colon Rectum 2002;45:1016-22. [Crossref] [PubMed]
- Lynn PA, Olsson C, Zagorodnyuk V, et al. Rectal intraganglionic laminar endings are transduction sites of extrinsic mechanoreceptors in the guinea pig rectum. Gastroenterology 2003;125:786-94. [Crossref] [PubMed]
- Ng KS, Brookes SJ, Montes-Adrian NA, et al. Electrophysiological characterization of human rectal afferents. Am J Physiol Gastrointest Liver Physiol 2016;311:G1047-55. [Crossref] [PubMed]
- Gunterberg B, Kewenter J, Petersén I, et al. Anorectal function after major resections of the sacrum with bilateral or unilateral sacrifice of sacral nerves. Br J Surg 1976;63:546-54. [Crossref] [PubMed]
- Musial F, Crowell MD. Rectal adaptation to distention: implications for the determination of perception thresholds. Physiol Behav 1995;58:1145-8. [Crossref] [PubMed]
- Rao SS, Read NW, Davison PA, et al. Anorectal sensitivity and responses to rectal distention in patients with ulcerative colitis. Gastroenterology 1987;93:1270-5. [Crossref] [PubMed]
- Loening-Baucke V, Metcalf AM, Shirazi S. Anorectal manometry in active and quiescent ulcerative colitis. Am J Gastroenterol 1989;84:892-7. [PubMed]
- Krol R, Hopman WP, Smeenk RJ, et al. Increased rectal wall stiffness after prostate radiotherapy: relation with fecal urgency. Neurogastroenterol Motil 2012;24:339-e166. [Crossref] [PubMed]
- Rasmussen OO, Petersen IK, Christiansen J. Anorectal function following low anterior resection. Colorectal Dis 2003;5:258-61. [Crossref] [PubMed]
- Chan CL, Lunniss PJ, Wang D, et al. Rectal sensorimotor dysfunction in patients with urge faecal incontinence: evidence from prolonged manometric studies. Gut 2005;54:1263-72. [Crossref] [PubMed]
- Chan CL, Scott SM, Williams NS, et al. Rectal hypersensitivity worsens stool frequency, urgency, and lifestyle in patients with urge fecal incontinence. Dis Colon Rectum 2005;48:134-40. [Crossref] [PubMed]
- Rasmussen O, Christensen B, Sørensen M, et al. Rectal compliance in the assessment of patients with fecal incontinence. Dis Colon Rectum 1990;33:650-3. [Crossref] [PubMed]
- Sun WM, Donnelly TC, Read NW. Utility of a combined test of anorectal manometry, electromyography, and sensation in determining the mechanism of 'idiopathic' faecal incontinence. Gut 1992;33:807-13. [Crossref] [PubMed]
- Salvioli B, Bharucha AE, Rath-Harvey D, et al. Rectal compliance, capacity, and rectoanal sensation in fecal incontinence. Am J Gastroenterol 2001;96:2158-68. [Crossref] [PubMed]
- Siproudhis L, El Abkari M, El Alaoui M, et al. Low rectal volumes in patients suffering from fecal incontinence: what does it mean? Aliment Pharmacol Ther 2005;22:989-96. [Crossref] [PubMed]
- Andrews C, Bharucha AE, Seide B, et al. Rectal sensorimotor dysfunction in women with fecal incontinence. Am J Physiol Gastrointest Liver Physiol 2007;292:G282-9. [Crossref] [PubMed]
- Corsetti M, Cesana B, Bhoori S, et al. Rectal hyperreactivity to distention in patients with irritable bowel syndrome: role of distention rate. Clin Gastroenterol Hepatol 2004;2:49-56. [Crossref] [PubMed]
- Gladman MA, Lunniss PJ, Scott SM, et al. Rectal hyposensitivity. Am J Gastroenterol 2006;101:1140-51. [Crossref] [PubMed]
- Ohkubo H, Takatsu T, Yoshihara T, et al. Difference in Defecation Desire Between Patients With and Without Chronic Constipation: A Large-Scale Internet Survey. Clin Transl Gastroenterol 2020;11:e00230. [Crossref] [PubMed]
- Vollebregt PF, Burgell RE, Hooper RL, et al. Clinical Impact of Rectal Hyposensitivity: A Cross-Sectional Study of 2,876 Patients With Refractory Functional Constipation. Am J Gastroenterol 2021;116:758-68. [Crossref] [PubMed]
- Hoffmann BA, Timmcke AE, Gathright JB Jr, et al. Fecal seepage and soiling: a problem of rectal sensation. Dis Colon Rectum 1995;38:746-8. [Crossref] [PubMed]
- Buser WD, Miner PB Jr. Delayed rectal sensation with fecal incontinence. Successful treatment using anorectal manometry. Gastroenterology 1986;91:1186-91. [Crossref] [PubMed]
- Hancke E, Schürholz M. Impaired rectal sensation in idiopathic faecal incontinence. Int J Colorectal Dis 1987;2:146-8. [Crossref] [PubMed]
- Sun WM, Read NW, Miner PB. Relation between rectal sensation and anal function in normal subjects and patients with faecal incontinence. Gut 1990;31:1056-61. [Crossref] [PubMed]
- Rasijeff AMP, García-Zermeño K, Di Tanna GL, et al. Systematic review and meta-analysis of anal motor and rectal sensory dysfunction in male and female patients undergoing anorectal manometry for symptoms of faecal incontinence. Colorectal Dis 2022; Epub ahead of print. [Crossref] [PubMed]
- Mundet L, Cabib C, Ortega O, et al. Defective Conduction of Anorectal Afferents Is a Very Prevalent Pathophysiological Factor Associated to Fecal Incontinence in Women. J Neurogastroenterol Motil 2019;25:423-35. [Crossref] [PubMed]
- Burgell RE, Lelic D, Carrington EV, et al. Assessment of rectal afferent neuronal function and brain activity in patients with constipation and rectal hyposensitivity. Neurogastroenterol Motil 2013;25:260-7, e167-8.
- Miller R, Lewis GT, Bartolo DC, et al. Sensory discrimination and dynamic activity in the anorectum: evidence using a new ambulatory technique. Br J Surg 1988;75:1003-7. [Crossref] [PubMed]
- Miller R, Bartolo DC, Cervero F, et al. Anorectal sampling: a comparison of normal and incontinent patients. Br J Surg 1988;75:44-7. [Crossref] [PubMed]
- Lium R, Porter JE. Observations on the etiology of ulcerative colitis: III. The distribution of lesions and its possible significance. Am J Pathol 1939;15:73-78.3.
- Sun WM, Read NW, Miner PB, et al. The role of transient internal sphincter relaxation in faecal incontinence? Int J Colorectal Dis 1990;5:31-6. [Crossref] [PubMed]
- Akervall S, Fasth S, Nordgren S, et al. Rectal reservoir and sensory function studied by graded isobaric distension in normal man. Gut 1989;30:496-502. [Crossref] [PubMed]
- Vasudevan SP, Scott SM, Gladman MA, et al. Rectal hyposensitivity: evaluation of anal sensation in female patients with refractory constipation with and without faecal incontinence. Neurogastroenterol Motil 2007;19:660-7. [Crossref] [PubMed]
- Vasudevan SP. Rectal hyposensitivity: clinical and physiological impact on patients with chronic constipation. PhD Thesis, University of London; 2013.
- Sangwan YP, Coller JA, Barrett RC, et al. Distal rectoanal excitatory reflex: a reliable index of pudendal neuropathy? Dis Colon Rectum 1995;38:916-20. [Crossref] [PubMed]
- Zbar AP, Aslam M, Gold DM, et al. Parameters of the rectoanal inhibitory reflex in patients with idiopathic fecal incontinence and chronic constipation. Dis Colon Rectum 1998;41:200-8. [Crossref] [PubMed]
- Gardette B, Gonella J. Electromyography in vivo of orthosympathetic control of cat colon. J Physiol (Paris) 1974;68:671-92. [PubMed]
- Hultén L. Extrinsic nervous control of colonic motility and blood flow. An experimental study in the cat. Acta Physiol Scand Suppl 1969;335:1-116. [PubMed]
- Tong WD, Ridolfi TJ, Kosinski L, et al. Effects of autonomic nerve stimulation on colorectal motility in rats. Neurogastroenterol Motil 2010;22:688-93. [Crossref] [PubMed]
- McNeil NI, Rampton DS. Is the rectum usually empty?--A quantitative study in subjects with and without diarrhea. Dis Colon Rectum 1981;24:596-9. [Crossref] [PubMed]
- Welch PB, Plant OH. A graphic study of the muscular activity of the colon, with special reference to its response to feeding. Am J Med Sci 1926;172:261-8. [Crossref]
- Lin AY, Du P, Dinning PG, et al. High-resolution anatomic correlation of cyclic motor patterns in the human colon: Evidence of a rectosigmoid brake. Am J Physiol Gastrointest Liver Physiol 2017;312:G508-15. [Crossref] [PubMed]
- Dinning PG, Wiklendt L, Maslen L, et al. Quantification of in vivo colonic motor patterns in healthy humans before and after a meal revealed by high-resolution fiber-optic manometry. Neurogastroenterol Motil 2014;26:1443-57. [Crossref] [PubMed]
- Moreno-Osset E, Bazzocchi G, Lo S, et al. Association between postprandial changes in colonic intraluminal pressure and transit. Gastroenterology 1989;96:1265-73. [Crossref] [PubMed]
- Bazzocchi G, Ellis J, Villanueva-Meyer J, et al. Effect of eating on colonic motility and transit in patients with functional diarrhea. Simultaneous scintigraphic and manometric evaluations. Gastroenterology 1991;101:1298-306. [Crossref] [PubMed]
- Cook IJ, Furukawa Y, Panagopoulos V, et al. Relationships between spatial patterns of colonic pressure and individual movements of content. Am J Physiol Gastrointest Liver Physiol 2000;278:G329-41. [Crossref] [PubMed]
- Ritchie JA, Truelove SC, Ardan GM, et al. Propulsion and retropulsion of normal colonic contents. Am J Dig Dis 1971;16:697-704. [Crossref] [PubMed]
- Heitmann PT, Mohd Rosli R, Maslen L, et al. High-resolution impedance manometry characterizes the functional role of distal colonic motility in gas transit. Neurogastroenterol Motil 2022;34:e14178. [Crossref] [PubMed]
- Bampton PA, Dinning PG, Kennedy ML, et al. Spatial and temporal organization of pressure patterns throughout the unprepared colon during spontaneous defecation. Am J Gastroenterol 2000;95:1027-35. [Crossref] [PubMed]
- Kern F Jr, Abbot FK, Almy TP. Action of Acetyl-Beta-Methylcholine Chloride (Mecholyl) On The Human Colon. Am J Med 1949;7:418. [Crossref]
- KERN F Jr. The motility of the distal colon in nonspecific ulcerative colitis. Gastroenterology 1951;19:492-503. [Crossref] [PubMed]
- Berenson MM, Avner DL. Alcohol inhibition of rectosigmoid motility in humans. Digestion 1981;22:210-5. [Crossref] [PubMed]
- Keane C, Paskaranandavadivel N, Vather R, et al. Altered colonic motility is associated with low anterior resection syndrome. Colorectal Dis 2021;23:415-23. [Crossref] [PubMed]
- Vather R, O'Grady G, Arkwright JW, et al. Restoration of normal colonic motor patterns and meal responses after distal colorectal resection. Br J Surg 2016;103:451-61. [Crossref] [PubMed]
- Vather R, O'Grady G, Lin AY, et al. Hyperactive cyclic motor activity in the distal colon after colonic surgery as defined by high-resolution colonic manometry. Br J Surg 2018;105:907-17. [Crossref] [PubMed]
- Lin AY, Dinning PG, Milne T, et al. The "rectosigmoid brake": Review of an emerging neuromodulation target for colorectal functional disorders. Clin Exp Pharmacol Physiol 2017;44:719-28. [Crossref] [PubMed]
- Patton V, Wiklendt L, Arkwright JW, et al. The effect of sacral nerve stimulation on distal colonic motility in patients with faecal incontinence. Br J Surg 2013;100:959-68. [Crossref] [PubMed]
- Boyle DJ, Knowles CH, Lunniss PJ, et al. Efficacy of sacral nerve stimulation for fecal incontinence in patients with anal sphincter defects. Dis Colon Rectum 2009;52:1234-9. [Crossref] [PubMed]
- Enomoto H, Nishizawa Y, Inamori K, et al. Sacral neuromodulation for the prevention of a permanent stoma in patients with severe defecation disorder following intersphincteric resection. Surg Today 2021;51:1379-86. [Crossref] [PubMed]
- Ong K, Bordeianou L, Brunner M, et al. Changing paradigm of sacral neuromodulation and external anal sphincter repair for faecal incontinence in specialist centres. Colorectal Dis 2021;23:710-5. [Crossref] [PubMed]
- Drake MJ, Kanai A, Bijos DA, et al. The potential role of unregulated autonomous bladder micromotions in urinary storage and voiding dysfunction; overactive bladder and detrusor underactivity. BJU Int 2017;119:22-9. [Crossref] [PubMed]
- De Groat WC. Neurophysiology of the pelvic organs. In: Rushton D. editor. Handbook of Neuro-Urology. New York: Marcel Dekker Inc.; 1994:55-93.
- Holstege G, Sie JAM. The central control of the pelvic floor. In: Pemberton JH, Swash M, Henry MM. editors. The Pelvic Floor: its function and disorders. London: WB Saunders; 2002:94-102.
- Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nat Rev Neurosci 2008;9:453-66. [Crossref] [PubMed]
- Christianson JA, Liang R, Ustinova EE, et al. Convergence of bladder and colon sensory innervation occurs at the primary afferent level. Pain 2007;128:235-43. [Crossref] [PubMed]
- Kruse MN, Mallory BS, Noto H, et al. Modulation of the spinobulbospinal micturition reflex pathway in cats. Am J Physiol 1992;262:R478-84. [PubMed]
- Athwal BS, Berkley KJ, Hussain I, et al. Brain responses to changes in bladder volume and urge to void in healthy men. Brain 2001;124:369-77. [Crossref] [PubMed]
- Blok BF, Willemsen AT, Holstege G. A PET study on brain control of micturition in humans. Brain 1997;120:111-21. [Crossref] [PubMed]
- Hobday DI, Aziz Q, Thacker N, et al. A study of the cortical processing of ano-rectal sensation using functional MRI. Brain 2001;124:361-8. [Crossref] [PubMed]
- Nour S, Svarer C, Kristensen JK, et al. Cerebral activation during micturition in normal men. Brain 2000;123:781-9. [Crossref] [PubMed]
- Halani PK, Andy UU, Rao H, et al. Regions of the brain activated in bladder filling vs rectal distention in healthy adults: A meta-analysis of neuroimaging studies. Neurourol Urodyn 2020;39:58-65. [Crossref] [PubMed]
- DUTHIE HL. GAIRNS FW. Sensory nerve-endings and sensation in the anal region of man. Br J Surg 1960;47:585-95. [PubMed]
- Gillespie JI, van Koeveringe GA, de Wachter SG, et al. On the origins of the sensory output from the bladder: the concept of afferent noise. BJU Int 2009;103:1324-33. [Crossref] [PubMed]
- Chan CL, Ponsford S, Swash M. The anal reflex elicited by cough and sniff: validation of a neglected clinical sign. J Neurol Neurosurg Psychiatry 2004;75:1449-51. [Crossref] [PubMed]
- Craggs M, McFarlane J. Neuromodulation of the lower urinary tract. Exp Physiol 1999;84:149-60. [Crossref] [PubMed]
- Lindström S, Fall M, Carlsson CA, et al. The neurophysiological basis of bladder inhibition in response to intravaginal electrical stimulation. J Urol 1983;129:405-10. [Crossref] [PubMed]
- Woock JP, Yoo PB, Grill WM. Mechanisms of reflex bladder activation by pudendal afferents. Am J Physiol Regul Integr Comp Physiol 2011;300:R398-407. [Crossref] [PubMed]
- Osman NI, Chapple CR. Fowler's syndrome--a cause of unexplained urinary retention in young women? Nat Rev Urol 2014;11:87-98. [Crossref] [PubMed]
- Petros P, Abendstein B, Swash M. Retention of urine in women is alleviated by uterosacral ligament repair: implications for Fowler's syndrome. Cent European J Urol 2018;71:436-43. [PubMed]
- Lynn PA, Brookes SJ. Function and morphology correlates of rectal nerve mechanoreceptors innervating the guinea pig internal anal sphincter. Neurogastroenterol Motil 2011;23:88-95, e9.
- Martens FM, Heesakkers JP, Rijkhoff NJ. Surgical access for electrical stimulation of the pudendal and dorsal genital nerves in the overactive bladder: a review. J Urol 2011;186:798-804. [Crossref] [PubMed]
- Worsøe J, Fynne L, Laurberg S, et al. Electrical stimulation of the dorsal clitoral nerve reduces incontinence episodes in idiopathic faecal incontinent patients: a pilot study. Colorectal Dis 2012;14:349-55. [Crossref] [PubMed]
- Worsøe J, Fynne L, Laurberg S, et al. The acute effect of dorsal genital nerve stimulation on rectal wall properties in patients with idiopathic faecal incontinence. Colorectal Dis 2011;13:e284-92. [Crossref] [PubMed]
- Peters KM, Feber KM, Bennett RC. A prospective, single-blind, randomized crossover trial of sacral vs pudendal nerve stimulation for interstitial cystitis. BJU Int 2007;100:835-9. [Crossref] [PubMed]
- Peters KM, Feber KM, Bennett RC. Sacral versus pudendal nerve stimulation for voiding dysfunction: a prospective, single-blinded, randomized, crossover trial. Neurourol Urodyn 2005;24:643-7. [Crossref] [PubMed]
- George AT, Dudding TC, Gurmany S, et al. Pudendal nerve stimulation for bowel dysfunction in complete cauda equina syndrome. Ann Surg 2014;259:502-7. [Crossref] [PubMed]
Cite this article as: Knowles CH, Dinning P, Scott SM, Swash M, de Wachter S. New concepts in the pathophysiology of fecal incontinence. Ann Laparosc Endosc Surg 2022;7:15.


