Imaging modalities for pelvic floor disorders
Introduction
Pelvic floor (PF) is commonly divided into three vertical compartments (anterior, middle, and posterior), however it rather works as a mechanical apparatus and must be considered as one horizontal compartment (1,2). Pelvic floor disorders (PFD), as pelvic organs prolapse (POP), voiding, defecatory and sexual dysfunctions, urinary or anal incontinences, chronic pelvic pain can develop after obstetric trauma, pelvic surgery, aging, and hormonal changes and frequently coexist (3-10).
Physical examination is often unable to detect the anatomical damages in the “complex” pelvis. An anterior or posterior vaginal wall bulging found at the physical examination may be scored by the POP quantification system. However, it is difficult for the clinician to accurately detect how many organs fill the “sack” (bladder, uterus, rectum, sigmoid colon, small bowel) in multicompartmental prolapse and which anatomical structures of support are damaged (9,10). In addition, a single symptom can be also related to “occult” conditions, often clinically underestimated. These causes, if not identified, may cause failure of the treatment or recurrence of the symptom.
Imaging modalities visualize clinically “occult” abnormalities and let to correlate radiological findings to symptoms and to clinical findings (11-14). Technological innovations have further improved the accuracy, however for a comprehensive overview of both the anatomy and functionality of the PF, it is of great importance the integration of different procedures.
This article aims to review the various imaging techniques in the assessment of anal incontinence (AI) and obstructive defecation syndrome (ODS), and their role in the management of these conditions.
Anal incontinence
Anal incontinence is a disorder that affects adult of different age groups and both sexes and may severely affect quality of life. It is defined as “the recurrent, uncontrolled, involuntary loss of feces, rectal gas or flatus or the loss of mucus only” (15). Different mechanisms can be related to the occurrence of AI: anal sphincter lesions, pelvic nerves damage, stool consistency, reduced rectal compliance, accelerated colonic transit. The imaging modalities for the evaluation of anal sphincter integrity are ultrasonography (endoanal—EAUS; endovaginal—EVUS; transperineal—TPUS), and magnetic resonance imaging (MRI) (16).
Endoanal ultrasonography
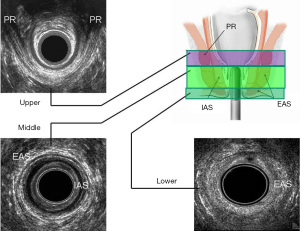
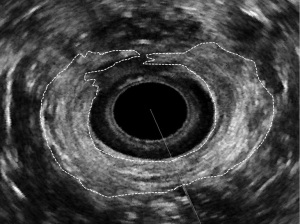
EAUS is performed with multifrequency, high-resolution, 360° rotational transducers, that differs in acquisition of 3D data (freehand or automatic) (16). After the acquisition, the 3D-data can be archived for off-line analysis. The anal canal is divided into three ultrasonographic levels of assessment: (I) the upper level, where the puborectalis muscle is visualized (PR); (II) the middle level, with the external (EAS) and internal anal sphincter (IAS); (III) the lower level, where only the subcutaneous part of the EAS can be identified (11,16) (Figure 1).
Lesions of the IAS, EAS and of the PR muscle may be isolated or combined. However, a sphincter damage is not necessarily the cause of AI (17-19). AI may be related to pudendal neuropathy (20) or primary degeneration of the IAS (21). Thakar and Sultan (22) reported that the severity of AI correlates with the size of the defects; whereas, Voyvodic et al. (23) didn’t find a relationship between sphincter injuries and the severity of incontinence symptoms.
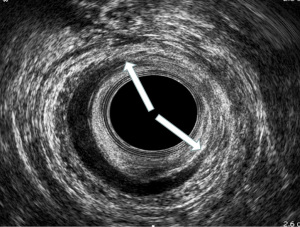
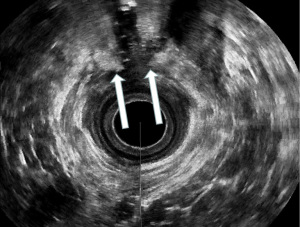
The 6th International Consultation on Incontinence (ICI, Tokyo 2017), has defined EAUS the “gold standard” technique for the assessment of anal sphincters integrity (24). It can help to differentiate incontinent patients with sphincter injuries (defects, scarring, thinning, thickening, and atrophy) from those with intact anal muscles (22,25-29) (Figures 2,3). EAUS is also useful to detect undiagnosed sphincter tears (previously defined as occult), found up to 33% of females after their first vaginal delivery (30-32).
In patients with AI, EAUS is indicated to identify damages to the anal sphincter and/or to the PF muscles. Dobben et al. (32) demonstrated physical examination was unable to detect IAS lesions and inaccurate (true positive rate 36%) to determine EAS defects lesser than 90°. Gold et al. (27) demonstrated that US had 100% sensitivity and specificity in locating the sphincter injury, and 90% accuracy in defining the topography of the lesion. Deen et al. (33) compared ultrasonographic and surgical findings in 44 patients with AI. They found 100% agreement for EAS defects and 95.5% agreement for IAS lesions. Sultan et al. (28) reported that all damages visualized by EAUS were confirmed at the time of surgery in 12 patients with AI who underwent sphincter repair. In 22 incontinent females, Sentovich et al. (34) reported 100% accuracy of EAUS in identifying the sphincter injury.
The most frequent mechanism leading to AI is childbirth trauma. Obstetric anal sphincter injuries (OASIS) is the definition of lesion that occur during vaginal delivery. Third-degree tears involve the external sphincter (3a <50% and 3b >50% of EAS thickness) or both the EAS and IAS (3c). Fourth-degree tears are injury to perineum also involving the anal epithelium. The incidence of OASIS in primiparous women ranges between 27–35% at EAUS performed within 2 months after the delivery. A new damage to the anal sphincter occur between 4% and 8.5% of multiparous females (35). OASIS increases the risk to develop AI either after childbirth or later in life. The prevalence of AI due to OASIS might be even higher. After primary sphincter repair, it develops in 15% up to 61% (mean 39%) of cases (35).
Obstetric trauma always involves the sphincters in the anterior part of the anal canal. Donnelly et al. (36) EAUS reported OASIS in 35% of first vaginal deliveries. In a review by Sultan et al. (30) EAUS found sphincter lesions in 35% of 79 primiparous females and 9 complained of incontinence to stool. In patients who delivered by cesarean section no sphincter defects were identified. Deen et al. (17) found ultrasonographic anal sphincter defects in 87% of 46 women with postpartum AI. De Parades et al. (31), in a prospective study, demonstrated that forceps delivery did not statistically increase the risk of OASIS. In this study, anal lesions were shown by EAUS in <13% of 93 healthy females after forceps delivery and were not related to AI. Perineal tear was the only significant predictive factor for anal sphincter injury. Pinta et al. (37) assessed potential risks factors associated with sphincter injury during vaginal delivery. In this study, 52 females who had had 3° and 4° OASIS were compared with 51 primiparous females who hadn’t experienced perineal lesions. EAUS detected persistent EAS defects in 39 women (75%) in the rupture group, whereas only 10 females (20%) in the control group had EAS lesions (P<0.001). Risk factor for OASIS was abnormal presentation. Oberwalder et al. (38) found occult sphincter damages at EAUS in 71% of females with late-onset AI after delivery. AI occurred at a median age of 61.5 years. The routine use of EAUS in asymptomatic patients after childbirth is controversial (24). Currently, screening women after vaginal delivery to detect occult sphincter has not been recommended. Sioutis et al. (39) reported 7% of over estimation of 3° OASIS. EAUS may play a role after sphincter reconstruction to detect residual injuries and to manage subsequent pregnancies (40) (Figure 4). Anyhow there are no systematic reviews or randomized controlled trials that suggest the best method of follow-up after OASIS.
EAUS is also useful to evaluate the outcome of sphincteroplasty (41-43), because 25–50% of patients have persistent AI due often to sub optimal repair of the anal sphincter tears. Ultrasound has demonstrated a high rate of residual defects after reconstruction. Savoye-Collet et al. (41) reported that the absence of ultrasonographic residual defect after overlapping anterior repair improved symptoms of AI in 86% (18/21) of patients. Eight of ten females with persistent sphincter defect still complained of significant incontinence symptoms. Likewise, Dobben et al. (42) also found that residual ultrasonographic EAS defects were related to worse outcome compared to patients with complete EAS reconstruction (P=0.003). Starck et al. (43) reported that the size of the defect after primary repair of OASIS increased over time and was associated to AI.
EAUS is also useful to assess the clinical efficacy of other surgical treatments (44). 3D-EAUS is able to visualize the silicone injected into the intersphincteric space to treat AI due to IAS lesion. de la Portilla et al. (45), demonstrated the correct location of all the implants at 3 months. After 24 months, 75% (33/37) of implants were still in an adequate position. In this study, recurrent incontinent symptoms affecting the majority of patients at 1-year follow-up, were not related to the number and position of the implanted agent.
Transperineal and endovaginal ultrasonography
The use of exoanal approaches, such as TPUS and EVUS remains controversial (46-50). The first description of the vaginal route to visualize the anal sphincter complex was published in 1994 by Sultan (46), in this study the 360° rotating probe used for EAUS was similarly used for EVUS, providing a clear visualization of the anal mucosa, EAS and IAS. In a prospective, observational study, 3D-EVUS detected damages of the pubovisceral muscle (PVM) in 27% of incontinent females after vaginal delivery. Severity of symptoms was correlated to the worsening of the PVM lesion and to the increased size of the levator hiatus (LH) (51). However, in another retrospective study the extent of the PVM deficiency was not significantly associated with the development of AI (52).
Transperineal US is frequently used by the urogynecologists to assess the anal sphincters. Its functional modality performed at rest, on maximal Valsalva maneuver and on PF muscle contraction (PFMC). The traditional convex, multifrequency (3 and 6 MHz) transducers with at least 70° field of view provide two-dimensional images (2D-TPUS) of the PF. In the mid-sagittal plane, the symphysis pubis (SP), all pelvic organs (bladder, urethra, vagina, uterus, anal canal and rectum) and the PR muscle are visualized. 3D-TPUS is performed with an electronic volumetric transducer, 4–8 MHz frequency, using mechanical sector technology. Compared to 2D-TPUS, this modality provides tomographic or multi-slice imaging, e.g., in the axial plane, and allows to evaluate the anal sphincters and the attachment of the levator ani muscle (LA) to the inferior pubic rami and to measure the biometric indices of the LH at rest and during Valsalva. 4D-TPUS indicates the real-time acquisition of 3D data.
Defects of at least 30° in circumference which are present in at least 4/6 tomographic slices are considered LA trauma (Figure 5). In a study on 78 females, LA damages found with 3D-TPUS had good correlation with symptoms (53). However, in another study, the same Authors did not find a significant correlation between the number of tomographic slices where the LA appeared damaged and the severity of incontinence (54). In a prospective, randomized controlled trial, TPUS was used to detect occult OASIS. The rate of occult tear increased from 3.5% (clinical finding) to 11.5% (ultrasonographical finding) (55).
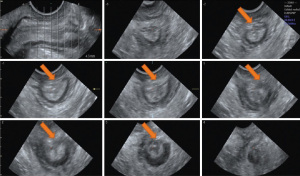
A few studies compared EVUS and TPUS with EAUS (56,57). Despite low sensitivity for the identification of sphincter defects (44% for EVUS and 50% for TPUS), these techniques, when used in combination with EAUS, provide further information on PF muscles and LH damages. In women with a history of OASIS, EVUS and TPUS are suitable to screen for an intact sphincter if EAUS is not available. In a cross-sectional study including 563 women who delivered their first child, defects of EAS and IAS were found on TPUS in 10% and 1% of cases after normal vaginal delivery, in 32% and 7% of cases after forceps delivery and in 15% and 4% of cases after vacuum delivery, respectively (58). When defects are found, women should have EAUS to verify the diagnosis (59).
MRI
The accuracy of endoanal MRI is consistent with EAUS in the detection of EAS lesions (60,61). In a retrospective study on 22 patients with AI undergoing anterior anal sphincter repair (62), endoanal MRI showed a better agreement with surgery than EAUS both for EAS (к MRI 0.85 vs. EAUS 0.53) and IAS defects (к MRI 0.64 vs. EAUS 0.49). However, in a prospective study on 52 patients, the agreement between endoanal MRI and EAUS and the final diagnosis made by an expert panel was fair (62%) (63). MRI was inferior in diagnosing IAS injuries. Agreement between these two procedures for detecting EAS lesions was also fair (κ=0.24; 146 patients/61%) in a multicenter study on 237 patients was found (64). Thirty-six patients underwent anterior sphincter repair. In this group, no significant difference between the two modalities were found (P=0.23). Sensitivity and positive predictive value were 81% and 89% for endoanal MRI and 90% and 85% for EAUS, respectively.
In patients who had ineffective sphincter repair, imaging can identify the cause of the failure. In a study on 30 incontinent patients, intact overlap and <20% fat tissue in the EAS at endoanal MRI, was associated to a better clinical outcome (65). Further, preserved EAS thickness correlated significantly with better surgical outcome. EAUS was superior to endoanal MRI in identifying residual EAS defects, which may be due to the limited experience with postoperative MRI.
The most important role of endoanal MRI is to evaluate and grade EAS atrophy, that is related to AI. In a prospective study, endoanal MRI demonstrated EAS atrophy in 123/200 patients (62%) (66). Severe atrophy was found in 44 patients (22%) and was significantly associated to lower squeeze and increment pressures compared to 79 patients (40%) with mild atrophy. This was consistent with another study (67), that demonstrated a correlation between anal squeeze pressure and EAS volume and fat content. EAS atrophy found at endoanal MRI was predictor of poor functional results after sphincter reconstruction. In a study on 20 females who had sphincter repair, 8 patients with EAS atrophy at endoanal MRI had a significant worse outcome compared to women without atrophy (42). A further study on 30 patients also confirmed that a preserved EAS volume was associated with the improvement of incontinence after surgery (60).
In a comparative study, endoanal MRI detected EAS atrophy in 8 out 20 females, whereas EAUS was unable to demonstrate atrophy (67). In another study, no differences were reported between the two modalities in identifying EAS atrophy on 18 patients, but they differed in grading it (68). Finally, West et al. (69) reported that the agreement of EAUS and endoanal MRI for the assessment of EAS atrophy in 18 incontinent patients was poor. The difficulty in determining the outer border of EAS with ultrasound, probably impairs accurate evaluation in EAS atrophy.
EAUS and endoanal MRI are consistent in the diagnosis of EAS damages. EAUS has been recommended as the gold standard modality to assess the sphincter integrity in AI for the advantages of availability, lower costs, time efficiency and patient compliance over endoanal MRI. However, MRI is the preferred method to demonstrate EAS atrophy and must be performed when considering sphincter repair. External phased array MRI can be used as alternative to endoanal MRI. This is a time-efficient tool, which has the advantage of lesser discomfort compared to the procedures that use endoanal devices. Imaging modalities are not able to select patients’ candidates to PF rehabilitation, as reported in a study on 250 patients with AI (70).
Obstructed defecation (OD)
Constipation is a common disease with prevalence reported up to 80% of the population (71). It is related to infrequent bowel movements, excessive straining, sense of incomplete evacuation, failed or lengthy attempts to defaecate, use of digital maneuvers for evacuation of stools, abdominal bloating, and hard consistency of stools. It is distinguished into two main categories: slow-transit constipation and OD, which can co-exist in the same person (72). OD is described as the difficulty in evacuation or emptying the rectum and is associated to the sensation of incomplete evacuation and/or anorectal blockage (15). Through different morphological and functional causes of OD there are: rectocele, enterocele, intussusception, PF descent and anismus.
Imaging modalities for the evaluation of OD are evacuation proctography (EP), dynamic MRI (DMRI) defecography and PF ultrasound which includes TPUS, EVUS, EAUS and echodefecography (EDF).
EP
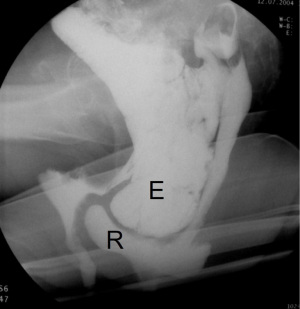
EP is a reliable, reproducible, cost-effective and highly accurate technique for the assessment of OD. It is performed in the sitting position using barium paste as rectal contrast. Oral contrast is administered one hour prior to evaluate the small bowel. The defecation process is assessed during evacuation of the contrast using X-ray. Disadvantages of this modality are: radiation exposure, invasiveness, time-consuming, embarrassment due to the necessity to defecate in a non-private setting, and visualization limited to the posterior PF compartment.
EP is useful to identify different pathological findings related to ODS such as abnormal PF descent, anismus, intussusception and rectal prolapse, rectocele, enterocele and sigmoidocele (Figure 6).
The Oxford criteria (73) provides a radiological classification of intussusception: Grade I: high recto-rectal intussusception, Grade II: low recto-rectal intussusception. In-folding descends onto the level of the rectocele; Grade III: high recto-anal intussusception; Grade IV: low recto-anal intussusception; Grade V: external rectal prolapse protruding through the anal canal.
A recent Cochrane (74) compared four imaging procedures used for the assessment OD. The meta-analyses included 39 studies covering 2,483 participants. To diagnose the posterior compartment disorders in OD patients, EP showed good sensitivity (rectocele 98%, enterocele 91%, intussusception 89% and PF descent 98%) and specificity (enterocele 96%, intussusception 92% and anismus 97%) in studies with high quality of evidence. However, the radiological findings of an anatomical defect not necessarily are related to symptoms because may be similarly visualized in asymptomatic individuals. There is no consensus on alternative investigation techniques even if DMRI defecography and ultrasound are validated for the studying of OD. In the population of women with symptoms of OD analyzed by the Cochrane, the other modalities did not meet the criteria to replace EP. DMRI defecography and TPUS met the criteria of a triage test, whereas the quality of evidence for EVUS and EDF was too low to define their clinical utilities.
EP remains the test of choice in cases of defecation disorders, but DMRI defecography and TPUS may be used as a screening test to reduce the number of EP. These two modalities identify healthy patients more reliably than EP, therefore when they are positive for the presence of an abnormality EP is not needed.
DMRI defecography
DMRI defecography has high sensitive to find the anatomic abnormalities in patients with OD. It is a dynamic investigation, and it allows to follow stools in their passage through the rectum and anus by using an endorectal contrast (ultrasound gel). Evacuation phase is performed in supine position and this is not like physiological defecation (75).
On DMRI defecography, rectocele appears as a protrusion of the anterior rectal wall and it can be graded by measuring the depth of the bulging (76,77) (Figure 7). Enterocele appears as the descent of intestinal loops or sigmoid colon into the rectovaginal space or below the pubococcygeal line (PCL), an imaginary link from the last horizontal coccygeal joint to the inferior border of the SP (76,78). Intussusception is described as a telescope image at DMRI defecography. Even if it is possible to distinguish between high and low rectal or anal intussusception through Oxford criteria, all kinds of intussusception are often grouped together (73). Anismus is diagnosed as a delay or incomplete expulsion of rectal contrast due to lack of opening of the anorectal angle (ARA) or anal canal. Kuijpers et al. described anismus as a persistent impression of the PR on the posterior rectal wall while Piloni et al. evidenced a paradoxical contraction (79-80). An incomplete or reduced release of the PR gives a more acute ARA, even if both normal and abnormal values have been reported in dyssynergy (81-83). Hainsworth et al. (84) and Pilkington et al. (85), also recognized a paradoxical pelvic floor contraction in anismus by looking at the subsequent frames. Position in the pelvic DMRI defecography is variable in the literature (85). Delemarre et al. (86) and Matsuoka et al. (87) performed the exam in the prone position and Fiaschetti et al. (88) used the upright position.
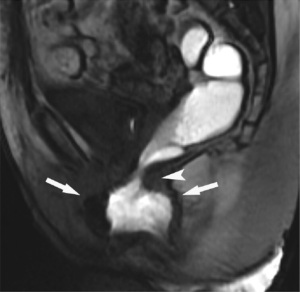
In the Cochrane by van Gruting et al. (74), high quality of evidence supported DMRI defecography as triage test in OD patients (90% specificity for rectocele, 99% for enterocele and 97% for intussusception). However, it did not meet the criteria to replace EP. Sensitivity of DMRI defecography with evacuation phase was higher than without for rectocele (94% vs. 65%) and enterocele (87% vs. 62%).
Pelvic floor ultrasound
Pelvic floor US provides real-time imaging of the pelvic structures, providing both static and dynamic information on the pelvic organs. It includes various techniques: TPUS is performed with a convex transducer placed on the perineum or translabial; EVUS is performed with a linear probe inserted into the vagina; echodefecography (EDF) is performed with a 360° rotating transducer inside the rectum and involves an evacuation phase. EVUS and EDF are performed with a high-frequency probe, which is closer to the area of interest than TPUS. High resolution provides better visualization of the PF anatomy (89).
Ultrasound is an ‘in-office’ examination and therefore can be performed by the clinician during the consultation allowing to correlate symptoms and clinical findings with the ultrasonographic findings. Advantages of US over EP and MRI are non-invasiveness, absence of ionizing radiation, relatively low cost, availability, lesser time consuming, better patient compliance, no need of bowel preparation or contrast medium. Disadvantages of US are the patient position, supine or left-lateral, that may limit the descent of pelvic organs during Valsalva, the absence of the evacuation phase as some abnormalities develops during the defecation process and the inability to assess the anterior and middle compartments when using EDF (90). In addition, PFUS is operator-dependent and needs adequate training.
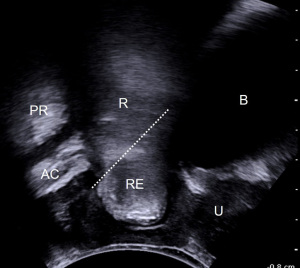
Rectocele is diagnosed by TPUS/EVUS/EDF as the protrusion over 10 mm of the anterior wall of the rectum during Valsalva (91-93) (Figure 8). The presence of a defect of the rectovaginal septum that looks like a sharp discontinuity of the anterior anorectal muscularis may also be detected (94). Stool trapping inside the rectal pocket, appearing as hyperechoic content or acoustic shadowing, is a typical finding of rectocele as cause of OD. However, the depth of the rectal bulging may be influenced by the stool consistency, and consequently may vary in the same patient. When there are no stools in the ampulla, the rectocele results smaller.
US classification of intussusception and rectal prolapse adopts the same Oxford criteria used for the radiological classification: Grade I: high intrarectal intussusception: the descending prolapse of the rectal wall stops before the upper half of PR muscle; Grade II: low intrarectal intussusception: the descending prolapse of the rectal wall stops before the lower half of PR muscle; Grade III: high rectoanal intussusception: prolapse of the rectal wall descends beyond PR muscle and onto the anal canal; Grade IV: low rectoanal intussusception: prolapse of the rectal wall descends into the anal canal but stops before the perineal body; Grade V: external rectal prolapse.
Enterocele is a slipping of the small bowel or sigmoid colon into the rectovaginal septum (94). Ultrasonographic findings are the visualization of intestinal loops, identified by peristalsis movement and hyperechoic content, into the vagina, anterior to the rectum and anal canal (Figure 9).
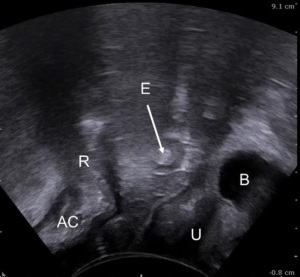
ARA measurement by US may be used to diagnose anismus. Narrowing of the ARA during Valsalva is associated with paradoxical contraction (95-97). During Valsalva maneuver in such a patient, the LH is also shortened in the anteroposterior dimension at TPUS. There are different criteria for diagnosis of PF descent: distance over 2.5 cm from the initial to the final position of anorectal junction (ARJ) during Valsalva (98), over 3.5 cm for Martellucci et al. (95), or when the PR muscle descends more than 2 cm on straining as described in Vitton et al. study (98).
According to the Cochrane by van Gruting et al. (74), high quality of evidence demonstrated that TPUS met the criteria for a triage test in OD (specificity for rectocele 89%, enterocele 98%, intussusception 96%, sensitivity for anismus 92%), but not to replace EP. Rectal contrast did not increase the sensitivity of TPUS for rectocele (92% vs. 81%), enterocele (90% vs. 67%) and intussusception (90% vs. 61%) that resulted lower than EP.
Very low to moderate quality of evidence showed that EVUS can be recommended as triage test (specificity for rectocele 76%, enterocele 97% and intussusception 93%; sensitivity for anismus 84%), but cannot replace EP.
Very low quality of evidence showed that EDF met the criteria to replace EP for the diagnosis of intussusception (sensitivity 89% and specificity 92%). With low to very low quality of evidence, EDF met the criteria for a triage test for diagnosis of rectocele (specificity 89%), enterocele (specificity 97%) and anismus (sensitivity 87%).
In OD patients, ultrasonographic techniques did not meet the criteria to replace EP. TPUS met the criteria of a triage test for the diagnosis of rectocele, enterocele, intussusception and anismus. Quality of evidence was too low to draw conclusions on EVUS and EDF. Ultrasonography, less embarrassing than EP, has a higher sensibility to identify healthy patients. It can be used as a first approach to reduce the number of women that must have an EP for correct diagnosis of OD.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Lucia Oliveira, Steven D. Wexner and Sarah A. Vogler) for the series “The Pelvic Floor and Anorectal Disorders” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://ales.amegroups.com/article/view/10.21037/ales-21-44/coif). The series “The Pelvic Floor and Anorectal Disorders” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- DeLancey JO. The anatomy of the pelvic floor. Curr Opin Obstet Gynecol 1994;6:313-6. [Crossref] [PubMed]
- Norton PA. Pelvic floor disorders: the role of fascia and ligaments. Clin Obstet Gynecol 1993;36:926-38. [Crossref] [PubMed]
- Boyles SH, Weber AM, Meyn L. Procedures for pelvic organ prolapse in the United States, 1979-1997. Am J Obstet Gynecol 2003;188:108-15. [Crossref] [PubMed]
- Boyles SH, Weber AM, Meyn L. Procedures for urinary incontinence in the United States, 1979-1997. Am J Obstet Gynecol 2003;189:70-5. [Crossref] [PubMed]
- Wu JM, Matthews CA, Conover MM, Pate V, Jonsson FM. Lifetime risk of stress urinary incontinence or pelvic organ prolapse surgery. Obstet Gynecol. 2014;123:1201-6. [Crossref] [PubMed]
- Luber KM, Boero S, Choe JY. The demographics of pelvic floor disorders: current observations and future projections. Am J Obstet Gynecol 2001;184:1496-501; discussion 1501-3. [Crossref] [PubMed]
- Wu JM, Vaughan CP, Goode PS, et al. Prevalence and trends of symptomatic pelvic floor disorders in U.S. women. Obstet Gynecol 2014;123:141-8. [Crossref] [PubMed]
- Barber MD, Maher C. Epidemiology and outcome assessment of pelvic organ prolapse. Int Urogynecol J 2013;24:1783-90. [Crossref] [PubMed]
- DeLancey JO. The hidden epidemic of pelvic floor dysfunction: achievable goals for improved prevention and treatment. Am J Obstet Gynecol 2005;192:1488-95. [Crossref] [PubMed]
- Maglinte DD, Kelvin FM, Fitzgerald K, et al. Association of compartment defects in pelvic floor dysfunction. AJR Am J Roentgenol 1999;172:439-44. [Crossref] [PubMed]
- Santoro GA, Wieczorek AP, Dietz HP, et al. State of the art: an integrated approach to pelvic floor ultrasonography. Ultrasound Obstet Gynecol 2011;37:381-96. [Crossref] [PubMed]
- Hainsworth AJ, Solanki D, Schizas AM, et al. Total pelvic floor ultrasound for pelvic floor defaecatory dysfunction: a pictorial review. Br J Radiol 2015;88:20150494. [Crossref] [PubMed]
- Hainsworth AJ, Pilkington SA, Grierson C, et al. Accuracy of integrated total pelvic floor ultrasound compared to defaecatory MRI in females with pelvic floor defaecatory dysfunction. Br J Radiol 2016;89:20160522. [Crossref] [PubMed]
- Groenendijk AG, Birnie E, de Blok S, et al. Clinical-decision taking in primary pelvic organ prolapse; the effects of diagnostic tests on treatment selection in comparison with a consensus meeting. Int Urogynecol J Pelvic Floor Dysfunct 2009;20:711-9. [Crossref] [PubMed]
- Sultan AH, Monga A, Lee J, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female anorectal dysfunction. Int Urogynecol J 2017;28:5-31. [Crossref] [PubMed]
- Santoro GA, Fortling B. The advantages of volume rendering in three-dimensional endosonography of the anorectum. Dis Colon Rectum 2007;50:359-68. [Crossref] [PubMed]
- Deen KI, Kumar D, Williams JG, et al. The prevalence of anal sphincter defects in fecal incontinence. A prospective endosonic study. Gut. 1993;34:685-8. [Crossref] [PubMed]
- Abramowitz L, Sobhani I, Ganansia R, et al. Are sphincter defects the cause of anal incontinence after vaginal delivery? Results of a prospective study. Dis Colon Rectum 2000;43:590-6; discussion 596-8. [Crossref] [PubMed]
- Felt-Bersma RJ, van Baren R, Koorevaar M, et al. Unsuspected sphincter defects shown by anal endosonography after anorectal surgery. A prospective study. Dis Colon Rectum 1995;38:249-53. [Crossref] [PubMed]
- Snooks SJ, Setchell M, Swash M, et al. Injury to innervation of pelvic floor sphincter musculature in childbirth. Lancet 1984;2:546-50. [Crossref] [PubMed]
- Vaizey CJ, Kamm MA, Bartram CI. Primary degeneration of the internal anal sphincter as a cause of passive faecal incontinence. Lancet 1997;349:612-5. [Crossref] [PubMed]
- Thakar R, Sultan AH. Anal endosonography and its role in assessing the incontinent patient. Best Pract Res Clin Obstet Gynaecol 2004;18:157-73. [Crossref] [PubMed]
- Voyvodic F, Rieger NA, Skinner S, et al. Endosonographic imaging of anal sphincter injury: does the size of the tear correlate with the degree of dysfunction? Dis Colon Rectum 2003;46:735-41. [Crossref] [PubMed]
- Bliss DJ, Mimura T, Berghmans B, et al. Assessment and conservative management of faecal incontinence and quality of life in adults. In: Abrams P, Cardozo L, Wagg A, et al. editors. Incontinence, ICUD ICS, 6th Edition. Bristol: International Continence Society; 2017: 1993-2085.
- Stoker J, Halligan S, Bartram CI. Pelvic floor imaging. Radiology 2001;218:621-41. [Crossref] [PubMed]
- Frudinger A, Halligan S, Bartram CI, et al. Female anal sphincter: age-related differences in asymptomatic volunteers with high-frequency endoanal US. Radiology 2002;224:417-23. [Crossref] [PubMed]
- Gold DM, Halligan S, Kmiot WA, et al. Intraobserver and interobserver agreement in anal endosonography. Br J Surg 1999;86:371-5. [Crossref] [PubMed]
- Sultan AH, Kamm MA, Talbot IC, et al. Anal endosonography for identifying external sphincter defects confirmed histologically. Br J Surg 1994;81:463-5. [Crossref] [PubMed]
- Nielsen MB, Hauge C, Pedersen JF, et al. Endosonographic evaluation of patients with anal incontinence: findings and influence on surgical management. AJR Am J Roentgenol 1993;160:771-5. [Crossref] [PubMed]
- Sultan AH, Kamm MA, Hudson CN, et al. Anal-sphincter disruption during vaginal delivery. N Engl J Med 1993;329:1905-11. [Crossref] [PubMed]
- de Parades V, Etienney I, Thabut D, et al. Anal sphincter injury after forceps delivery: myth or reality? A prospective ultrasound study of 93 females. Dis Colon Rectum 2004;47:24-34. [Crossref] [PubMed]
- Dobben AC, Terra MP, Deutekom M, et al. Anal inspection and digital rectal examination compared to anorectal physiology tests and endoanal ultrasonography in evaluating fecal incontinence. Int J Colorectal Dis 2007;22:783-90. [Crossref] [PubMed]
- Deen KI, Kumar D, Williams JG, et al. Anal sphincter defects. Correlation between endoanal ultrasound and surgery. Ann Surg 1993;218:201-5. [Crossref] [PubMed]
- Sentovich SM, Wong WD, Blatchford GJ. Accuracy and reliability of transanal ultrasound for anterior anal sphincter injury. Dis Colon Rectum 1998;41:1000-4. [Crossref] [PubMed]
- Harvey MA, Pierce M, Alter JE, et al. Obstetrical Anal Sphincter Injuries (OASIS): Prevention, Recognition, and Repair. J Obstet Gynaecol Can 2015;37:1131-48. Erratum in: J Obstet Gynaecol Can 2016;38:421. [Crossref] [PubMed]
- Donnelly V, Fynes M, Campbell D, et al. Obstetric events leading to anal sphincter damage. Obstet Gynecol 1998;92:955-61. [PubMed]
- Pinta TM, Kylänpää ML, Salmi TK, et al. Primary sphincter repair: are the results of the operation good enough? Dis Colon Rectum 2004;47:18-23. [Crossref] [PubMed]
- Oberwalder M, Dinnewitzer A, Baig MK, et al. The association between late-onset fecal incontinence and obstetric anal sphincter defects. Arch Surg 2004;139:429-32. [Crossref] [PubMed]
- Sioutis D, Thakar R, Sultan AH. Overdiagnosis and rising rate of obstetric anal sphincter injuries (OASIS): time for reappraisal. Ultrasound Obstet Gynecol 2017;50:642-7. [Crossref] [PubMed]
- Fitzpatrick M, Cassidy M, Barassaud ML, et al. Does anal sphincter injury preclude subsequent vaginal delivery? Eur J Obstet Gynecol Reprod Biol 2016;198:30-4. [Crossref] [PubMed]
- Savoye-Collet C, Savoye G, Koning E, et al. Anal endosonography after sphincter repair: specific patterns related to clinical outcome. Abdom Imaging 1999;24:569-73. [Crossref] [PubMed]
- Dobben AC, Terra MP, Deutekom M, et al. The role of endoluminal imaging in clinical outcome of overlapping anterior anal sphincter repair in patients with fecal incontinence. AJR Am J Roentgenol 2007;189:W70-7. [Crossref] [PubMed]
- Starck M, Bohe M, Valentin L. The extent of endosonographic anal sphincter defects after primary repair of obstetric sphincter tears increases over time and is related to anal incontinence. Ultrasound Obstet Gynecol 2006;27:188-97. [Crossref] [PubMed]
- Soerensen MM, Lundby L, Buntzen S, et al. Intersphincteric injected silicone biomaterial implants: a treatment for faecal incontinence. Colorectal Dis 2009;11:73-6. [Crossref] [PubMed]
- de la Portilla F, Vega J, Rada R, et al. Evaluation by three-dimensional anal endosonography of injectable silicone biomaterial (PTQ) implants to treat fecal incontinence: long-term localization and relation with the deterioration of the continence. Tech Coloproctol 2009;13:195-9. [Crossref] [PubMed]
- Sultan AH, Loder PB, Bartram CI, et al. Vaginal endosonography. New approach to image the undisturbed anal sphincter. Dis Colon Rectum 1994;37:1296-9. [Crossref] [PubMed]
- Frudinger A, Bartram CI, Kamm MA. Transvaginal versus anal endosonography for detecting damage to the anal sphincter. AJR Am J Roentgenol 1997;168:1435-8. [Crossref] [PubMed]
- Roos AM, Abdool Z, Sultan AH, et al. The diagnostic accuracy of endovaginal and transperineal ultrasound for detecting anal sphincter defects: The PREDICT study. Clin Radiol 2011;66:597-604. [Crossref] [PubMed]
- Peschers UM, DeLancey JO, Schaer GN, et al. Exoanal ultrasound of the anal sphincter: normal anatomy and sphincter defects. Br J Obstet Gynaecol 1997;104:999-1003. [Crossref] [PubMed]
- Meriwether KV, Hall RJ, Leeman LM, et al. The relationship of 3-D translabial ultrasound anal sphincter complex measurements to postpartum anal and fecal incontinence. Int Urogynecol J 2015;26:1191-9. [Crossref] [PubMed]
- Murad-Regadas SM, Fernandes GO, Regadas FS, et al. Assessment of pubovisceral muscle defects and levator hiatal dimensions in women with faecal incontinence after vaginal delivery: is there a correlation with severity of symptoms? Colorectal Dis 2014;16:1010-8. [Crossref] [PubMed]
- Rostaminia G, White D, Quiroz LH, et al. 3D pelvic floor ultrasound findings and severity of anal incontinence. Int Urogynecol J 2014;25:623-9. [Crossref] [PubMed]
- Dietz HP. Quantification of major morphological abnormalities of the levator ani. Ultrasound Obstet Gynecol 2007;29:329-34. [Crossref] [PubMed]
- Dietz HP, Shek KL. Tomographic ultrasound imaging of the pelvic floor: which levels matter most? Ultrasound Obstet Gynecol 2009;33:698-703. [Crossref] [PubMed]
- Ozyurt S, Aksoy H, Gedikbasi A, et al. Screening occult anal sphincter injuries in primigravid women after vaginal delivery with transperineal use of vaginal probe: a prospective, randomized controlled trial. Arch Gynecol Obstet 2015;292:853-9. [Crossref] [PubMed]
- Oom DM, West RL, Schouten WR, et al. Detection of anal sphincter defects in female patients with fecal incontinence: a comparison of 3-dimensional transperineal ultrasound and 2-dimensional endoanal ultrasound. Dis Colon Rectum 2012;55:646-52. [Crossref] [PubMed]
- Meriwether KV, Hall RJ, Leeman LM, et al. Anal sphincter complex: 2D and 3D endoanal and translabial ultrasound measurement variation in normal postpartum measurements. Int Urogynecol J 2015;26:511-7. [Crossref] [PubMed]
- Guzmán Rojas RA, Salvesen KÅ, Volløyhaug I. Anal sphincter defects and fecal incontinence 15-24 years after first delivery: a cross-sectional study. Ultrasound Obstet Gynecol 2018;51:677-83. [Crossref] [PubMed]
- Taithongchai A, van Gruting IMA, Volløyhaug I, et al. Comparing the diagnostic accuracy of 3 ultrasound modalities for diagnosing obstetric anal sphincter injuries. Am J Obstet Gynecol 2019;221:134.e1-9. [Crossref] [PubMed]
- deSouza NM, Puni R, Zbar A, et al. MR imaging of the anal sphincter in multiparous women using an endoanal coil: correlation with in vitro anatomy and appearances in fecal incontinence. AJR Am J Roentgenol 1996;167:1465-71. [Crossref] [PubMed]
- deSouza NM, Hall AS, Puni R, et al. High resolution magnetic resonance imaging of the anal sphincter using a dedicated endoanal coil. Comparison of magnetic resonance imaging with surgical findings. Dis Colon Rectum 1996;39:926-34. [Crossref] [PubMed]
- Rociu E, Stoker J, Eijkemans MJ, et al. Fecal incontinence: endoanal US versus endoanal MR imaging. Radiology 1999;212:453-8. [Crossref] [PubMed]
- Malouf AJ, Williams AB, Halligan S, et al. Prospective assessment of accuracy of endoanal MR imaging and endosonography in patients with fecal incontinence. AJR Am J Roentgenol 2000;175:741-5. [Crossref] [PubMed]
- Dobben AC, Terra MP, Slors JFM, et al. External anal sphincter defects in patients with fecal incontinence. Comparison of endoanal MR imaging and endoanal US. Radiology 2007;242:463-71. [Crossref] [PubMed]
- Terra MP, Deutekom M, Beets-Tan RG, et al. Relationship between external anal sphincter atrophy at endoanal magnetic resonance imaging and clinical, functional, and anatomic characteristics in patients with fecal incontinence. Dis Colon Rectum 2006;49:668-78. [Crossref] [PubMed]
- Williams AB, Bartram CI, Modhwadia D, et al. Endocoil magnetic resonance imaging quantification of external anal sphincter atrophy. Br J Surg 2001;88:853-9. [Crossref] [PubMed]
- Briel JW, Stoker J, Rociu E, et al. External anal sphincter atrophy on endoanal magnetic resonance imaging adversely affects continence after sphincteroplasty. Br J Surg 1999;86:1322-7. [Crossref] [PubMed]
- Cazemier M, Terra MP, Stoker J, et al. Atrophy and defects detection of the external anal sphincter: comparison between three-dimensional anal endosonography and endoanal magnetic resonance imaging. Dis Colon Rectum 2006;49:20-7. [Crossref] [PubMed]
- West RL, Dwarkasing S, Briel JW, et al. Can three-dimensional endoanal ultrasonography detect external anal sphincter atrophy? A comparison with endoanal magnetic resonance imaging. Int J Colorectal Dis 2005;20:328-33. [Crossref] [PubMed]
- Terra MP, Deutekom M, Dobben AC, et al. Can the outcome of pelvic-floor rehabilitation in patients with fecal incontinence be predicted? Int J Colorectal Dis 2008;23:503-11. [Crossref] [PubMed]
- Forootan M, Bagheri N, Darvishi M. Chronic constipation: A review of literature. Medicine (Baltimore) 2018;97:e10631. [Crossref] [PubMed]
- Lembo A, Camilleri M. Chronic constipation. N Engl J Med 2003;349:1360-8. [Crossref] [PubMed]
- Collinson R, Cunningham C, D'Costa H, et al. Rectal intussusception and unexplained faecal incontinence: findings of a proctographic study. Colorectal Dis 2009;11:77-83. [Crossref] [PubMed]
- van Gruting IM, Stankiewicz A, Thakar R, et al. Imaging modalities for the detection of posterior pelvic floor disorders in women with obstructed defaecation syndrome. Cochrane Database Syst Rev 2021;9:CD011482. [PubMed]
- Bertschinger KM, Hetzer FH, Roos JE, et al. Dynamic MR imaging of the pelvic floor performed with patient sitting in an open-magnet unit versus with patient supine in a closed-magnet unit. Radiology 2002;223:501-8. [Crossref] [PubMed]
- Lienemann A, Anthuber C, Baron A, et al. Dynamic MR colpocystorectography assessing pelvic-floor descent. Eur Radiol 1997;7:1309-17. [Crossref] [PubMed]
- Mellgren A, Bremmer S, Johansson C, et al. Defecography. Results of investigations in 2,816 patients. Dis Colon Rectum 1994;37:1133-41. [Crossref] [PubMed]
- Pescatori M, Spyrou M, Pulvirenti d'Urso A. A prospective evaluation of occult disorders in obstructed defecation using the 'iceberg diagram'. Colorectal Dis 2006;8:785-9. [Crossref] [PubMed]
- Kuijpers HC, Bleijenberg G. The spastic pelvic floor syndrome. A cause of constipation. Dis Colon Rectum 1985;28:669-72. [Crossref] [PubMed]
- Piloni V, Tosi P, Vernelli M. MR-defecography in obstructed defecation syndrome (ODS): technique, diagnostic criteria and grading. Tech Coloproctol 2013;17:501-10. [Crossref] [PubMed]
- Ferrante SL, Perry RE, Schreiman JS, et al. The reproducibility of measuring the anorectal angle in defecography. Dis Colon Rectum 1991;34:51-5. [Crossref] [PubMed]
- Halligan S, Bartram CI, Park HJ, et al. Proctographic features of anismus. Radiology 1995;197:679-82. [Crossref] [PubMed]
- Shorvon PJ, McHugh S, Diamant NE, et al. Defecography in normal volunteers: results and implications. Gut 1989;30:1737-49. [Crossref] [PubMed]
- Hainsworth AJ, Solanki D, Morris SJ, et al. Is there any association between symptoms and findings on imaging in pelvic floor defaecatory dysfunction? A prospective study. Colorectal Dis 2021;23:237-45. [Crossref] [PubMed]
- Pilkington SA, Nugent KP, Brenner J, et al. Barium proctography vs magnetic resonance proctography for pelvic floor disorders: a comparative study. Colorectal Dis 2012;14:1224-30. [Crossref] [PubMed]
- Delemarre JB, Kruyt RH, Doornbos J, et al. Anterior rectocele: assessment with radiographic defecography, dynamic magnetic resonance imaging, and physical examination. Dis Colon Rectum 1994;37:249-59. [Crossref] [PubMed]
- Matsuoka H, Desai MB, Wexner SD, et al. A pilot assessment of whether external coil MRI is useful to assess evacuatory disorders. Int J Colorectal Dis 2000;15:91-5. [Crossref] [PubMed]
- Fiaschetti V, Pastorelli D, Squillaci E, et al. Static and dynamic evaluation of pelvic floor disorders with an open low-field tilting magnet. Clin Radiol 2013;68:e293-300. [Crossref] [PubMed]
- Wieczorek AP, Stankiewicz A, Santoro GA, et al. Pelvic floor disorders: role of new ultrasonographic techniques. World J Urol 2011;29:615-23. [Crossref] [PubMed]
- Regadas FS, Haas EM, Abbas MA, et al. Prospective multicenter trial comparing echodefecography with defecography in the assessment of anorectal dysfunction in patients with obstructed defecation. Dis Colon Rectum 2011;54:686-92. [Crossref] [PubMed]
- Shobeiri SA, White D, Quiroz LH, et al. Anterior and posterior compartment 3D endovaginal ultrasound anatomy based on direct histologic comparison. Int Urogynecol J 2012;23:1047-53. [Crossref] [PubMed]
- Beer-Gabel M, Teshler M, Schechtman E, et al. Dynamic transperineal ultrasound vs. defecography in patients with evacuatory difficulty: a pilot study. Int J Colorectal Dis 2004;19:60-7. [Crossref] [PubMed]
- Dietz HP, Steensma AB. Posterior compartment prolapse on two-dimensional and three-dimensional pelvic floor ultrasound: the distinction between true rectocele, perineal hypermobility and enterocele. Ultrasound Obstet Gynecol 2005;26:73-7. [Crossref] [PubMed]
- Beer-Gabel M, Assoulin Y, Amitai M, et al. A comparison of dynamic transperineal ultrasound (DTP-US) with dynamic evacuation proctography (DEP) in the diagnosis of cul de sac hernia (enterocele) in patients with evacuatory dysfunction. Int J Colorectal Dis 2008;23:513-9. [Crossref] [PubMed]
- Martellucci J, Naldini G. Clinical relevance of transperineal ultrasound compared with evacuation proctography for the evaluation of patients with obstructed defaecation. Colorectal Dis 2011;13:1167-72. [Crossref] [PubMed]
- Murad-Regadas SM, Regadas FS, Rodrigues LV, et al. A novel three-dimensional dynamic anorectal ultrasonography technique (echodefecography) to assess obstructed defecation, a comparison with defecography. Surg Endosc 2008;22:974-9. [Crossref] [PubMed]
- Murad-Regadas SM, dos Santos D, Soares G, et al. A novel three-dimensional dynamic anorectal ultrasonography technique for the assessment of perineal descent, compared with defaecography. Colorectal Dis 2012;14:740-7. [Crossref] [PubMed]
- Vitton V, Vignally P, Barthet M, et al. Dynamic anal endosonography and MRI defecography in diagnosis of pelvic floor disorders: comparison with conventional defecography. Dis Colon Rectum 2011;54:1398-404. [Crossref] [PubMed]
Cite this article as: Santoro GA, Colangelo AC, Pelizzo P, Cian R, Zanus G. Imaging modalities for pelvic floor disorders. Ann Laparosc Endosc Surg 2022;7:13.