Early gastric cancer: results in a Western area without a population-based screening program and minimal invasive treatment
Introduction
Forlì, a medium-sized Italian town with a population of about 120,000, is situated in a high incidence area for gastric cancer by Western standards. Recent epidemiological data for this area highlighted a gastric cancer incidence of 27.2 males and 14.7 females per 100,000 inhabitants (1,2). Although no mass screening has ever been proposed for residents, clinicians and general practitioners (GPs) work closely together to detect signs of early gastric disease. High priority is given to the patient’s first endoscopy, which GPs can rapidly organize on the basis of common healthcare protocols (3).
The aim of this study is to present our experience in early gastric cancer (EGC) or dysplasia detection and treatment suggesting a close collaboration between different hospital units such as gastroenterology, pathology and general surgery.
Moreover, we underline the importance of the missing lesions detected by endoscopic services as a quality index.
We present the following article in accordance with the STROBE reporting checklist (available at https://ales.amegroups.com/article/view/10.21037/ales-21-30/rc).
Methods
Morgagni-Pierantoni Hospital in Forlì serves a catchment area of 180,000 inhabitants. The Gastroenterology Unit of the hospital, the only one in the area, performs an average of 5,000 gastroscopies each year. Close cooperation with GPs permits timely access to the endoscopy service when patients present with suspicious symptoms. First-step endoscopy, often associated with chromoendoscopy or flexible spectral imaging color enhancement (FICE) on all suspicious lesions are carefully mapped and biopsied. A second-look endoscopy after few days may be required to confirm suspected findings. This approach permits a high number of dysplastic lesions or EGCs to be detected (4-8). The incidence of missed lesions, defined as patients who had undergone on endoscopy within 3 years before the diagnosis (9), was used to assess endoscopic accuracy.
After histologic confirmation of biopsy specimens with Lauren classification (10), if all criteria for endoscopic dissection [as indicated by the Japanese Gastric Cancer Association (JGCA)] are satisfied, endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD) can be proposed (3,11). Patients considered as radically treated with endoscopy undergo at our institution, a follow-up consisting of endoscopic check up every 3 months for the first year and every 2 years thereafter. All other patients are submitted to gastrectomy with D2 lymphatic dissection according to JGCA guidelines and considered for robotic access. Subtotal gastrectomy is performed for distal EGC when free resection margins can be guaranteed at a distance of 2 cm from the lesion, while total gastrectomy is performed for tumors in the upper third of the stomach.
We report our experience on gastric cancer during the period 2001–2011. Morbidity and mortality rates within the first 30 days of endoscopic or surgical treatments are reported. All patients treated were submitted to follow-up and those who died for non-cancer-related causes were considered as censored.
Statistical analysis
Data were analyzed using MedCalc Statistical Software version 15.8 (MedCalc Software bvba, Ostend, Belgium; https://www.medcalc.org; 2015). Continuous variables were shown as median while categorical data were presented as numbers and percentages. Survival was analysed using the Kaplan-Meier product limit method; all P values were based on two-sided testing (threshold value =0.05) (12).
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional/regional/national ethics/committee/ethics board of Romagna (protocol code 5707/2020, 03/07/2020) and individual consent for this retrospective analysis was waived.
Results
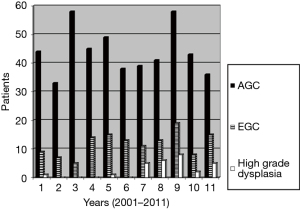
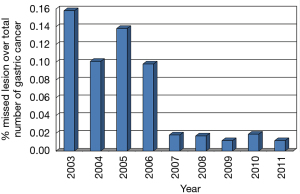
During the period 2001–2011, 51,381 patients underwent gastroscopy at the Gastroenterological Unit of our hospital and 641 gastric cancers or high-grade dysplasias (HGDs) were diagnosed, representing 1.2% of all the endoscopies performed. Taking into consideration false negative endoscopies, the number of missed lesions decreased from 15.8% in 2003 to 1.6% in 2011 (Figure 1). The missing lesions were generally intestinal forms (70% of cases), diagnosed with a delay of 16 months in 2003 increased to 25 months in 2011; generally, they were diagnosed as advanced stage (≥ T2) in 92.6%. One hundred and eighty (28%) gastric cancers detected by our endoscopists were early lesions, of which 41 were HGDs and 139 EGCs (Figure 2). Lesion’s characteristics are summarised in Table 1. Endoscopic gastric resection was first performed at our hospital in 2006 and 51 patients underwent the procedure during the study period, 13 submitted to EMR and 38 to ESD (Figure 3).
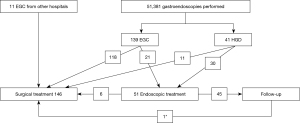
Table 1
| Characteristics | No. of EMRs performed | No. of ESDs performed | No. of patients surgically treated |
|---|---|---|---|
| Total | 13 | 38 | 146 |
| T | |||
| T1a | 6 | 13 | 64 |
| T1b | 0 | 2 | 71 |
| High-grade dysplasia | 7 | 23 | 11 |
| Site | |||
| Upper third | 0 | 0 | 19 |
| Middle third | 4 | 5 | 45 |
| Lower third | 8 | 33 | 78 |
| Stump anastomosis | 1 | 0 | 4 |
| Size, mm | |||
| ≤10 | 3 | 3 | 23 |
| >10, ≤20 | 8 | 27 | 34 |
| >20, ≤30 | 3 | 5 | 41 |
| >30, ≤40 | 0 | 2 | 29 |
| >40 | 0 | 1 | 19 |
| Macroscopic type | |||
| I, IIa | 11 | 30 | 39 |
| IIb, IIc | 2 | 8 | 76 |
| III | 0 | 0 | 31 |
| Histotype | |||
| Intestinal | 6 | 15 | 107 |
| Diffuse | 0 | 0 | 28 |
| Mix | 0 | 0 | 0 |
| High-grade dysplasia | 7 | 23 | 11 |
EMR, endoscopic mucosal resection; ESD, endoscopic submucosal dissection.
One case of perforation occurred among patients treated endoscopically. The patient was submitted to laparoscopic suturing of the perforation whilst waiting for the histological diagnosis, which confirmed that the endoscopic treatment had been radical. Two patients showed delayed bleeding, successfully treated by endoscopic haemostasis. No deaths occurred in the endoscopically-treated group.
On the basis of histology, 45 of the 51 patients submitted to EMR/ESD for neoplastic lesions were considered as radically cured, while the remaining 6 patients, not fulfilling JGCA criteria, underwent further surgical treatment. During the same period, 146 patients (135 referred by our endoscopist, including the above 6 endoscopically treated cases and 11 patients diagnosed elsewhere) not satisfying criteria for endoscopic resection were surgically treated. One hundred and forty-six were surgically treated and of these, 121 patients with EGC of the middle or distal third of the stomach were submitted to subtotal gastrectomy and 25 to total gastrectomy.
Twenty-five of the surgically treated patients underwent robotic surgery considered as a better option to dissect lymphatic D2 stations with a few number of conversions. A median of 26 (range, 9–60) lymph nodes were removed and a median of 7 stations were dissected (not considering stations where only fatty tissue was detected).
No deaths occurred within the first 30 days. However, one patient with preoperative kidney and respiratory co-morbidities died from respiratory failure 2 months later after hospital re-admission. Morbidity for surgically resected patients was 18.2%. Pneumonia, bleeding, medically treated anastomotic leaks, pancreatic leaks and embolism were the most important surgical morbidities reported.
All patients underwent follow-up for a median of 32.6 (range, 1–132) months.
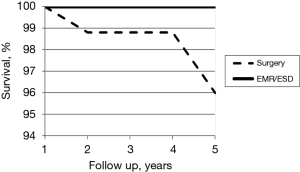
During that period, HGD was diagnosed in one of the endoscopically treated patients, while EGC was detected in another patient 2 years after ESD. The latter was probably a metachronous cancer, highlighting the importance of close monitoring of all patients. All endoscopically treated patients were alive after 5 years, while those surgically treated had a 5-year survival of 96% (P=0.63) (Figure 1).
Discussion
Mass gastric cancer screening programs such as those used in Japan have greatly increased the number of EGCs detected. However, such programs are not feasible in western countries because of the high costs involved and the generally lower and gradually decreasing incidence of this tumor. A careful endoscopic policy with enough time for each endoscopy, chromoendoscopy or FICE and multidisciplinary discussion of the suspected patients may increase the number of EGC lesions. Moreover, continuous check-up of the missing lesions may improve accuracy and quality in endoscopic service; indeed, our study shows that better results, may be observed also where a lot of early lesions are already detected (Figure 2).
The number of missed lesions in our opinion may be taken into account as quality index of an endoscopy service.
This index, which can be reduced by improving technical skills and service organization, is a reliable indicator of the quality of the endoscopy service. Innovative endoscopic tools currently used in our Endoscopy unit for suspicious lesions are chromoendoscopy and novel imaging techniques such as spectral analysis. Appropriate staff training is also crucial and facilitated by quality improvement projects based around staff training in the use of colour enhancement for endoscopy and endoscopic magnification, weekly workshops using educational DVDs (13) and regular seminars using internal photo libraries to discuss the most significant endoscopic examinations performed in our Endoscopy Unit. Using this method, each member of the team has become aware of his/her own detection rate of gastric dysplasia and EGC.
A better understanding of mucosal surface alterations has also changed the way in which biopsies are obtained. Although randomized biopsies are often taken (14), it is now believed to be more useful to obtain biopsy specimens from suspicious areas revealed by white light and advanced endoscopy. Early diagnosis, albeit an expensive and often time-consuming procedure, is the most important prognostic factor in gastric cancer (15,16) and represents an important objective for endoscopists, clinicians and the healthcare system in general.
EMR/ESD is the best treatment for EGC, especially with regard to quality of life, but strict criteria must be satisfied during patient selection and close collaboration is needed between endoscopists, surgeons and pathologists. If conditions for endoscopic treatment are met, these procedures result in lower morbidity and mortality and a better quality of life than those obtained by surgical treatment. Nonetheless, follow-up is mandatory. All other patients must be surgically treated, and our own patients surgically treated at our hospital during the study period showed a 5-year survival of 96% (Figure 4). Good 10-year follow-up results from surgical treatment were also seen in previous larger studies carried out by our group, with 89% survival, 14% morbidity and 1.3% perioperative mortality (15,16).
Mini-invasive surgery is now the preferred treatment for early lesions and robot-assisted surgical procedures is our preferred treatment for this patient. Although limited lymphatic dissection is proposed by Japanese surgeons for this subgroup (3), we prefer to perform D2 dissection to avoid the risk of undertreatment which represents a high-risk factor for patients who could otherwise be cured. The risk of morbidity and mortality, albeit low, must of course be taken into account in surgical treatment, but this is the only option that can guarantee lymphatic dissection and long-term survival when lymph node metastases are suspected.
We are aware of this retrospective study’s limitations with a low number of patients, but it represents sone of the few western experience with a good number of cases observed in a single center.
Conclusions
Screening is not the only means of achieving good rates of EGC detection. Close collaboration with GPs who facilitate rapid access to the first endoscopy, specific training to increase the skill of endoscopists, and accuracy of the patient’s first endoscopy, can lead to a reduction in the number of missed lesions, which could be proposed as a sort of service quality index. Our findings confirm that endoscopic treatments can also be performed in western countries and that a mini-invasive surgical treatment may be proposed for all patients without endoscopic criteria for radical resection.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Stefano Rausei and Simone Giacopuzzi) for the series “Minimally Invasive Surgery and Gastric Cancer: Where Are We Now?” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://ales.amegroups.com/article/view/10.21037/ales-21-30/rc
Data Sharing Statement: Available at https://ales.amegroups.com/article/view/10.21037/ales-21-30/dss
Peer Review File: Available at https://ales.amegroups.com/article/view/10.21037/ales-21-30/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://ales.amegroups.com/article/view/10.21037/ales-21-30/coif). The series “Minimally Invasive Surgery and Gastric Cancer: Where Are We Now?” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional/regional/national ethics/committee/ethics board of Romagna (protocol code 5707/2020, 03/07/2020) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- AIRTUM-AIOM. I Numeri Del Cancro Gastrico in Italia. 2011th edition. Intermedia editore, 2011.
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [Crossref] [PubMed]
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 2011;14:113-23. [Crossref] [PubMed]
- Kato M, Kaise M, Yonezawa J, et al. Magnifying endoscopy with narrow-band imaging achieves superior accuracy in the differential diagnosis of superficial gastric lesions identified with white-light endoscopy: a prospective study. Gastrointest Endosc 2010;72:523-9. [Crossref] [PubMed]
- Ohashi A, Niwa Y, Ohmiya N, et al. Quantitative analysis of the microvascular architecture observed on magnification endoscopy in cancerous and benign gastric lesions. Endoscopy 2005;37:1215-9. [Crossref] [PubMed]
- Tajiri H, Doi T, Endo H, et al. Routine endoscopy using a magnifying endoscope for gastric cancer diagnosis. Endoscopy 2002;34:772-7. [Crossref] [PubMed]
- Otsuka Y, Niwa Y, Ohmiya N, et al. Usefulness of magnifying endoscopy in the diagnosis of early gastric cancer. Endoscopy 2004;36:165-9. [Crossref] [PubMed]
- Simone A, Casadei A, De Vergori E, et al. Rescue endoscopy to identify site of gastric dysplasia or carcinoma found at random biopsies. Dig Liver Dis 2011;43:721-5. [Crossref] [PubMed]
- Yalamarthi S, Witherspoon P, McCole D, et al. Missed diagnoses in patients with upper gastrointestinal cancers. Endoscopy 2004;36:874-9. [Crossref] [PubMed]
- Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. an attempt at a histo-clinical classification. Acta Pathol Microbiol Scand 1965;64:31-49. [Crossref] [PubMed]
- Gotoda T, Yanagisawa A, Sasako M, et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer 2000;3:219-25. [Crossref] [PubMed]
- Kaplan EL, Meier P. Nonparametric Estimation from Incomplete Observations. J Am Stat Assoc 1958;53:457-81. [Crossref]
- Hamanaka H, Gotoda T, Yeh R, et al. Diagnosis and Treatment of Early Gastric Cancer; ASGE Endoscopic Learning Library DVD, 2005.
- Rabenstein T, May A, Gossner L, et al. Invisible gastric carcinoma detected by random biopsy: long-term results after photodynamic therapy. Endoscopy 2008;40:899-904. [Crossref] [PubMed]
- Folli S, Morgagni P, Roviello F, et al. Risk factors for lymph node metastases and their prognostic significance in early gastric cancer (EGC) for the Italian Research Group for Gastric Cancer (IRGGC). Jpn J Clin Oncol 2001;31:495-9. [Crossref] [PubMed]
- Roviello F, Rossi S, Marrelli D, et al. Number of lymph node metastases and its prognostic significance in early gastric cancer: a multicenter Italian study. J Surg Oncol 2006;94:275-80; discussion 274. [Crossref] [PubMed]
Cite this article as: Morgagni P, Vittimberga G, Casadei A, Manzi I, Framarini M, D’Acapito F, Saragoni L. Early gastric cancer: results in a Western area without a population-based screening program and minimal invasive treatment. Ann Laparosc Endosc Surg 2022;7:2.