Minimally invasive gastrectomy after neoadjuvant chemotherapy: a literature review
Introduction
Background
Gastric cancer is one of the most common malignant diseases and the third cause of cancer-related deaths worldwide (1). Its prevalence varies widely among countries. The highest incidence occurs in East Asia, whereas in Western countries, the incidence is <10 cases per 100,000 inhabitants (2).
In recent decades, much progress has been made in managing gastric cancer. While gastrectomies are the mainstay of treatment, perioperative chemotherapy has been shown to enhance oncologic outcomes in patients with locally advanced gastric cancer (LAGC) in Western countries compared with surgery alone (3,4). Minimally invasive (MI) surgical approaches for early cancers have been standardised.
In 1994, Kitano et al. performed the first reported laparoscopic distal gastrectomy (LDG) with a modified D1 lymph node dissection (5). Many studies followed this first experience, demonstrating the feasibility of MI gastrectomies and comparing their advantages and disadvantages to open surgery (6-9). The outcomes of these studies showed that laparoscopic gastrectomies (LGs) allow faster recovery, less pain, shorter hospital stays, an improved postoperative quality of life, and equal outcomes of morbidity and mortality compared with those of open gastrectomies (OGs) (6-9). Therefore, laparoscopies are widely used, mainly in Eastern countries, to treat distal early gastric cancers. Several randomized studies have confirmed their safety and advantages, and LG has been introduced in the Japanese Gastric Cancer Treatment Guidelines (10) for treating stage I distal cancers.
Studies applying MI gastrectomies for advanced cancers have also been conducted. A multicentre randomized controlled trial (RCT) of stage II/III gastric cancer (JLSSG0901) from the Japanese Laparoscopic Surgery Study Group (JLSGG) was conducted to confirm the feasibility of LDG in terms of technical safety and short-term surgical outcomes (11). No statistical differences were found between LDG and traditional surgery.
The CLASS-01 trial (12), a multicentre randomized clinical trial, examined the surgical and oncological safety of LDG for LAGC. The trial showed no statistically significant differences in the 3-year disease-free survival rates between OG and LG for advanced cancers (77.8% vs. 76.5%). The laparoscopic group had a similar complication rate and a faster postoperative recovery compared with that of OG. Additionally, the KLASS-02 (13) trial, a phase-III multicentre RCT in Korea, revealed no difference in terms of oncological radicality of the procedures (i.e., the number of lymph nodes retrieved and R0 resections). Patients’ postoperative courses were significantly improved after LDG, with shorter postoperative hospital stays in this group.
Because of these findings, LDG is now considered noninferior to OG in terms of oncologic outcomes and beneficial in its postoperative course in patients with LAGC. However, most studies on this topic were conducted in Eastern countries, and patients included in these trials were typically not submitted to perioperative or neoadjuvant therapies because perioperative treatments are not standard in those countries. Therefore, uncertainty remains in recommending the optimal surgical approach for patients with LAGC after neoadjuvant or perioperative treatment (14-16). Preoperative chemotherapy could affect the normal tissue planes owing to profibrotic reactions induced by the oncologic agents and to cytotoxicity, which might complicate dissection during a laparoscopic lymphadenectomy (17,18). We present the following article in accordance with the Narrative Review reporting checklist (available at https://ales.amegroups.com/article/view/10.21037/ales-21-28/rc).
Objective
In Western countries, interest is increasing in determining the safety and efficacy of LG after perioperative chemotherapy. This review was conducted to assess the results of MI surgery for advanced gastric cancer after neoadjuvant perioperative treatment. We present the following article in accordance with the narrative review reporting checklist.
Methods
We conducted a systematic search of the electronic medical databases, including a comprehensive analysis of the PubMed, EMBASE and Cochrane databases, to identify all relevant publications on MI surgery for advanced gastric cancer. All articles published until January 2021 were eligible.
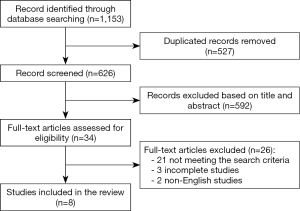
Search terms included “minimally invasive”, “gastrectomy”, “laparoscopic”, “neoadjuvant therapy”, and “perioperative treatment”. The references of relevant articles were considered as additional articles. After rejecting nonrelevant papers, articles published in languages other than English, and incomplete articles, eight studies were included in this analysis (Figure 1). The search was conducted by three authors (S De Pascale, A d’Amore, F Ascari).
Results
Eight papers published on this topic fulfilled the search criteria (Table 1).
Table 1
| Country | Publication date | Study design | Surgery type | Study aims | |
|---|---|---|---|---|---|
| Z Li (19) | China | 2016 | Prospective | Distal gastrectomy | To evaluate the perioperative safety and efficacy of LDG following NAC in a prospective cohort study |
| Z Li (20) | China | 2019 | Randomized | Distal gastrectomy | To evaluate short-term outcomes of patients with LAGC who received either LDG or open distal gastrectomy |
| N Wang (21) | China | 2019 | Retrospective | Distal, proximal and total gastrectomies | To evaluate postoperative safety and long-time survival after LG compared with that of OG after NAC |
| N van der Wielen (22) | Europe | 2020 | Multicentre, international randomized | Total gastrectomy with D2 | Non-inferiority of MITG compared to OTG after NAC with regard to oncological quality of the resection, postoperative outcomes and survival |
| K Yamamoto (23) | Japan | 2020 | Retrospective | Total and subtotal gastrectomies | To evaluate safety and clinical impact of MIS as conversion surgery after chemotherapy for stage IV GC |
| S Zhang (24) | China | 2020 | Retrospective | Total and distal gastrectomy | To evaluate the outcomes of LG after FLOT |
| Y Yan (25) | China, USA | 2021 | Multicentre retrospective | Total and distal gastrectomy | To evaluate the effect of NAC on postoperative outcomes in advanced GC treated with minimally invasive surgery |
| A van der Veen (26) | Netherlands (EU) | 2021 | Randomized | Total and distal gastrectomy | To verify whether laparoscopic gastrectomies lead to shorter hospital stays and fewer postoperative complications with comparable postoperative mortality, lymph node yields, and R0 resection rates |
LDG, laparoscopic distal gastrectomy; NAC, neoadjuvant chemotherapy; LAGC, locally advanced gastric cancer; LG, laparoscopic gastrectomy; OG, open gastrectomy; MITG, minimally invasive total gastrectomy; OTG, open total gastrectomy; MIS, minimally invasive surgery.
Case-control studies
Five case-control trials, four prospective (19,20,22) and one retrospective (21), compared LG and OG after neoadjuvant therapy. Among the prospective studies, patients’ distribution into groups was randomly established in three studies and guided by patient preference in one study. A total of 732 patients were enrolled in these trials: 276 underwent MI surgery; 456 underwent open surgery.
Three studies were conducted in Asia. In two of them (19,20), all patients underwent either laparoscopic or laparotomic distal gastrectomy; in the third trial (21), both distal and total gastrectomies were included. Two randomized trials in Europe (one in the Netherlands and one in several European countries) compared LG and OG. The STOMACH trial included only patients requiring total gastrectomies after neoadjuvant chemotherapy (NAC) (22). The LOGICA trial included patients with both early and advanced gastric cancers treated with either a distal or a total gastrectomy to reflect the daily practice in Western countries (26); 77% of patients had advanced gastric cancer in the laparoscopic group, and 75% had advanced gastric cancer in the open group. In the laparoscopic group, 67% of patients underwent NAC; in the open group, 78% of patients underwent NAC (Table 2).
Table 2
| MIG | OG | |
|---|---|---|
| Patients n° | 115 | 112 |
| LAGC | 88 (77%) | 84 (75%) |
| Preoperative chemotherapy | 77 (67%) | 87 (78%) |
| Surgery with curative intent | 109 | 107 |
| Total gastrectomy | 48 (41.7%) | 43 (39.1%) |
| Distal gastrectomy | 59 (51.3%) | 64 (58.2%) |
| Other resection | 1 | 0 |
| Postoperative chemotherapy | 41 (35.7%) | 44 (40%) |
| Time to postoperative chemotherapy (day) | 45 | 50 |
MIG, minimally invasive gastrectomy; OG, open gastrectomy.
Preoperative chemotherapy regimens differed among the studies and within some of the studies. Nonhomogeneous neoadjuvant treatments were not analysed. Patients’ baseline demographic and clinical characteristics were similar in all studies. All trials analysed the surgical, postoperative and oncological results of each group; however, long-term oncological results were available only in some of the studies.
Operative results
Oncological radicality of the procedure was defined as complete resection of the primary tumour, achievement of cancer-free resection margins (R0), and an adequate lymphadenectomy (27). Appropriate lymphadenectomy was considered to be D2 dissection with at least 15 lymph nodes retrieved. All studies reported the number of lymph nodes harvested (Table 3). Both groups met the criteria for adequate lymphadenectomy in all studies. The numbers of harvested lymph nodes did not differ between the groups.
Table 3
| Patients n° | Operative time (min) | Blood loss (mL) | No. of lymph nodes | R0 rate, n (%) | |
|---|---|---|---|---|---|
| STOMACH trial (22) | MIG: 47 OG: 49 | MIG: 244 (IQR 198-293); OG: 200 (IQR 164-245); p= 0.005 | MIG: 171 (IQR 64–300); OG: 200 (IQR 100–400); P=0.45 | MIG: 40.7 (SD 16.3); OG:44.3 (SD 16.7); P=0.20 | MIG: 44 (93.6%); OG: 48 (98%); P=0.6 |
| Z. Li (19) | MIG: 20 OG: 24 | MIG: 214 (±42.2); OG: 200 (±52.5); P=0.34 | MIG: 94 (±36); OG: 97.9 (±52.1); P=0.77 | MIG: 24.7 (±8.3); OG:24.6 (±10.0); P=0.96 | MIG: 20 (100%); OG: 24 (100%); P>0.05 |
| Z. Li (20) | MIG: 45; OG: 50 | MIG: 224.8 (SD 35.8); OG: 182.9 (SD 44.8); P<0.001 | MIG: 87 (IQR, 60–150); OG: 100 (IQR, 58–200); P=0.22 | MIG: 31 (IQR, 24–38); OG: 33 (IQR, 28–41); P=0.43 | MIG: 44 (98%); OG: 46 (92%); P=0.37 |
| N. Wang (21) | MIG: 49; OG: 221 | MIG: 221.5 (SD 69.9); OG: 201.1 (SD 56.7); P=0.06 | MIG: 260.2 (SD 232.1); OG: 241 (SD 186.3); P=0.59 | MIG: 32.9 (±13.6); OG: 30 (±14); P=0.19 | Not reported |
| LOGICA trial (26) | MIG: 115; OG: 112 | MIG: 216 (SD 68.8); OG: 182 (SD 53.7); P<0.01 | MIG: 150 (IQR, 50–250); OG: 300 (IQR, 150–508); P=0.001 | MIG: 29 (IQR, 21–37); OG: 29 (IQR, 22–39); P=0.48 | MIG: 103 (95.4%); OG: 102 (95.3%); P=1.0 |
| K. Yamamoto (23) | MIG: 41; OG: 53 | MIG: 339 (IQR, 155–607); OG: 266 (IQR, 154–470); P=0.039 | MIG: 10 (IQR, 0–430); OG: 520 (IQR, 85–1,555); P<0.001 | MIG: D2 dissection (87.8%); OG: D2 dissection (79.2%); P=0.12 | MIG: 28 (68.3%); OG: 36 (67.9%); P=0.64 |
| S. Zhang (24) | MIG: 23 | MIG: 255 (IQR 195-310) | MIG: 105 (IQR, 50–350) | MIG: 25 (IQR 19-38) | MIG: 21 (91.3%) |
| KLASS 02 RCT (13) | MIG: 513; OG: 498 | MIG: 227; OG: 165; P=0.001 | MIG: 153; OG: 230; P<0.001 | MIG: 46.6; OG:46.9; P=0.74 | MIG: 503 (99.1%); OG: 491 (98.6%); P=0.3 |
| CLASS 01 (12) | MIG: 519; OG: 520 | MIG: 217; OG: 186; P=0.001 | MIG: 105; OG: 117; P<0.001 | MIG: 36.1 (SD 16.7); OG: 36.9 (SD 16.1); P=0.7 | MIG:100%; OG: 100% |
SD, standard deviation; IQR, interquartile range; MIG, minimally invasive gastrectomy; OG, open gastrectomy.
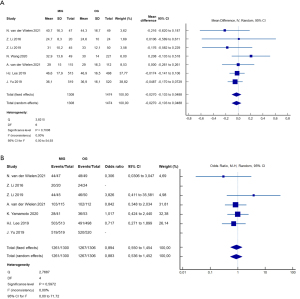
In the STOMACH trial, three of 47 patients in the laparoscopic group had positive margins compared with one of 49 in the open group (P=n.s.). Li et al. and the LOGICA trial both reported similar positive margin incidences in both groups (P=n.s.) (20,26). Wang et al. did not report the R0 resection rate (21). In one study, all patients had R0 resections (19) (Figure 2).
Estimated blood loss was lower in the laparoscopic groups, but the mean operative time was slightly longer. All studies reported longer operative times for the MI group; however, only four studies found statistically significant differences (20-22,26).
Postoperative results
One study reported that the overall complication rate within 30 postoperative days was significantly lower in the laparoscopic group than in the open group (20% vs. 46%; P=0.007) (20); however, severe complications (Clavien-Dindo grade III or higher) were similar in both groups. No differences were noted between the groups in the other studies, neither for overall complication rate nor for Clavien-Dindo grade III or higher. Postoperative recovery times were comparable between the two groups in four of the five studies. One of the five reported a significantly shorter postoperative stay for the laparoscopic group (P<0.05) (20) (Table 4, Figure 3).
Table 4
| First aerofluxus time (days) | Complications | Clavien-Dindo grade III–V | Length of stay (days) | Time to postoperative chemotherapy (days) | Cycles of completed adjuvant therapy | 12-month survival (%) | 3-year survival (%) | |
|---|---|---|---|---|---|---|---|---|
| STOMACH trial (22) | Unreported | MIG: 16 (34%); OG: 21 (42.9%); P=0.4 | MIG: 8 (17%); OG: 6 (12.2%); P>0.05 | MIG: 8 (IQR, 7–9); OG: 8 (IQR, 7–11); P=0.33 | Unreported | Unreported | MIG: 86%; OG: 90.4%; P=0.7 | Unreported |
| Z. Li (19) | MIG: 3.2 (±0.9); OG: 3.9 (±0.9); P=0.012 | MIG: 3 (15%); OG: 2 (8.3%); P=0.39 | MIG: 2 (10%); OG: 0 (0%) | MIG: 11 (IQR, 9–12.5); OG: 10 (IQR, 10–12.5); P=0.914 | Unreported | Unreported | Unreported | Unreported |
| Z. Li (20) | MIG: 3.3; OG: 3.2; P=0.62 | MIG: 9 (20%); OG: 23 (46%); P=0.007 | MIG: 6 (13%); OG: 2 (4%); P=0.47 | MIG: 9 (IQR, 8–10); OG: 9 (IQR, 8–13); P=0.10 | MIG: 37 [34–50]; OG: 39 [34–44]; P=0.67 | MIG: 5 cycles; OG: 4 cycles; P=0.06 | unreported | Unreported |
| N. Wang (21) | Unreported | MIG: 6 (12.2%); OG: 26 (11.8%); P=0.75 | MIG: 3 (6%); OG: 8 (4%); P>0.05 | MIG: 11.1 (SD 4.4); OG: 13 (SD 7.3); P=0.02 | Unreported | Unreported | MIG: 89.6%; OG: 81.6%; P>0.05 | MIG: 75.6%; OG: 55.9%; P>0.05 |
| LOGICA trial (26) | MIG: 4; OG: 4; P=0.74 (first defecation) | MIG: 50 (43.5%); OG: 46 (41.8%); P=0.907 | MIG: 19 (16.4%); OG: 25 (22.8%); P=0.33 | MIG: 7; OG: 7; P=0.30 | MIG: 45 (IQR 38-60.75); OG: 50 (IQR 41-57); P=0.415 | MIG: 41 (35%); OG: 44 (40%); P=0.49 | MIG: 76%; OG: 78%; P=0.74 | unreported |
| K. Yamamoto (23) | Unreported | MIG: 4 (9.8%) (CD >2); OG:13 (24.5%) (CD>2); P=0.058 | MIG: 0; OG: 4 (7.6%); P=0.072 | MIG: 8 (IQR, 6–15); OG: 12 (IQR, 6–100); P<0.0001 | MIG: 25 [16–60]; OG: 39 [15–123]; P=0.0008 | MIG: 95.1%; OG: 90.6%; P=0.39 | MIG: 95%; OG: 75%; P=0.028 | MIG: 75%; OG: 35%; P=0.028 |
| S. Zhang (24) | MIG: 2.7 [1–6] | MIG: 7 (30.4%) | MIG: 1 (4.3%) | MIG: 13.2 (IQR, 8–31) | Unreported | Unreported | Unreported | Unreported |
| KLASS 02 RCT (13) | MIG: 3.5; OG: 3.7; P=0.025 | MIG:85 (16.3%); OG: 120 (24.9%); P=0.003 | MIG: 45 (8.8%); OG: 56 (11.2%); P>0.05 | MIG: 8.1; OG: 9.3; P=0.003 | Unreported | Unreported | Unreported | Unreported |
| CLASS 01(12) | MIG: 3.5; OG: 3.6; P=0.11 | MIG: 79 (15.5%); OG: 67 (12.9%); P=0.28 | MIG: 18 (3.5%); OG: 14 (2.8%); P>0.05 | MIG: 10.8; OG: 11.3; P=0.001 | Not reported | Not reported | Not reported | MIG: 83%; OG: 85%; P>0.05 |
SD, standard deviation; IQR, interquartile range; MIG, minimally invasive gastrectomy; OG, open gastrectomy.
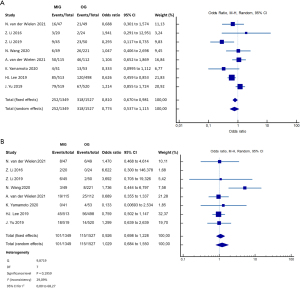
Postoperative chemotherapy
Two studies considered the influence of the access route on postoperative chemotherapy. Li et al. (20) reported that patients who underwent laparoscopic surgery were more likely to complete more cycles of postoperative chemotherapy and less likely to discontinue it because of adverse effects.
The LOGICA trial (26) revealed no significant difference in postoperative chemotherapy rates between the groups. The laparoscopic group had a slightly shorter interval between surgery and adjuvant therapy than did the open-surgery group (P=n.s.).
Long-term results
Long-term follow-up is ongoing in two studies. The only available data on 3-year disease-free survival (DFS) and overall survival (OS) were from the retrospective study: 3- and 5-year OS rates were 75.6% and 65.8% in the laparoscopic group and 55.9% and 49.7% in the open group, respectively. These rates did not significantly differ (22). The LOGICA and STOMACH trials reported that the 1-year OS did not differ between the groups. Longer follow-up is ongoing in both trials.
Other studies
Three studies examined the relationship between NAC and MI surgery for LAGC but lacked a straight comparison between laparoscopic and open surgery after NAC (23-25).
LG and conversion surgery
Yamamoto et al. (23) retrospectively analysed the outcomes of patients who underwent conversion surgery after chemotherapy for stage IV gastric cancer to determine the feasibility of an MI approach in this setting. Ninety-four patients were included; 41 underwent LG, and 53 underwent OG. Patients in the OG group had larger tumours with peritoneal metastasis or required splenectomies. The other background characteristics were comparable between the groups. Data regarding operative factors and postoperative outcomes were collected, and few significant differences were observed. Operative times were longer in the laparoscopic group, but operative blood loss was consistently lower. Hospital stays were significantly shorter in the laparoscopic group.
OS and DFS were calculated over an 18-month observational period. The most relevant prognostic factor was R0 resection, which was achieved in nearly 70% of patients and equally distributed between the groups. However, patients in the laparoscopic group had higher DFS and OS rates than did the OG group. Although the baseline disease stages were not comparable, MI surgery was not detrimental in terms of OS after conversion surgery. Moreover, the interval from surgery to postoperative chemotherapy was significantly shorter in the MI group than in the open group.
Effect of preoperative chemotherapy on LG
Zhang et al. (24) designed a study to clarify the effects of the FLOT regimen in patients with LAGC and determine its effect on subsequent LG. Twenty-three patients were enrolled; all received four cycles of FLOT completed at least 4 weeks before surgery. According to tumour site, 12 patients underwent total gastrectomies, and 11 underwent distal gastrectomies. The median number of lymph nodes retrieved was 25, and the R0 rate was 91.4%. Six patients reported overall complications (26%), with one severe (grade III) (4.3 %). Data on the operative time, intraoperative bleeding, first flatus and hospital stay were similar to those reported in the case-control trials.
Yan et al. (25) compared the postoperative outcomes of patients undergoing MI surgery alone or combined with NAC for LAGC. They enrolled 673 patients: 112 in the NAC + surgery cohort and 561 in the surgery upfront cohort. After 1:1 propensity score-matching, 97 patients were included in each cohort. The two groups did not significantly differ in terms of intra- and postoperative data. Significantly more lymph nodes were retrieved in the NAC + surgery group (Table 5).
Table 5
| Neoadjuvant chemotherapy + surgery | Upfront surgery | P value | |
|---|---|---|---|
| Patients n° | 97 | 97 | |
| Mean number of lymph nodes | 35.7 (±12.6) | 31.9 (±13.8) | 0.037 |
| R0 rate, number (%) | Unreported | Unreported | |
| Mean operative time (min) | 358.7 (±101.9) | 324.3 (±93.4) | 0.04 |
| Mean blood loss (mL) | 93.6 (±99.7) | 115.4 (±116.5) | 0.2 |
| First aerofluxus time | 4.2 | 4.4 | 0.39 |
| Complications | 29 (30%) | 26 (26.8%) | 0.34 |
| Clavien-Dindo grade III/IV | 5 (5.1%) | 5 (5.1%) | – |
| Length of stay (days) | 7.5 (± 4.6) | 7.7 (±6.2) | 0.13 |
Discussion
Although an MI approach to early gastric cancer is considered a safe surgical procedure and extensively accepted, the role of laparoscopy in LAGC is controversial. Technical issues related to tumour size, possible infiltration of other organs, demanding extensive resections, and the requirement of a D2 dissection make the MI approach challenging. In recent years, relevant data have emerged from high-quality trials conducted in Eastern countries (11-13). These trials assessed the feasibility of LDG, even in LAGC, showing comparable oncological outcomes and better postoperative outcomes for LG than for open surgery relative to postoperative pain and recovery time. However, patients included in these trials were treated with primary surgery, whereas in Western countries, most LAGC is treated with NAC (14-17). Neoadjuvant therapies are aimed at improving localized disease control and long-term survival. The effects of neoadjuvant therapies on subsequent surgeries conducted via MI approaches are unclear.
Only eight studies were analysed in this review. Only a few were randomized, and most were not homogeneous in the type of preoperative chemotherapy used. In this review, the R1 and R2 rates were comparable between the open and laparoscopic groups and in the percentages reported in the CLASS01 and KLASS02 trials. Only the STOMACH trial reported slightly but non-significantly higher R1 resection rates in the MI group than in the open group (6% vs. 2%). Notably, this was the only trial that analysed total gastrectomies for proximal tumours.
Preoperative chemotherapy does not seem to directly affect lymphadenectomies during LG; the number of retrieved lymph nodes in these studies did not differ between open and laparoscopic resections. Moreover, postoperative complication rates, mainly CD >3 were similar in both groups.
Pretreated patients lost less blood during LG, but this was counterbalanced by a significantly longer operative time. These results are consistent with those reported in the CLASS-01 and KLASS-02 trials. Thus, NAC does not appear to directly affect the surgical difficulty of the intervention. These studies demonstrated no clear superiority of the MI approach in terms of postoperative morbidity.
The authors of the LOGICA trial determined the health-related quality of life (HRQoL) at different time points after surgery, testing their patients with standardized questionnaires. The groups did not significantly differ at any scheduled time points.
Notably, the STOMACH trial evaluated the results of MI and open gastrectomies in terms of HRQoL [paper submitted]. Here too, no differences were noted in HRQoL scores between the two groups. Importantly, in this trial, the number of patients who were fit enough after surgery to receive adjuvant chemotherapy was higher in the MI group than in the open group. Two other studies reported similar results in that patient who underwent MI gastrectomies were more likely to complete postoperative chemotherapy in terms of number of cycles and time between surgery and adjuvant therapy (20,23). Conversely, the LOGICA trial found no difference in the number of completed cycles but reported a slightly shorter interval between surgery and the beginning of adjuvant therapy (2). A study of colon cancer reported similar benefits in the laparoscopic cohort (28). Whether these results can be translated to advanced gastric cancer remains uncertain.
As stated, the overall and severe complications rates appeared comparable between both groups. It remains to be determined whether laparoscopy, owing to visual magnification, better exposure, and more delicate manipulation, can mitigate the increased risk of surgical complications induced by the chemotherapy-associated tissue fibrosis and disrupted anatomical planes (17,18,29-32). These studies reported no difference in the overall rate, type, or severity of postoperative complications among patients who either received or did not receive NAC.
The effect of MI surgery after NAC on long-term oncological outcomes remains uncertain. Four studies reported a long-term follow-up (21,22,23,26). In these studies, DFS and OS after MI gastrectomies were comparable to those of open surgery.
Conclusions
Few studies have addressed the issue of laparoscopic surgery after NAC. The results of these studies indicate that NAC does not adversely influence MI gastrectomy results. The MI approach, even after NAC, may facilitate postoperative chemotherapy in terms of timing and number of cycles.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Stefano Rausei and Simone Giacopuzzi) for the series “Minimally Invasive Surgery and Gastric Cancer: Where Are We Now?” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://ales.amegroups.com/article/view/10.21037/ales-21-28/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://ales.amegroups.com/article/view/10.21037/ales-21-28/coif). The series “Minimally Invasive Surgery and Gastric Cancer: Where Are We Now?” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Rawla P, Barsouk A. Epidemiology of gastric cancer: global trends, risk factors and prevention. Prz Gastroenterol 2019;14:26-38. [Crossref] [PubMed]
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11-20. [Crossref] [PubMed]
- Dikken JL, van Sandick JW, Maurits Swellengrebel HA, et al. Neo-adjuvant chemotherapy followed by surgery and chemotherapy or by surgery and chemoradiotherapy for patients with resectable gastric cancer (CRITICS). BMC Cancer 2011;11:329. [Crossref] [PubMed]
- Kitano S, Iso Y, Moriyama M, et al. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc 1994;4:146-8. [PubMed]
- Azagra JS, Goergen M, De Simone P, et al. Minimally invasive surgery for gastric cancer. Surg Endosc 1999;13:351-7. [Crossref] [PubMed]
- Zeng YK, Yang ZL, Peng JS, et al. Laparoscopy-assisted versus open distal gastrectomy for early gastric cancer: evidence from randomized and nonrandomized clinical trials. Ann Surg 2012;256:39-52. [Crossref] [PubMed]
- Kim W, Kim HH, Han SU, et al. Decreased Morbidity of Laparoscopic Distal Gastrectomy Compared With Open Distal Gastrectomy for Stage I Gastric Cancer: Short-term Outcomes From a Multicenter Randomized Controlled Trial (KLASS-01). Ann Surg 2016;263:28-35. [Crossref] [PubMed]
- Songun I, Putter H, Kranenbarg EM, et al. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol 2010;11:439-49. [Crossref] [PubMed]
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer 2021;24:1-21.
- Inaki N, Etoh T, Ohyama T, et al. A Multi-institutional, Prospective, Phase II Feasibility Study of Laparoscopy-Assisted Distal Gastrectomy with D2 Lymph Node Dissection for Locally Advanced Gastric Cancer (JLSSG0901). World J Surg 2015;39:2734-41. [Crossref] [PubMed]
- Yu J, Huang C, Sun Y, et al. Effect of Laparoscopic vs Open Distal Gastrectomy on 3-Year Disease-Free Survival in Patients With Locally Advanced Gastric Cancer: The CLASS-01 Randomized Clinical Trial. JAMA 2019;321:1983-92. [Crossref] [PubMed]
- Lee HJ, Hyung WJ, Yang HK, et al. Short-term Outcomes of a Multicenter Randomized Controlled Trial Comparing Laparoscopic Distal Gastrectomy With D2 Lymphadenectomy to Open Distal Gastrectomy for Locally Advanced Gastric Cancer (KLASS-02-RCT). Ann Surg 2019;270:983-91. [Crossref] [PubMed]
- Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol 2011;29:1715-21. [Crossref] [PubMed]
- Knight G, Earle CC, Cosby R, et al. Neoadjuvant or adjuvant therapy for resectable gastric cancer: a systematic review and practice guideline for North America. Gastric Cancer 2013;16:28-40. [Crossref] [PubMed]
- Al-Batran SE, Homann N, Schmalenberg H, et al. Perioperative chemotherapy with docetaxel, oxaliplatin, and fluorouracil/leucovorin (FLOT) versus epirubicin, cisplatin, and fluorouracil or capecitabine (ECF/ECX) for resectable gastric or gastroesophageal junction (GEJ) adenocarcinoma (FLOT4-AIO): a multicenter, randomized phase 3 trial. J Clin Oncol 2017;35:4004. [Crossref]
- Claassen YHM, Hartgrink HH, Dikken JL, et al. Surgical morbidity and mortality after neoadjuvant chemotherapy in the CRITICS gastric cancer trial. Eur J Surg Oncol 2018;44:613-9. [Crossref] [PubMed]
- Téoule P, Trojan J, Bechstein W, et al. Impact of Neoadjuvant Chemotherapy on Postoperative Morbidity after Gastrectomy for Gastric Cancer. Dig Surg 2015;32:229-37. [Crossref] [PubMed]
- Li Z, Shan F, Wang Y, et al. Laparoscopic versus open distal gastrectomy for locally advanced gastric cancer after neoadjuvant chemotherapy: safety and short-term oncologic results. Surg Endosc 2016;30:4265-71. [Crossref] [PubMed]
- Li Z, Shan F, Ying X, et al. Assessment of Laparoscopic Distal Gastrectomy After Neoadjuvant Chemotherapy for Locally Advanced Gastric Cancer: A Randomized Clinical Trial. JAMA Surg 2019;154:1093-101. [Crossref] [PubMed]
- Wang N, Zhou A, Jin J, et al. Open vs. laparoscopic surgery for locally advanced gastric cancer after neoadjuvant therapy: Short-term and long-term survival outcomes. Oncol Lett 2020;20:861-7. [Crossref] [PubMed]
- van der Wielen N, Straatman J, Daams F, et al. Open versus minimally invasive total gastrectomy after neoadjuvant chemotherapy: results of a European randomized trial. Gastric Cancer 2021;24:258-71. [Crossref] [PubMed]
- Yamamoto K, Omori T, Hara H, et al. Minimally invasive surgery is feasible after preoperative chemotherapy for stage IV gastric cancer. Ann Gastroenterol Surg 2020;4:396-404. [Crossref] [PubMed]
- Zhang S, Yan D, Sun Q, et al. FLOT Neoadjuvant Chemotherapy Followed by Laparoscopic D2 Gastrectomy in the Treatment of Locally Resectable Advanced Gastric Cancer. Can J Gastroenterol Hepatol 2020;2020:1702823. [Crossref] [PubMed]
- Yan Y, Yang A, Lu L, et al. Impact of Neoadjuvant Therapy on Minimally Invasive Surgical Outcomes in Advanced Gastric Cancer: An International Propensity Score-Matched Study. Ann Surg Oncol 2021;28:1428-36. [Crossref] [PubMed]
- van der Veen A, Brenkman HJF, Seesing MFJ, et al. Laparoscopic Versus Open Gastrectomy for Gastric Cancer (LOGICA): A Multicenter Randomized Clinical Trial. J Clin Oncol 2021;39:978-89. [Crossref] [PubMed]
- Smyth EC, Verheij M, Allum W, et al. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27:v38-49. [Crossref] [PubMed]
- Kim RH, Kavanaugh MM, Caldito GC. Laparoscopic colectomy for cancer: Improved compliance with guidelines for chemotherapy and survival. Surgery 2017;161:1633-41. [Crossref] [PubMed]
- Haskins IN, Kroh MD, Amdur RL, et al. The Effect of Neoadjuvant Chemoradiation on Anastomotic Leak and Additional 30-Day Morbidity and Mortality in Patients Undergoing Total Gastrectomy for Gastric Cancer. J Gastrointest Surg 2017;21:1577-83. [Crossref] [PubMed]
- Chang AY, Foo KF, Koo WH, et al. Phase II study of neo-adjuvant chemotherapy for locally advanced gastric cancer. BMJ Open Gastroenterol 2016;3:e000095. [Crossref] [PubMed]
- Kawamura Y, Satoh S, Suda K, et al. Critical factors that influence the early outcome of laparoscopic total gastrectomy. Gastric Cancer 2015;18:662-8. [Crossref] [PubMed]
- An JY, Kim KM, Kim YM, et al. Surgical complications in gastric cancer patients preoperatively treated with chemotherapy: their risk factors and clinical relevance. Ann Surg Oncol 2012;19:2452-8. [Crossref] [PubMed]
Cite this article as: d’Amore A, De Pascale S, Ascari F, Bertani E, Fumagalli Romario U. Minimally invasive gastrectomy after neoadjuvant chemotherapy: a literature review. Ann Laparosc Endosc Surg 2022;7:10.