Indocyanine green—a potential to explore: narrative review
Introduction
The advantages of laparoscopic surgery have been extensively proved when compared to open surgery. However, the minimally invasive approach is a treatment option for stage I gastric cancers and the non-inferiority of the technique has been proved in both distal and total gastrectomy (1,2). To date, there is no evidence to recommend the laparoscopic approach in stage II–IV cancers, as it is associated with several complications like anastomotic leakage and visceral injuries. Moreover, extension of the lymphadenectomy and other technical issues are still debated (3,4).
Recently, indocyanine green (ICG)-enhanced fluorescence was introduced in laparoscopic surgery to improve the view and provide detailed anatomical information during surgery (5). Indocyanine-green is a sterile, anionic, water soluble but relatively hydrophobic, tricarbocyanine molecule, which was approved for clinical use in 1959 by the Food and Drug Administration (FDA) (6). After intravenous injection, ICG rapidly bounds to plasma protein, especially lipoproteins, with minimal leakage into the interstitium. This dye has no known metabolites. It is rapidly extracted by the liver without modifications and excreted in unconjugated form in the bile about eight minutes after injection, depending on liver functionality and vascularization (6,7). When injected outside blood vessels (e.g., into the normal tissue close to tumours), ICG binds to proteins and is found into the lymph, reaching the nearest lymph node usually within 15 minutes. After one to two hours it binds to the regional lymph nodes and is deposited into macrophages (8-10). The standard dose commonly administered in clinical practice is 0.1–0.5 mg/mL/kg, which is well below the toxicity level and has practically no adverse effects (6). ICG becomes fluorescent when excited either by a laser beam (11), or by near-infrared light at about 820 nm and longer wave lengths (12). The fluorescence released by ICG can be detected using specifically designated scopes and cameras.
ICG fluorescence imaging has found several different applications in gastric cancer surgery aiming in reducing complications, such as anastomotic leakage, clarifying anatomic landmarks and as a guidance for lymphadenectomy. To date, the clinical and experimental applications of ICG are: ICG-fluorescence guided angiography for perfusion control, lymphography for sentinel nodes detection and lymphatic mapping, intraoperative tumour identification, and intraoperative identification of peritoneal carcinomatosis. Although widespread, these intraoperative applications are off label, as ICG in not approved by FDA as a surgical guidance.
The purpose of this review article is to describe all the possible applications of ICG imaging in gastric cancer surgery, including advantages and weakness of each technique through a critical review of the literature.
We present the following article in accordance with the Narrative Review reporting checklist (available at https://ales.amegroups.com/article/view/10.21037/ales-21-5/rc).
Methods
Literature review was performed in January 2021 using the PubMed database and Google scholar, using the following keywords: “indocyanine green”, “gastric cancer”, “laparoscopy”, “anastomotic leakage”, “perfusion” “sentinel nodes”, “lymphatic mapping”, “tumour identification”. Only English articles were included. After identification of relevant articles, the abstracts were read to select eligible article for full text revision. References of identified articles were searched for additional relevant articles. Case reports and editorial letters were excluded.
ICG-fluorescence guided angiography for perfusion control
Anastomotic leakage is one of the most serious complication of gastric cancer surgery, associated with high morbidity and mortality. The incidence varies according to authors, from 5% to 9% (13-15). In a Korean retrospective multicentre study including 1.485 patients who underwent gastrectomy for gastric cancer, the morbidity rate was 14% and the mortality rate was 0.6%, with incidence of anastomotic leakage of 1.3% (13). Nagasako et al. reported anastomotic complications in 37 (9.3%) of 400 patients after laparoscopic assisted gastrectomy (16). Moreover, abdominal complications, like anastomotic leakage or intra-abdominal abscess, affect initiation and continuation of adjuvant chemotherapy. A multicenter analysis including 2,954 patients, reported that time between surgery and initiation of adjuvant chemotherapy was significantly longer for patients with post-operative complication compared with those without (P<0.0001) (17). Even if anastomotic leakage is a multifactorial complication associated with several risk factors, blood perfusion is one of the most important and there is no standardised method for assessing it. In order to evaluate intraoperative anastomotic perfusion, surgeons often rely on bowel colour, bleeding, pulsation or presence of peristalsis and temperature. All these methods present limitations, because they are based on totally subjective evaluation and intraoperative ischemic bowel demarcation may require extended time. Karliczek’s prospective study showed that global clinical risk assessment of anastomotic leakage by the operating surgeon has low predictive value with underestimation of the risk of anastomotic leakage (sensitivity of 61.3% and specificity of 88.5%) (18). Other techniques to determine anastomotic perfusion, like Doppler-flowmetry, are time consuming and not very convincing (19).
Due to the ability of ICG to become fluorescent once excited, this dye can be injected intravenously to obtain a real-time angiography in order to evaluate vascular anatomy and bowel perfusion. ICG angiography has also several advantages as high tissue permeability and real-time imaging. This method can obtain more stable results than Doppler and it is not influenced by patients’ individual features. In addition, it is a real time technique able to replace the lack of tactile sensation during laparoscopic surgery. According to literature, ICG-fluorescence angiography (FA) is considered safe, feasible and does not require extensive learning curve to be applied. To date, no complications attributable to the use of the technique were recorded, so it is easily performed. The mean added surgical time is about less than 5 minutes to the total duration of the surgical procedure (20).
Degett et al. published a systematic review on ICG fluorescence angiography for gastrointestinal anastomosis. More in detail, 17 cohort studies were included in the review encountering 1,206 patients in the ICG group, with fluorescent angiography, and 396 in the control group, without fluorescent angiography. Intraoperative ICG-FA assessment of colorectal anastomoses was associated with reduced risk of anastomotic leakage (n=23/693) compared to standard procedure with no ICG-FA assessment (n=19/223). The anastomotic leakage rate in patients with esophagogastric anastomosis of the ICG group was 14% (n=30/214). No studies including patients with esophagogastric anastomosis had a control group without ICG fluorescent angiography (14).
In a prospective study including 30 patients, Huh et al. showed the potential role of ICG fluorescent angiography using a near-infrared (NIR) camera system to assess anastomotic vascular perfusion in laparoscopic gastric cancer. ICG was injected intravenously at a concentration of 2.5 mg/mL immediately after anastomosis. The perfusion was assessed through the adopted perfusion score of fluorescence activity, which ranged from 1 to 5 (1= no uptake, and 5= iso-fluorescent to all other segments). No change in the surgical plan occurred due to high clinical scores. One case of anastomotic leakage was recorded, probably due to a focal perfusion defect around the gastroduodenostomy identified through the NIR mode during review of the surgical video (21).
However, one of the limits of ICG fluorescence angiography is the lack of objectivity. Thus, recently, many studies tried to reach objective quantification of fluorescence intensity and to find a threshold to predict anastomotic leakage. A Dutch prospective cohort study of patients who underwent esophagectomy reported that time between ICG injection and enhancement of the stumps was predictive for anastomotic leakage (P=0.174, area under the curve =0.731). The cut-off value of 98 seconds was identified (specificity: 98%) (22). A recent review about the optimal methodology to perform quantitative ICG angiography reported that fluorescence intensity parameters (maximum intensity and relative maximum intensity) are unstable and do not reflect complication rate. Instead, inflow parameters (time-to-peak, slope, and t1/2max) are resilient in clinical setting and significantly associated with anastomotic leakage. But it is necessary to use a body-mass adjusted ICG dose and find standardization of the methodology to establish a gold standard within the field of quantitative indocyanine green angiography (Q-ICG) (23).
Most of the studies published on the use of ICG fluorescent angiography in gastric surgery focuses on the presence, or absence, of tissue perfusion to minimize the risk of anastomotic leakage. Conversely, Mori et al. showed that the difference of time between ICG fluorescence appearance on two different sides of the anastomosis was a useful predictor of anastomotic leakage in gastric cancer surgery (24).
Moreover, ICG fluorescent angiography has different surgical applications in gastric surgery. It can be used not only to assess perfusion in order to reduce anastomotic leakage, but also to clarify vascular anatomy through intraoperative real time imaging that allows vessels’ identification. For instance, ICG angiography can be used to visualize the infra-pyloric artery during pylorus-preserving gastrectomy (25).
Therefore, according to literature there have been many reports and some systematic reviews on the effectiveness of ICG fluorescence imaging in gastric surgery. To date, the power of the studies published and the evidence are limited, so large multicentre trials are necessary before stating the superiority of the technique when compared with standard procedures. Furthermore, a quantitative evaluation of the gastric perfusion seems to be more precise than the qualitative one, identifying some factors which are significantly associated with a lower incidence of anastomotic leakage.
ICG fluorescence lymphography for sentinel nodes biopsy and lymphatic mapping
The major field of application of ICG is fluorescence lymphography for both early gastric cancer (T1) and gastric cancer (T2–T4).
To date, according to European Society for Medical Oncology (ESMO) guidelines and Japanese gastric cancer treatment guidelines, endoscopic resection [endoscopic mucosal resection (EMR) or endoscopic submucosal (ESD)] is indicated in case of T1a, well differentiated, ≤2 cm, non-ulcerated tumours. Indeed, these cases have less than 1% probability of lymph node metastases. ESD have less strictly indications and can be performed in case of larger or non-differentiated tumours according to patients age and comorbidities, in order to reduce operative risks (3,4). Surgical gastrectomy with lymphadenectomy is the recommended treatment for gastric cancer with no indication for ESD (4).
It is a well-known concept that adequate lymphadenectomy is associated with improved long-term disease specific survival and accurate staging of gastric cancer (26). Indeed, the accuracy of detecting lymph node metastases during preoperative staging is inadequate (27). Nevertheless, the complex gastric vascular anatomy and lymphatic drainage increase difficulty in performing lymphadenectomy especially during minimally invasive surgery. On the one hand, it is complex to dissect a high number of lymph nodes without increasing morbidity and mortality (28). On the other hand, futile extensive lymphadenectomy in patients without metastatic lymph nodes is unfavourable (26). In order to reduce morbidity associated with extensive lymphadenectomy, the concept of sentinel node (SN) has been introduced in clinical practice of gastric cancer surgery (29), after it was firstly proposed in breast cancer by Morton et al. Sentinel lymph nodes are the first lymph nodes to receive lymphatic flow from a tumour, so they are thought to be the primary site of micrometastasis. Thus, the pathological status of SNs can predict the status of all regional lymph nodes. In case of SNs negative for metastases, unnecessary lymphadenectomy can be avoided (30). Many authors demonstrated feasibility and utility of sentinel nodes biopsy in gastric cancer (31). However, this concept has been controversial in gastric cancer as lymphatic flow of the stomach is complex (32). A total of 30 cases should be performed in order to learn the technique for gastric cancer (29). Kusano et al. introduced the use of ICG fluorescence lymphography for SNs mapping in clinical practice for gastric cancer (33). Some technical issues regarding ICG guided SNs mapping should be analysed.
Several tracers have been used to perform SNs biopsy in gastric cancer: radiocolloids and dyes, like methylene blue and ICG. The combination of a radioactive tracer and a dye, in the dual tracer technique, is associated with higher sensitivity and specificity if compared with single tracer technique (34). Moreover, the dual tracer technique has been confirmed even in minimally invasive gastric cancer surgery (35). However, all tracers have some limits. Methylene blue deteriorates quickly and has low tissue penetration. Radiocolloid is expensive, time-consuming, has low sensitivity in detecting SNs near the injection site and the effectiveness of gamma probe detection is limited by the directional limitation of the laparoscopic surgical device. Conversely, ICG has a higher tissue penetration and lower incidence of allergies than methylene blue. This dye has no radioactivity and is less time consuming than radioactive tracer. Other advantages are the direct visualization of lymphatic canals and primary drainage lymphatic areas and high stability (36,37). It has been demonstrated that ICG is a suitable tracer for laparoscopic SNs mapping in gastric cancer (38,39).
Skubleny et al. published a meta-analysis including 10 studies, for a total of 643 patients, to evaluate the diagnostic utility of ICG for SNs mapping. The authors found identification rate of 99%, sensitivity of 87%, specificity of 100% and accuracy of 98.3%. The relatively low sensitivity was explained by heterogeneity of the studies included in the analysis. A subgroup analysis including studies published after 2010 demonstrated sensitivity of 93% (38). Another meta-analysis published by He et al. on the diagnostic value of IGC SNs mapping in gastric cancer included 13 studies, for a total of 971 patients, and identified pooled sensitivity of 94% and pooled specificity of 100% (39).
As reported from previous studies, indication for local resection associated with SNs dissection should be limited to clinical T1, less than 4 cm in size (29,31,40). In their prospective study, Kitagawa et al. concluded that SNs mapping should be avoided in T2 tumour because they are associated with high number of metastases. As the incidence of lymph nodes metastases increases with tumour size and stage, from 5% in T1a, 20% in T1b to 50% in T2, the risk to have false negative SNs and skip metastases is high in T2 tumours (29). Moreover, the number of skip metastases is higher in tumours more than 4 cm in size (41). Indeed, greater sizes of the tumour and advanced stages present massive lymph node metastases and blockage of lymphatic channel so the tracer cannot reach the lymph nodes resulting in false negative SNs biopsy.
The injection method is as important as the tracer itself. Two methods have been described for SNs mapping in gastric cancer: endoscopic submucosal injection and intraoperative subserosal injection. To date, the standard procedure is the endoscopic submucosal injection (29). Skubleny et al. found higher diagnostic odd ratio and sensitivity in submucosal injection group compared with subserosal injection group (942 vs. 31.18 and 93% vs. 56% respectively) (38). In He et al. meta-analysis, the pooled sensitivity of the submucosal injection subgroup was considerably higher than the subserosal one (98% vs. 40%) (39). ICG dilution with distilled water varies among the studies. A safe dose used for diagnostic procedures is 0.1–0.5 mg/kg. ICG dilution is injected through endoscopic needle into the submucosal layer in four different points around the tumour. The amount of ICG dilution injected varies (usually from 2 to 4 mL) according to concentration. These results show that high concentration probably results in decreased resolution (38,39,42). Timing injection varies among authors: from 24 hours before surgery to immediate imaging. The most diffused timing is to start the dissection 20 minutes after injection allowing clear visualization of lymphatic nodes and canals. In He et al. study, the sensitivity of 20 minutes after subgroup was higher than that of immediate imaging or imaging with preoperative injection (98% vs. 70%) (39).
After visualization of fluorescent SNs, the biopsy can be performed through the lymphatic basin dissection or the pick-up method. Lymphatic basin dissection is a selective lymphadenectomy allowing en bloc dissection of the whole basin. Gastric lymphatic basins are defined according to the five directions along the main arteries: left gastric artery, right gastric artery, left gastroepiploic artery, right gastroepiploic artery and posterior gastric artery. It has been shown that the first technique is superior to the second one for minimizing the risk of missed SNs (diagnostic accuracy 92.3% vs. 50% respectively). Indeed, metastatic lymph nodes of the drainage basin are analysed even if fluorescent negative, reducing the risk of false negative (43). In the meta-analysis published by He et al., the sensitivity of lymphatic basis dissection subgroup was slightly higher than the pick-up one even if the difference was not statistically significant (96% vs. 94%) (39).
The main technical issue associated with SNs mapping in gastric cancer is the intraoperative diagnosis of lymph node metastases at the micrometastasis level. Usually, intraoperative diagnosis is made by analysing the largest dimension of the frozen section by hematoxylin-eosin (H&E) staining. However, the prospective JCOG0302 multicentre study evaluating the feasibility of ICG SNs mapping in T1 gastric cancer was abandoned due to the high false negative rate (46%) of intraoperative histological examination using a single plane (44). Alternative methods have been proposed in order to solve this issue like immunohistochemistry (IHC), reverse transcriptase-polymerase chain reaction, one-step nucleic acid amplification assay (45). Yano et al. compared H&E with IHC in 130 patients who underwent ICG SNs mapping for gastric cancer. Fifteen patients diagnosed as N0 with H&E were found to be metastatic (micrometastases) with IHC and all the positive nodes were included in the SNs (46).
One of the limits of ICG is the lack of objectivity. This limitation can be overcome by objective evaluation methods. In a case series of 17 patients who underwent SNs mapping for gastric cancer, Okubo et al. compared the radioisotope uptake with the fluorescence intensity evaluated through the imaging software of the hyper eye medical system. The authors found a significant correlation between radioisotope uptake and fluorescence intensity (47).
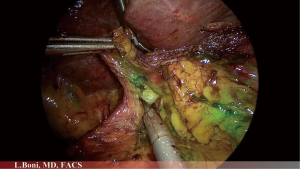
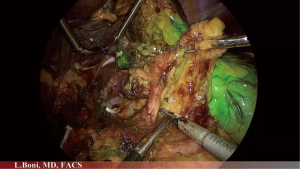
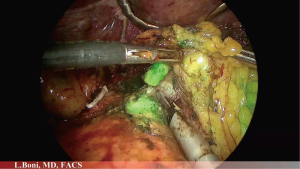
ICG can be used not only in case of early gastric cancer but also in case of T2–T4 tumours to perform lymphatic mapping (Figures 1-3). A fundamental step in gastric cancer surgery is lymphadenectomy, since the removal of an adequate number of lymph nodes improves accuracy of staging, regional disease control and patient survival. According to 8th edition of the UICC/AJCC TNM staging system, at least 16 lymph nodes are required to perform a correct staging but the desirable amount is of 30 or more. To date, according to ESMO and Japanese gastric cancer treatment guidelines it remains to be shown if laparoscopic surgery can achieve the same results as open surgery requiring D2 lymphadenectomy (3,4). Indeed, minimally invasive lymphadenectomy along greater vessel is technically demanding. As a consequence, it seems plausible that ICG fluorescent lymphatic mapping improves identification of lymph nodes. The use of ICG to facilitate lymphatic mapping and lymphadenectomy is still in preliminary stages of clinical practice but with good results.
In order to perform lymphatic mapping, ICG dilution is injected submucosally into 4 sites around the tumour through endoscopy one day before surgery. ICG concentration and amount of dilution injected vary among authors (48-51).
Chen et al. reported a randomized clinical trial including 258 patients who underwent laparoscopic D2 gastrectomy with or without ICG mapping. The number of lymph nodes retrieved was significantly higher in the ICG group than in the non-ICG group (50.5 vs. 42.0 lymph nodes respectively, P<0.001). Significantly more perigastric and extraperigastric lymph nodes were harvested in the ICG group than in the non-ICG group. Moreover, the lymph node noncompliance rate was lower in the ICG group than in non ICG group (31.8% vs. 57.4% respectively, P<0.001). There was no difference in post-operative complications (48).
In a case series published by Baiocchi et al. 13 patients underwent fluorescence guided lymphadenectomy for gastric cancer. In this series, 81.48% of lymphatic stations were fluorescent. There were no IGC negative node metastases and all 54 positive lymph nodes were fluorescent (49).
Kim et al. published a study investigating the completeness of lymphadenectomy under conventional light through examination of each lymphatic station with ICG fluorescent lymphography to identify extra fluorescent tissue. The authors concluded that ICG provides additional lymph nodes detection during lymphadenectomy for gastric cancer especially in the infra-pyloric area (52).
Kwon et al. published a study including 40 patients (IGC group) who underwent robotic radical gastrectomy with fluorescence-guided lymphadenectomy. The results of the ICG group were compared with 40 historical control patients without ICG-guided lymphadenectomy. A mean of 23.9 fluorescent lymph nodes were harvested among a mean of 48.9 overall lymph nodes retrieved in the ICG group. The mean number of overall lymph nodes harvested was significantly higher in the ICG group than in the control group (48.9 vs. 35). All metastatic lymph nodes of the ICG group were fluorescent (50). In a case matched cohort study, Cianchi et al. compared two groups of 37 patients who underwent robotic gastrectomy with and without ICG fluorescent lymphography. The mean total number of harvested lymph nodes was significantly higher in the ICG group than in the non-ICG group (50.8% vs. 40.1%) (51).
Limitations of the technique are the fluorophore, which is currently non-specific to cancerous tissue, and the limited depth of penetration of the imaging system.
Nevertheless, prior to any routine clinical application, further validation is necessary to define the utility of fluorescence lymphatic mapping and if the lymph nodes identified with ICG fluorescence imaging are reflective of patient’s lymphatic basin status. Subsequently, the technique of both sentinel lymph nodes mapping in early gastric cancer and lymphatic mapping in gastric cancer should be standardized.
Intraoperative tumour identification
In last years, minimally invasive surgery for gastric cancer has spread worldwide. To date, according to ESMO guidelines and Japanese gastric cancer treatment guidelines, the technique can be considered a treatment option for stage I cancer, but there is no evidence for more advanced cancer (3,4). Due to the intense screening programme, early detection of small tumours is increasing in Asian countries. If open surgery allows identification of small gastric tumours through palpation when they are not visible, this possibility is denied during minimally invasive surgery. As a consequence, several methods of intraoperative localization for small lesions have been proposed. One of the most used methods is endoscopic tattooing with India Ink, but it can lead to inaccurate localization caused by faint or diffused tattooing, perforation and peritonitis due to intraperitoneal spraying of the ink. In addition, the charcoal, an India ink component, is not approved in some countries like in Korea. Intraoperative endoscopy is another diffused method to localize gastrointestinal tumours, but it requires the presence of specialised personnel and equipment and extended operative time. Moreover, swelling of the stomach can eventually interfere with laparoscopy. Placement of endoscopic clips is also possible, but they are not visible outside the stomach nor palpable. All these methods are associated with considerable limitations. This has motivated researchers to look for more efficient alternatives.
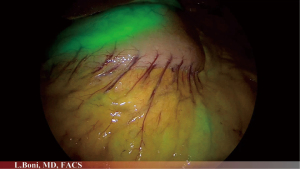
Recently, new methods have been developed as endoscopic clip with a light-emitting diode, endoclips with radio frequency identification, and endoscopic ICG tattooing (53). Endoscopic tattooing using ICG (Figure 4) skips the limits of the India ink and is also approved in Korea. The ICG tattooing site could be seen 1–8 days after marking, and then it will be completely absorbed (54). To date, there is no literature consensus on ICG dose and dilution required for endoscopic submucosal gastrointestinal ICG injection for tattooing (53,55,56). Ushimaru et al. published a retrospective analysis of patients with gastric cancer undergoing elective laparoscopic distal gastrectomy after endoscopic submucosal ICG injection the day before surgery. He proved that ICG fluorescence is useful in laparoscopic gastric cancer surgery to establish the surgical resection line. However, even these recent techniques have some limitations such as the need for a power source and the possibility of electromagnetic interference. Moreover, after ICG submucosal injection the precise tumour location may be difficult, because the dye often spreads into the submucosal layer.
Thus, effort has been put into combining the exact location of endoscopic clips with the advantages of ICG fluorescence imaging resulting in ICG-equipped clip. This tool does not require independent energy source, is not associated with electromagnetic interference and can easily point out tumour location. Takeyama et al. developed a method for applying fluorescence-coated endoscopic clips to visualise specific locations inside the colon during laparoscopic surgery, testing the procedure in both in vivo porcine model and ex vivo human colon tissues (56). Hyun et al. proposed an endoscopic fluorescent band ligation method to localize small tumour during gastric or colorectal surgery. It is based on fluorescent rubber band, made of ICG and liquid rubber solution, placed endoscopically on the mucosae of porcine stomach and colon and easily identified by near infrared fluorescence laparoscopic system in real time. This method seems to be superior to Takeyama’s one. As a matter of fact, on the one hand, it may be difficult to detect ICG fluorescence emitting from narrow and short clip through the stomach wall because of its thickness. On the other hand, fluorescent band can be applied at any sites of gastrointestinal tract and the intensity of fluorescent band is brighter than fluorescent clip’s one. Indeed, the ligating band containing fluorochromes can be located close to gastrointestinal serosal wall and can be maintained in place from 1 to 4 weeks, with a similar duration of endoscopic clips (57). Barberio et al. proposed fluorescent over-the-scope clips (FOSC) in a porcine model experiment, using a novel biocompatible fluorescent dye, as long-lasting marker instead of ICG tattoos, with similar wavelength sensitivities but an increased intensity if compared to ICG. When using endoscopic marker clips, the risk of jamming the jaws of the stapler during laparoscopic resections should be always considered (58).
Hayashi et al. described a tissue marker detectable by both X-ray computed tomography (CT) scan and near-infrared fluorescence systems. The marker is in giant cluster-like vesicles’ form, made up of liposomes, comprised phospholipids and a NIR fluorescent dye (an ICG derivative), and emulsions (phospholipids, oily radiographic contrast medium and polyglycerol-polyricinoleate). This vesicular dispersion was tested in an animal model through endoscopic injection. The authors concluded that injection sites are identified by CT scan one hour after administration and they are still fluorescent at NIR laparoscopy up to 18 hours later (59).
In order to increase the fluorescence efficiency and stability and to prolong the detection time, different ICG binding agents were proposed. Recently, Lee et al. showed promising results through in vivo animal studies, in mouse and porcine models, using injectable alginate hydrogel containing ICG-human serum albumin (HSA) complex (60). Other studies focused on the use of antibodies labeled with a fluorescent marker. Several studies reported that conjugated anti-MUC1 antibody with ICG-sulfo-Osu as fluorescent dye is related to nearly specific infrared fluorescence in cancer tissues (61). Muguruma et al. developed a molecular imaging method able to generate strong fluorescence if activated by cancer proteins. They used infrared fluorescence endoscope (IRFE) and anti-human carcinoembryonic antigen (CEA) antibody and MUC1 antibody labeled with ICG-N-hydroxysulfosuccinimide ester (ICG-sulfo-OSu) and 3-ICG-acyl-1,3-thiazolidine-2-thione (ICG-ATT) as infrared fluorescent-labeling reagents. The authors concluded that the fluorescence intensity of the ICG-ATT-labeled MUC1 antibody is stronger than the ICG-sulfo-OSu-labeled MUC1 antibody, as reported by literature evidence (62).
Another application of marking through ICG is in the field of endoscopic resections (EMR or ESD). The use of infrared electronic endoscope system to recognise mucosal and submucosal infiltration was already been studied (63,64). The relationship between positive fluorescence after intravenous injection of 0.01 mg/kg ICG and tumour invasion was studied by Kimura et al. (65). The authors used IRFE with a charge coupled device (CCD) at the tip of the endoscope providing high-quality images to observe superficial gastric tumours. ICG fluorescence was positive in 8 out of 10 gastric cancers with submucosal invasion (80%) and in 1 out of 20 adenomas or intramucosal gastric cancers (5%) (P<0.01). Submucosal invasion has higher correlation with fluorescence than with macroscopic morphology and histopathologic differentiation (63-65). This data could influence method of tumour diagnosis, as cancer is defined more by molecular characteristics, rather than by morphology (66).
Therefore, the future of endoscopic gastric cancer diagnosis could be a combination of biomarkers and instrumental technology. Molecular imaging called also immunoscopy, bioendoscopy, and optical biopsy, is the most novel imaging concept in the field of endoscopy.
Diagnostic and therapeutic application of ICG fluorescence in advanced gastric cancer
Other experimental diagnostic and therapeutic application of ICG in metastatic model of gastric cancer have been reported. Antibody or liposomal derivative can be bound to ICG to detect peritoneal metastases.
ICG-labeled antibody such as anti-human epidermal growth factor receptor (EGFR) or CEA antibodies can be used for detection of peritoneal micrometastases of 1–2 mm in size that cannot be seen by MRI o CT scan (67). Cheng et al. studied an integrin-targeting ICG method to detect peritoneal carcinomatosis in murine model of gastric tumour showing 93.93% of sensitivity, 100% of specificity and 93.93% of diagnostic accuracy (68). Hoshino et al. described a method based on NIR fluorescence imaging using a liposomal synthesized ICG liposomal derivative for detection of peritoneal nodules (69). A recent study by Pierangelo et al., introduced a promising intraoperative differentiation between inflammatory and malignant nodules using probe-based confocal laser endomicroscopy (pCLE). The technique was used immediately after surgery to perform ex vivo analysis through ICG application, and, in the future, it may allow surgeon to detect cancer in vivo (70).
Moreover, ICG-loaded lactosome has been used as diagnostic tool for peritoneal carcinosis after intravenous injection in animal models of gastric cancer and as photosensitizer of photodynamic therapy of animal model of peritoneal carcinomatosis and lymph nodes metastases of gastric cancer (71,72).
Conclusions
According to this review, ICG fluorescence imaging is a promising technique in minimally invasive gastric cancer surgery. Intraoperative ICG fluorescence angiography is feasible and safe and allows to assess gastric perfusion before performing upper GI anastomosis.
Several studies demonstrated the usefulness of ICG fluorescence lymphography in order to perform the sentinel lymph node mapping associated to endoscopic or laparoscopic resection of early gastric cancer, and lymphatic mapping to simplify lymphadenectomy during minimally invasive surgery and increase the number of lymph nodes harvested. Furthermore, some studies reported the possibility to identify gastric cancer peritoneal carcinomatosis through antibody or liposomal labelled ICG. Further studies are needed to prove the advantages the ICG guided minimally invasive gastric cancer surgery.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Stefano Rausei and Simone Giacopuzzi) for the series “Minimally Invasive Surgery and Gastric Cancer: Where Are We Now?” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://ales.amegroups.com/article/view/10.21037/ales-21-5/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://ales.amegroups.com/article/view/10.21037/ales-21-5/coif). The series “Minimally Invasive Surgery and Gastric Cancer: Where Are We Now?” was commissioned by the editorial office without any funding or sponsorship. LB served as an unpaid editorial board member of Annals of Laparoscopic and Endoscopic Surgery from June 2019 to May 2021. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kim HH, Han SU, Kim MC, et al. Effect of Laparoscopic Distal Gastrectomy vs Open Distal Gastrectomy on Long-term Survival Among Patients With Stage I Gastric Cancer: The KLASS-01 Randomized Clinical Trial. JAMA Oncol 2019;5:506-13. [Crossref] [PubMed]
- Katai H, Mizusawa J, Katayama H, et al. Single-arm confirmatory trial of laparoscopy-assisted total or proximal gastrectomy with nodal dissection for clinical stage I gastric cancer: Japan Clinical Oncology Group study JCOG1401. Gastric Cancer 2019;22:999-1008. [Crossref] [PubMed]
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer 2021;24:1-21.
- Smyth EC, Verheij M, Allum W, et al. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27:v38-49. [Crossref] [PubMed]
- Schaafsma BE, Mieog JS, Hutteman M, et al. The clinical use of indocyanine green as a near-infrared fluorescent contrast agent for image-guided oncologic surgery. J Surg Oncol 2011;104:323-32. [Crossref] [PubMed]
- Alander JT, Kaartinen I, Laakso A, et al. A review of indocyanine green fluorescent imaging in surgery. Int J Biomed Imaging 2012;2012:940585. [Crossref] [PubMed]
- Ishizawa T, Fukushima N, Shibahara J, et al. Real-time identification of liver cancers by using indocyanine green fluorescent imaging. Cancer 2009;115:2491-504. [Crossref] [PubMed]
- Tajima Y, Murakami M, Yamazaki K, et al. Sentinel node mapping guided by indocyanine green fluorescence imaging during laparoscopic surgery in gastric cancer. Ann Surg Oncol 2010;17:1787-93. [Crossref] [PubMed]
- Korn JM, Tellez-Diaz A, Bartz-Kurycki M, et al. Indocyanine green SPY elite-assisted sentinel lymph node biopsy in cutaneous melanoma. Plast Reconstr Surg 2014;133:914-22. [Crossref] [PubMed]
- Tanaka E, Choi HS, Fujii H, et al. Image-guided oncologic surgery using invisible light: completed pre-clinical development for sentinel lymph node mapping. Ann Surg Oncol 2006;13:1671-81. [Crossref] [PubMed]
- Daskalaki D, Fernandes E, Wang X, et al. Indocyanine green (ICG) fluorescent cholangiography during robotic cholecystectomy: results of 184 consecutive cases in a single institution. Surg Innov 2014;21:615-21. [Crossref] [PubMed]
- Luo S, Zhang E, Su Y, et al. A review of NIR dyes in cancer targeting and imaging. Biomaterials 2011;32:7127-38. [Crossref] [PubMed]
- Kim MC, Kim W, Kim HH, et al. Risk factors associated with complication following laparoscopy-assisted gastrectomy for gastric cancer: a large-scale korean multicenter study. Ann Surg Oncol 2008;15:2692-700. [Crossref] [PubMed]
- Degett TH, Andersen HS, Gögenur I. Indocyanine green fluorescence angiography for intraoperative assessment of gastrointestinal anastomotic perfusion: a systematic review of clinical trials. Langenbecks Arch Surg 2016;401:767-75. [Crossref] [PubMed]
- Inokuchi M, Otsuki S, Fujimori Y, et al. Systematic review of anastomotic complications of esophagojejunostomy after laparoscopic total gastrectomy. World J Gastroenterol 2015;21:9656-65. [Crossref] [PubMed]
- Nagasako Y, Satoh S, Isogaki J, et al. Impact of anastomotic complications on outcome after laparoscopic gastrectomy for early gastric cancer. Br J Surg 2012;99:849-54. [Crossref] [PubMed]
- Kanda M, Ito S, Mochizuki Y, et al. Multi-institutional analysis of the prognostic significance of postoperative complications after curative resection for gastric cancer. Cancer Med 2019;8:5194-201. [Crossref] [PubMed]
- Karliczek A, Harlaar NJ, Zeebregts CJ, et al. Surgeons lack predictive accuracy for anastomotic leakage in gastrointestinal surgery. Int J Colorectal Dis 2009;24:569-76. [Crossref] [PubMed]
- Nachiappan S, Askari A, Currie A, et al. Intraoperative assessment of colorectal anastomotic integrity: a systematic review. Surg Endosc 2014;28:2513-30. [Crossref] [PubMed]
- Boni L, Macina S, David G, ICG-Enhanced fluorescence-guided laparoscopic surgery 2nd Edition. Endo Press GmbH, 2019.
- Huh YJ, Lee HJ, Kim TH, et al. Efficacy of Assessing Intraoperative Bowel Perfusion with Near-Infrared Camera in Laparoscopic Gastric Cancer Surgery. J Laparoendosc Adv Surg Tech A 2019;29:476-83. [Crossref] [PubMed]
- Slooter MD, de Bruin DM, Eshuis WJ, et al. Quantitative fluorescence-guided perfusion assessment of the gastric conduit to predict anastomotic complications after esophagectomy. Dis Esophagus 2021;34:doaa100.
- Lütken CD, Achiam MP, Svendsen MB, et al. Optimizing quantitative fluorescence angiography for visceral perfusion assessment. Surg Endosc 2020;34:5223-33. [Crossref] [PubMed]
- Mori M, Shuto K, Hirano A, et al. A Novel Parameter Identified Using Indocyanine Green Fluorescence Angiography may Contribute to Predicting Anastomotic Leakage in Gastric Cancer Surgery. World J Surg 2020;44:2699-708. [Crossref] [PubMed]
- Kim M, Son SY, Cui LH, et al. Real-time Vessel Navigation Using Indocyanine Green Fluorescence during Robotic or Laparoscopic Gastrectomy for Gastric Cancer. J Gastric Cancer 2017;17:145-53. [Crossref] [PubMed]
- Kim JP, Lee JH, Kim SJ, et al. Clinicopathologic characteristics and prognostic factors in 10 783 patients with gastric cancer. Gastric Cancer 1998;1:125-33. [Crossref] [PubMed]
- Sim SH, Kim YJ, Oh DY, et al. The role of PET/CT in detection of gastric cancer recurrence. BMC Cancer 2009;9:73. [Crossref] [PubMed]
- Cuschieri A, Fayers P, Fielding J, et al. Postoperative morbidity and mortality after D1 and D2 resections for gastric cancer: preliminary results of the MRC randomised controlled surgical trial. The Surgical Cooperative Group. Lancet 1996;347:995-9. [Crossref] [PubMed]
- Kitagawa Y, Takeuchi H, Takagi Y, et al. Sentinel node mapping for gastric cancer: a prospective multicenter trial in Japan. J Clin Oncol 2013;31:3704-10. [Crossref] [PubMed]
- Morton DL, Wen DR, Wong JH, et al. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg 1992;127:392-9. [Crossref] [PubMed]
- Wang Z, Dong ZY, Chen JQ, et al. Diagnostic value of sentinel lymph node biopsy in gastric cancer: a meta-analysis. Ann Surg Oncol 2012;19:1541-50. [Crossref] [PubMed]
- Maruyama K, Sasako M, Kinoshita T, et al. Can sentinel node biopsy indicate rational extent of lymphadenectomy in gastric cancer surgery? Fundamental and new information on lymph-node dissection. Langenbecks Arch Surg 1999;384:149-57. [Crossref] [PubMed]
- Kusano M, Tajima Y, Yamazaki K, et al. Sentinel node mapping guided by indocyanine green fluorescence imaging: a new method for sentinel node navigation surgery in gastrointestinal cancer. Dig Surg 2008;25:103-8. [Crossref] [PubMed]
- Kitagawa Y, Fujii H, Kumai K, et al. Recent advances in sentinel node navigation for gastric cancer: a paradigm shift of surgical management. J Surg Oncol 2005;90:147-51; discussion 151-2. [Crossref] [PubMed]
- Huang L, Wei T, Chen J, et al. Feasibility and diagnostic performance of dual-tracer-guided sentinel lymph node biopsy in cT1-2N0M0 gastric cancer: a systematic review and meta-analysis of diagnostic studies. World J Surg Oncol 2017;15:103. [Crossref] [PubMed]
- Tajima Y, Yamazaki K, Masuda Y, et al. Sentinel node mapping guided by indocyanine green fluorescence imaging in gastric cancer. Ann Surg 2009;249:58-62. [Crossref] [PubMed]
- Kinami S, Oonishi T, Fujita J, et al. Optimal settings and accuracy of indocyanine green fluorescence imaging for sentinel node biopsy in early gastric cancer. Oncol Lett 2016;11:4055-62. [Crossref] [PubMed]
- Skubleny D, Dang JT, Skulsky S, et al. Diagnostic evaluation of sentinel lymph node biopsy using indocyanine green and infrared or fluorescent imaging in gastric cancer: a systematic review and meta-analysis. Surg Endosc 2018;32:2620-31. [Crossref] [PubMed]
- He M, Jiang Z, Wang C, et al. Diagnostic value of near-infrared or fluorescent indocyanine green guided sentinel lymph node mapping in gastric cancer: A systematic review and meta-analysis. J Surg Oncol 2018;118:1243-56. [Crossref] [PubMed]
- Shida A, Mitsumori N, Fujioka S, et al. Sentinel Node Navigation Surgery for Early Gastric Cancer: Analysis of Factors Which Affect Direction of Lymphatic Drainage. World J Surg 2018;42:766-72. [Crossref] [PubMed]
- Lee YJ, Jeong SH, Hur H, et al. Prospective Multicenter Feasibility Study of Laparoscopic Sentinel Basin Dissection for Organ Preserving Surgery in Gastric Cancer: Quality Control Study for Surgical Standardization Prior to Phase III Trial. Medicine (Baltimore) 2015;94:e1894. [Crossref] [PubMed]
- Xiong L, Gazyakan E, Yang W, et al. Indocyanine green fluorescence-guided sentinel node biopsy: a meta-analysis on detection rate and diagnostic performance. Eur J Surg Oncol 2014;40:843-9. [Crossref] [PubMed]
- Lee SE, Lee JH, Ryu KW, et al. Sentinel node mapping and skip metastases in patients with early gastric cancer. Ann Surg Oncol 2009;16:603-8. [Crossref] [PubMed]
- Miyashiro I, Hiratsuka M, Sasako M, et al. High false-negative proportion of intraoperative histological examination as a serious problem for clinical application of sentinel node biopsy for early gastric cancer: final results of the Japan Clinical Oncology Group multicenter trial JCOG0302. Gastric Cancer 2014;17:316-23. [Crossref] [PubMed]
- Shoji Y, Kumagai K, Kamiya S, et al. Prospective feasibility study for single-tracer sentinel node mapping by ICG (indocyanine green) fluorescence and OSNA (one-step nucleic acid amplification) assay in laparoscopic gastric cancer surgery. Gastric Cancer 2019;22:873-80. [Crossref] [PubMed]
- Yano K, Nimura H, Mitsumori N, et al. The efficiency of micrometastasis by sentinel node navigation surgery using indocyanine green and infrared ray laparoscopy system for gastric cancer. Gastric Cancer 2012;15:287-91. [Crossref] [PubMed]
- Okubo K, Uenosono Y, Arigami T, et al. Quantitative assessment of fluorescence intensity of ICG in sentinel nodes in early gastric cancer. Gastric Cancer 2018;21:776-81. [Crossref] [PubMed]
- Chen QY, Xie JW, Zhong Q, et al. Safety and Efficacy of Indocyanine Green Tracer-Guided Lymph Node Dissection During Laparoscopic Radical Gastrectomy in Patients With Gastric Cancer: A Randomized Clinical Trial. JAMA Surg 2020;155:300-11. [Crossref] [PubMed]
- Baiocchi GL, Molfino S, Molteni B, et al. Fluorescence-guided lymphadenectomy in gastric cancer: a prospective western series. Updates Surg 2020;72:761-72. [Crossref] [PubMed]
- Kwon IG, Son T, Kim HI, et al. Fluorescent Lymphography-Guided Lymphadenectomy During Robotic Radical Gastrectomy for Gastric Cancer. JAMA Surg 2019;154:150-8. [Crossref] [PubMed]
- Cianchi F, Indennitate G, Paoli B, et al. The Clinical Value of Fluorescent Lymphography with Indocyanine Green During Robotic Surgery for Gastric Cancer: a Matched Cohort Study. J Gastrointest Surg 2020;24:2197-203. [Crossref] [PubMed]
- Kim TH, Kong SH, Park JH, et al. Assessment of the Completeness of Lymph Node Dissection Using Near-infrared Imaging with Indocyanine Green in Laparoscopic Gastrectomy for Gastric Cancer. J Gastric Cancer 2018;18:161-71. [Crossref] [PubMed]
- Ushimaru Y, Omori T, Fujiwara Y, et al. The Feasibility and Safety of Preoperative Fluorescence Marking with Indocyanine Green (ICG) in Laparoscopic Gastrectomy for Gastric Cancer. J Gastrointest Surg 2019;23:468-76. [Crossref] [PubMed]
- Miyoshi N, Ohue M, Noura S, et al. Surgical usefulness of indocyanine green as an alternative to India ink for endoscopic marking. Surg Endosc 2009;23:347-51. [Crossref] [PubMed]
- Tanaka C, Kanda M, Funasaka K, et al. Detection of indocyanine green fluorescence to determine tumor location during laparoscopic gastrectomy for gastric cancer: Results of a prospective study. Asian J Endosc Surg 2020;13:160-7. [Crossref] [PubMed]
- Takeyama H, Hata T, Nishimura J, et al. A novel endoscopic fluorescent clip visible with near-infrared imaging during laparoscopic surgery in a porcine model. Surg Endosc 2014;28:1984-90. [Crossref] [PubMed]
- Hyun JH, Kim SK, Kim KG, et al. A novel endoscopic fluorescent band ligation method for tumor localization. Surg Endosc 2016;30:4659-63. [Crossref] [PubMed]
- Barberio M, Pizzicannella M, Spota A, et al. Preoperative endoscopic marking of the gastrointestinal tract using fluorescence imaging: submucosal indocyanine green tattooing versus a novel fluorescent over-the-scope clip in a survival experimental study. Surg Endosc 2021;35:5115-23. [Crossref] [PubMed]
- Hayashi H, Toyota T, Goto S, et al. Development of a non-blurring, dual-imaging tissue marker for gastrointestinal tumor localization. Surg Endosc 2015;29:1445-51. [Crossref] [PubMed]
- Lee SS, Kim H, Sohn DK, et al. Indocyanine green-loaded injectable alginate hydrogel as a marker for precision cancer surgery. Quant Imaging Med Surg 2020;10:779-88. [Crossref] [PubMed]
- Bando T, Muguruma N, Ito S, et al. Basic studies on a labeled anti-mucin antibody detectable by infrared-fluorescence endoscopy. J Gastroenterol 2002;37:260-9. [Crossref] [PubMed]
- Muguruma N, Ito S. Labeled anti-mucin antibody detectable by infrared-fluorescence endoscopy. Cancer Biomark 2008;4:321-8. [Crossref] [PubMed]
- Mataki N, Nagao S, Kawaguchi A, et al. Clinical usefulness of a new infrared videoendoscope system for diagnosis of early stage gastric cancer. Gastrointest Endosc 2003;57:336-42. [Crossref] [PubMed]
- Iseki K, Tatsuta M, Iishi H, et al. Effectiveness of the near-infrared electronic endoscope for diagnosis of the depth of involvement of gastric cancers. Gastrointest Endosc 2000;52:755-62. [Crossref] [PubMed]
- Kimura T, Muguruma N, Ito S, et al. Infrared fluorescence endoscopy for the diagnosis of superficial gastric tumors. Gastrointest Endosc 2007;66:37-43. [Crossref] [PubMed]
- Namikawa T, Iwabu J, Munekage M, et al. Evolution of photodynamic medicine based on fluorescence image-guided diagnosis using indocyanine green and 5-aminolevulinic acid. Surg Today 2020;50:821-31. [Crossref] [PubMed]
- Ito A, Ito Y, Matsushima S, et al. New whole-body multimodality imaging of gastric cancer peritoneal metastasis combining fluorescence imaging with ICG-labeled antibody and MRI in mice. Gastric Cancer 2014;17:497-507. [Crossref] [PubMed]
- Cheng H, Chi C, Shang W, et al. Precise integrin-targeting near-infrared imaging-guided surgical method increases surgical qualification of peritoneal carcinomatosis from gastric cancer in mice. Oncotarget 2017;8:6258-72. [Crossref] [PubMed]
- Hoshino I, Maruyama T, Fujito H, et al. Detection of peritoneal dissemination with near-infrared fluorescence laparoscopic imaging using a liposomal formulation of a synthesized indocyanine green liposomal derivative. Anticancer Res 2015;35:1353-9. [PubMed]
- Pierangelo A, Fuks D, Benali A, et al. Diagnostic accuracy of confocal laser endomicroscopy for the ex vivo characterization of peritoneal nodules during laparoscopic surgery. Surg Endosc 2017;31:1974-81. [Crossref] [PubMed]
- Tsujimoto H, Morimoto Y, Takahata R, et al. Theranostic Photosensitive Nanoparticles for Lymph Node Metastasis of Gastric Cancer. Ann Surg Oncol 2015;22:S923-8. [Crossref] [PubMed]
- Tsujimoto H, Morimoto Y, Takahata R, et al. Photodynamic therapy using nanoparticle loaded with indocyanine green for experimental peritoneal dissemination of gastric cancer. Cancer Sci 2014;105:1626-30. [Crossref] [PubMed]
Cite this article as: Bertani C, Cassinotti E, Della Porta M, Pagani M, Boni L, Baldari L. Indocyanine green—a potential to explore: narrative review. Ann Laparosc Endosc Surg 2022;7:9.