The value of laparoscopic intraoperative ultrasound of the liver by the surgeon
Introduction
Ultrasonography is an extremely cheap, safe and minimally invasive diagnostic tool in the hands of a skillful user. It has been an important instrument in abdominal surgery for more than 30 years (1). Intraoperative ultrasound (IOUS) is an important tool to surgeons in liver resections for several reasons. It assists the operator in identifying the surgical anatomy with real time imaging and it gives him information about the size and quantity of the tumors with also a good accuracy for detecting small lesions (2,3). Different studies have shown the added value of ultrasound during laparotomic liver surgery. Zacherl et al. found that IOUS changed surgical strategy in 22.8% of cases. The sensitivity of IOUS in a segment-by-segment analysis for colorectal liver metastasis was 95.2%, which was the highest amongst the diagnostic techniques including CT and MRI. Based on these findings, they concluded that IOUS even should be considered the gold standard for hepatic neoplasms (4).
Laparoscopic liver surgery is increasingly performed nowadays, and has gradually replaced laparotomic surgery for numerous indications. The first minimal invasive liver resection was reported in 1993 and laparoscopic liver surgery has gradually become widespread since then (5). Laparoscopic resection of liver tumors has proven to be safe; it has comparable oncologic outcomes to open liver resections and the perioperative outcomes are better (6-8). Although laparoscopic intraoperative ultrasound (LIOUS) is also considered essential and a standard tool in laparoscopic liver surgery, not much research has been done to confirm this (9).
A big advantage of (laparoscopic) IOUS is an unobstructed view of the liver without interference of costae or abdominal wall. All liver segments are available for ultrasonographic examination as a result of this direct contact and liver nodules can be identified with high sensitivity and specificity (10). The importance of ultrasound in laparoscopic surgery is even bigger than during open surgery due to the lack of palpation of the liver during laparoscopy.
In contrast, the main limitation is that LIOUS requires a more demanding handling technique due to several reasons. Ultrasound gives an additional dimension to work with, while spatial orientation is already more difficult during laparoscopic surgery. Furthermore, the fixed entry during laparoscopy makes it harder to get a right angulation, although a flexible laparoscopic ultrasound tip partly negates this problem. And lastly, the device is relatively expensive.
One of the first studies about LIOUS was done on pigs in 1998 and revealed a sensitivity of 80% and a specificity of 91% (11). Since then, several studies about LIOUS were performed but most of them are outdated. This is mainly because preoperative imaging techniques have become much better in the past two decades (e.g., multidetector helical CT, magnetic resonance spectroscopy and new contrast agents). The only relevant and recent study about LIOUS was done by Viganò et al, concluding that LIOUS is a reliable tool for staging liver tumors with a performance similar to open IOUS in detecting new nodules (12).
As mentioned, LIOUS is an indispensable adjunct in laparoscopic liver surgery, as also emphasized by the Southampton Consensus Guidelines for Laparoscopic Liver Surgery. In our centers it is performed routinely. Whereas ultrasound is often a specific expertise of the radiologist, the handling of laparoscopic instruments in a three-dimensional operating field is usually the expertise of the laparoscopic surgeon. In practice, we observed that radiologists often had problems in handling the laparoscopic ultrasound to satisfaction. We therefore trained our surgical staff in performing laparoscopic ultrasound of the liver. The aim of the study was to analyze the accuracy of LIOUS performed by ultrasound-trained and experienced laparoscopic surgeons instead of radiologists. We present the following article in accordance with the STARD reporting checklist (available at http://dx.doi.org/10.21037/ales-20-106).
Methods
Patients
Patients were retrospectively included between 2014 and 2018 in the Jeroen Bosch Ziekenhuis in Den Bosch. Inclusion criteria were a laparoscopic liver procedure due to a suspected malignancy and the usage of LIOUS.
LIOUS training
Both surgeons were trained by the center’s own radiologists in open perioperative hepatic ultrasound. They received additional on-site training and off-site training in cooperation with the ultrasound manufacturer. The surgeons were trained in using a L44LA Linear Laparoscopic 4 Way probe with a frequency range of 13–2 MHz, which is compatible with the Arietta system (Hitachi Medical Systems Europe). After a minimum of 10 cases executed with a supplier representative, LIOUS by the surgeon alone was implemented.
LIOUS
LIOUS was performed by one of both surgeons. A minimum of one 12mm trocar was used for probe introduction during surgery. The number of trocars depended on the resection that was going to be performed.
Characterization of liver lesions depended on different aspects. Patient characteristics were taken into account like age, symptoms, previous chemotherapeutic treatment and presence of liver disease as steatosis, hepatofibrosis and cirrhosis. Specific signs to regard on ultrasound (e.g., echogenicity, vascularisation) are broad and all aspects were taken into account for decision making. Cystic lesions were defined as anechoic with posterior acoustic enhancement and without thickening of the wall. Hemangiomas were defined as uniformly hyperechoic, presence of posterior echo enhancement and a well-defined margin. Focal nodular hyperplasia was defined as mostly isoechoic, with a typical doppler vascular pattern and a central scarring. Although malignant lesions vary in their presentation, they can be roughly defined as follows: a heterogeneous and rigid structure, an imprecise delineation, disrupture of normal liver architecture and they may have a pronounced circulatory signal. The bull’s eye, or target sign, is due to compressed normal tissue and proliferation of cancer cells and also indicates malignancy. Colorectal liver metastasis are usually calcified, which creates a shadow behind the lesion.
Primary outcome
The percentage of procedures that changed the preoperative surgical plan due to information from LIOUS was calculated. In addition, it was analyzed how the surgical plan was changed and whether the impact had positive or negative clinical implications. Information that could have also been obtained during inspection was not taken into account for the primary outcome.
Secondary outcomes
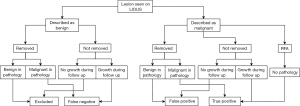
Accuracy of LIOUS was measured as sensitivity and positive predictive value. Positive predictive value was used instead of specificity because a true negative rate is undefinable in a per-lesion analysis. A per-lesion analysis was preferred in this study instead of a segment-by-segment analysis because every lesion is accounted for in this way. A cross-reference was performed using the pathology reports and follow-up data. A false positive lesion was defined as: malignant on LIOUS but benign in pathology results. False negative was defined as: non-malignant on LIOUS but malignant in pathology results (when the lesion was resected because it appeared malignant on other imaging modalities) or with obvious malignant growth during follow-up. The method of calculating false negative and positive rates is also shown in Figure 1. Unlike the primary outcome, lesions seen during inspection were counted as positive for LIOUS in the secondary outcome. All near satellite lesions were counted as one because they were often regarded as one in diagnostic reports. In the case of a hemi-hepatectomy, the whole resected specimen was counted as one positive result. The lesions in the future liver remnant were each separately analyzed. Pathological cross-reference was not possible in the case of ablative therapy. These lesions were not excluded. After neoadjuvant therapy, lesions were counted as non-malignant when no malignant cells were found in pathology.
The size of all benign and malignant lesions on LIOUS was noted. When a lesion was not measured on LIOUS, the size measured in pathology was used and otherwise the size on the most recent imaging (CT or MRI).
Collected information
The following information was collected: patient information (age during surgery, gender and liver disease); preoperative plan of surgery, type of surgery performed, blood loss and duration; information obtained during surgery through inspection and LIOUS, sonographer; type of malignancy and number, size and location of tumors seen in postoperative pathology results; follow up data regarding the liver lesions.
All procedures performed in this study were in accordance with the Declaration of Helsinki (as revised in 2013). Because of the retrospective nature of the research, the requirement for informed consent was waived.
Statistical analysis
For the descriptive statistics, normally distributed data is shown as mean with the standard deviation. Non-normally distributed data is shown as mean with the interquartile range. The 95% confidence intervals of the accuracy of LIOUS were calculated using Wilson score interval. The significance of the difference in size between the resected malignant lesions, the resected benign lesions and the non-resected malignant lesions was calculated using ANOVA with a P-value <0.05.
Results
Patient and surgery characteristics
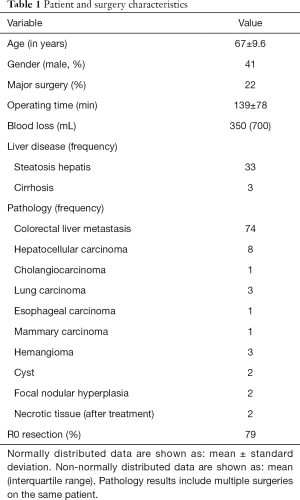
Ninety-one patients and 107 surgical procedures in which LIOUS was used, were included. No patients had to be excluded. All LIOUS were performed by a surgeon. Of all patients, 41% was male and the average age during surgery was 67 years old. Anatomic resection i.e., segmental resection was conducted in 36 cases, metastasectomy in 40 cases and in 9 cases they were combined. 50% of all resections included one or more lesions in deep segments (i.e., 1, 4a, 7 or 8). When defining major surgery as resection of ≥4 lesions or simultaneous other major surgery (e.g., colectomy), 22% met these criteria. 13 surgeries were right hemihepatectomies. The median blood loss was 350 mL (interquartile range: 700 mL) and the average operating time was 139 minutes (standard deviation: 78 minutes). On pathological examination, colorectal carcinoma was most frequent seen (n=74) followed by hepatocellular carcinoma (n=8). Thirty-three pathology reports showed a form of liver steatosis and 3 showed cirrhosis. All patients with hepatocellular carcinoma had steatosis or cirrhosis. After oncologic resection, 66 pathology reports showed R0 resection, 18 showed R1 resection and 4 were inconclusive. R1 resection was defined as a combined parameter of margin <1 mm and margin 1–3 mm. The percentage of R0 resection was 79%. All these results are shown in Table 1.
Full table
Primary outcome
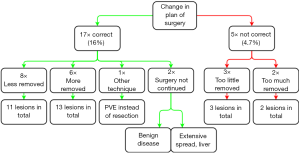
Pre-operative imaging was obtained in all 107 surgeries: 101 CT scans and 59 MRI scans. LIOUS changed the preoperative plan of surgery in 22 cases. The plan was changed 17 times (16%) for the benefit of the patient: during 8 surgical procedures, 11 lesions were accurately not removed because they seemed non-malignant on LIOUS and follow-up did not show malignancy at these sites over time. No biopsies were taken to confirm benignancy. 7 preoperative CT scans and 6 preoperative MRI scans were obtained in these cases and lesions were false positive on 3 CT scans and 5 MRI scans. Furthermore, 13 lesions during 6 procedures were additionally removed and were malignant in pathology. Only preoperative CT scans were obtained in these cases and no MRI scans. Also, one time, portal vein embolization was chosen instead of resection because resecting the lesions seemed not safe on LIOUS. And lastly, two surgeries were aborted due to information gained from LIOUS: one due to extensive spread of the disease in the liver, the other because LIOUS suspected benign cysts which were confirmed with a frozen section analysis (although CT and MRI showed suspected malignancy).
The plan of surgery was changed to the disadvantage of the patient during 5 surgeries (4.7%). Twice, an extra lesion was removed but appeared non-malignant in pathology. And three times, a lesion was not removed because it was not visible on LIOUS although pre-operative imaging suspected a malignancy (CT in all three cases and an additional MRI in one case). All three patients underwent additional procedures of which two were also scheduled to remove other lesions after local recurrence. All these results are summarized in Figure 2.
Secondary outcomes
Accuracy
A total of 165 lesions were described malignant in 107 LIOUS examinations. 22 benign lesions were wrongfully diagnosed as malignant and 6 lesions were missed using LIOUS. Sensitivity of LIOUS was 96% (92–98%) and positive predictive value was 88% (82–92%). The 95% confidence intervals were calculated using Wilson score interval.
Furthermore, 12 lesions were treated with ablative therapy so no tissue specimen was available for pathology in these cases. During a 1 to 4 years follow-up, no local recurrence was detected on the sites that were treated with ablative therapy.
The quantitative origin of false negative and false positive rates are shown in the Figure S1.
Size
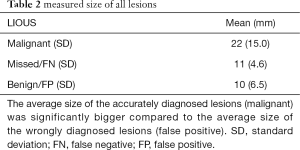
The average size measured of all malignant, false negative (missed) and false positive (benign) lesions was 22, 11 and 10 mm respectively. The average size of all false positive lesions was significantly smaller than the average size of all malignant lesions using ANOVA (P<0.05). The average size of all malignant lesions was not significantly different than the average size of all false negative lesions (P=0.11), but this last group contained only 6 lesions. These results are shown in Table 2.
Full table
Discussion
Of all 107 surgical procedures in which LIOUS was used, surgical strategy was changed for the benefit of the patient in 16% of cases, including 2 surgeries that were accurately prematurely ended. In 4.7% of all cases, surgery was changed to the disadvantage of the patient.
Sensitivity and positive predictive value were 96% and 88% respectively for LIOUS. The average size of all false positive and false negative lesions was smaller than the average size of all malignant lesions.
This study aids in filling the gap in research on LIOUS and contains, so far, the highest number of LIOUS in laparoscopic liver procedures in the past decade. In this study, LIOUS had a larger positive than negative impact on decision making during surgery. Viganò et al. already concluded in their study that LIOUS should be a reliable tool for staging liver tumors (12). This study also shows that a laparoscopic surgeon is able to perform LIOUS with good accuracy.
Limitations and strengths
Sensitivity could be overestimated in this study due to the lack of a gold standard when lesions were not resected i.e., whether a postoperative malignant lesion was new, or missed before, was not always evident. A surgeon would, on the other hand, resect a lesion when in doubt: which could increase false positive rate for LIOUS. Also, there was no pathology available when ablative therapy was performed, although ablation was only done in cases with little doubt about the nature of the lesion. And lastly, this is a retrospective study with inherent limitations like concomitant inferior level of evidence and risk for confounding.
Besides limitations in measuring accuracy, an advantage of this study compared to prospective studies is that it is a representation of daily practice. Per-lesion analysis with verification in pathological examination and follow-up data could be a correct way to retrospectively analyze accuracy of imaging for liver procedures.
Recommendations
The Southampton Consensus Guidelines describe LIOUS as a necessary tool, which is supported by this study and previous ones. LIOUS adds value to the diagnostic process around liver surgery for malignant indications besides CT and MRI and should always be considered in laparoscopic liver surgery. However, the prognostic and thereby clinical relevance need to be further investigated. A course for learning the skills required to perform LIOUS is important and could be implemented in the curriculum to becoming a surgeon.
Significantly more diagnostic mistakes were made with smaller lesions (see Table 2). Extra attention should be paid to these and especially sub-centimeter lesions.
Suggestions for further research
Future research could be aimed at improving technical quality of laparoscopic ultrasound: for example, image fusion between ultrasound and laparoscopy, 3D ultrasound and ultrasound guided navigation.
Conclusions
Of all liver procedures, 16% changed for the benefit of the patient due to LIOUS, including 2 surgeries that were accurately prematurely ended. Compared to 4.7% that changed to the disadvantage of the patient. Sensitivity and positive predictive value were 96% and 88% respectively. LIOUS is a reliable diagnostic tool and its added value was confirmed, as was in previous studies.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at http://dx.doi.org/10.21037/ales-20-106
Data Sharing Statement: Available at http://dx.doi.org/10.21037/ales-20-106
Peer Review File: Available at http://dx.doi.org/10.21037/ales-20-106
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales-20-106). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the Declaration of Helsinki (as revised in 2013). Because of the retrospective nature of the research, the requirement for informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Piccolboni D, Ciccone F, Settembre A, et al. The role of echo-laparoscopy in abdominal surgery: Five years’ experience in a dedicated center. Surg Endosc 2008;22:112-7. [Crossref] [PubMed]
- Garancini M, Gianotti L, Delitala A, et al. Intra-operative ultrasound: A review on its role in liver surgery for primitive and metastatic tumors. Minerva Chir 2016;71:201-13. [PubMed]
- Gouillat C, Ben-Hayoun E, Detry L, et al. Value of intraoperative ultrasonography in the surgical treatment of malignant tumors of the liver. Ann Chir 1991;45:534-9. [PubMed]
- Zacherl J, Scheuba C, Imhof M, et al. Current value of intraoperative sonography during surgery for Hepatic neoplasms. World J Surg 2002;26:550-4. [Crossref] [PubMed]
- Karagkounis G, Akyuz M, Guerron AD, et al. Perioperative and oncologic outcomes of minimally invasive liver resection for colorectal metastases: A case–control study of 130 patients. Surgery 2016;160:1097-103. [Crossref] [PubMed]
- Cheng Y, Zhang L, Li H, et al. Laparoscopic versus open liver resection for colorectal liver metastases: a systematic review. J Surg Res 2017;220:234-46. [Crossref] [PubMed]
- Lee W, Park J-H, Kim J-Y, et al. Comparison of perioperative and oncologic outcomes between open and laparoscopic liver resection for intrahepatic cholangiocarcinoma. Surg Endosc 2016;30:4835-40. [Crossref] [PubMed]
- Geller DA, Tsung A. Long-term outcomes and safety of laparoscopic liver resection surgery for hepatocellular carcinoma and metastatic colorectal cancer. J Hepatobiliary Pancreat Sci 2015 Oct;22(1:728-30.
- Abu Hilal M, Aldrighetti L, Dagher I, et al. The Southampton Consensus Guidelines for Laparoscopic Liver Surgery: From Indication to Implementation. Ann Surg 2018;268:11-8. [Crossref] [PubMed]
- Ellebæk SB, Fristrup CW, Mortensen MB. Intraoperative ultrasound as a screening modality for the detection of liver metastases during resection of primary colorectal cancer–A systematic review. Ultrasound Int Open 2017;3:E60-8. [Crossref] [PubMed]
- Foley EF, Kolecki R V, Schirmer BD. The accuracy of laparoscopic ultrasound in the detection of colorectal cancer liver metastases. Am J Surg 1998;176:262-4. [Crossref] [PubMed]
- Viganò L, Ferrero A, Amisano M, et al. Comparison of laparoscopic and open intraoperative ultrasonography for staging liver tumours. Br J Surg 2013;100:535-42. [Crossref] [PubMed]
Cite this article as: van der Steen K, Bosscha K, Lips DJ. The value of laparoscopic intraoperative ultrasound of the liver by the surgeon. Ann Laparosc Endosc Surg 2021;6:17.