
A minimally invasive approach to colon cancer resection improves time to adjuvant chemotherapy
Introduction
Colorectal cancer is the second leading cause of cancer death in the United States each year. For stage III colon cancer patients, the use of adjuvant chemotherapy after surgical resection has been associated with a survival benefit (1,2). However, even with the addition of oxaliplatin to the adjuvant chemotherapy regimen, the 6-year overall survival (OS) rates for resected stage III colon cancer are only 78.5% (2). Although the exact timing is unknown, timely initiation of adjuvant chemotherapy, generally defined by multiple trials as starting between 6–8 weeks following surgery, has been demonstrated to have an effect on OS and disease-free survival in multiple retrospective analyses. Specifically, in a meta-analysis looking at time to adjuvant chemotherapy (TTAC), a 4-week increase in TTAC led to a decrease in OS and disease-free survival among stage III colon cancer patients (3).
Recognizing factors leading to delays in initiating chemotherapy after colon cancer surgery is therefore critical to improve overall clinical outcomes in those with stage III colon cancer. Given the complex multispecialty coordination needed to get patients to chemotherapy, delays can occur at different steps in the process. We hypothesized that the minimally invasive approach to colon cancer is associated with timely initiation of adjuvant treatment. Accordingly, the primary objective of the study was to examine factors that influence TTAC.
The impact of some surgery specific variables has not been a focus of study for factors impacting TTAC. Minimally invasive surgery (laparoscopic or robotic) for colon patients has been shown to decrease the overall time of postoperative recovery with patients leaving the hospital and returning to regular activities in a shorter time period (4).Data also demonstrates that surgical subspecialty impacts survival in colorectal cancer. Patients operated on by specialists show improved 5-year survival and less postoperative mortality when compared with non-specialty trained surgeons (5). Despite these improvements, there has not been data to show if either surgeon specialty or surgical approach impact TTAC. We also aimed to evaluate the overall rate of adjuvant chemotherapy use for stage III colon cancer patients at a single institution, and to identify both patient and systems factors that may affect the time to initiation of this therapy.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/ales-20-95).
Methods
All stage III colon cancer patients who had surgery between January 2008 and March 2014 at a single academic, university hospital were identified and chart review performed. The hospital tumor registry identified patients diagnosed with stage IIIa, IIIb, or IIIc adenocarcinoma of the colon who were hospitalized and underwent surgery during the study period. Any patient who received chemotherapy >150 days after surgery (outliers), chose not to receive chemotherapy, was lost to follow-up, or was not a candidate for chemotherapy as determined by the treating medical oncologist was excluded from the final analysis.
Over the study time interval, there were a total of twelve colorectal surgeons and six surgical oncologists practicing at our institution, seven employed as full-time faculty and eleven in community practice. Colon surgery was also performed by eight acute care and general surgeons. There were two full-time medical oncologists in the university practice accepting colorectal cancer patients, and approximately 20 medical oncologists with privileges at the hospital not employed by the medical school.
The main focus of interest was TTAC, which was calculated from the time of surgery to the first dose of chemotherapy administered. Data regarding time to port placement and time to pathology report were also collected. TTAC was then compared based on various sociodemographic characteristics (age, gender, race, marital status, and type of insurance). The specialty of the surgeon, effect of surgical complications (intraoperative and postoperative including prolonged intubation, bleeding requiring transfusion, superficial surgical site infection (SSI), deep SSI, pulmonary embolus, renal failure, pneumonia, and/or a deep venous thrombosis), inpatient medical oncology consulting (yes or no), and type of medical oncology practice (academic versus private) on time to initiation of chemotherapy treatment were also analyzed.
In performing the statistical analysis, the Kolmogorov-Smirnov test for normality distribution of TTAC in these groups was performed. Because of abnormal distribution in all groups, data were presented as a median with interquartile range (IQR) and compared between subgroups by Wilcoxon rank-sum test for non-parametric data. To minimize the effect of various potential confounders on TTAC when comparing the different groups, we used the generalized linear modeling (SAS GENMOD procedure) with gamma model with log link and with adjustment for patient sociodemographic characteristics, type of surgical procedure, surgical complications, and provider characteristics above. A P value <0.05 was considered statistically significant.
Regarding the type of surgery performed (minimally invasive or open), we also analyzed the postoperative complication rate, body mass index (BMI), estimated blood loss (EBL), age, race, gender, and insurance specific to surgery type. For the continuous variables age and BMI, the variables were statistically normal so the Student’s t-test was used. For the other categorical variables the Wilcoxon rank-sum test was used. Counts and chi-square values were calculated.
All procedures performed in this study were in accordance with the Declaration of Helsinki (as revised in 2013). Institutional Review Board (IRB) approval for this study was obtained from Rutgers Robert Wood Johnson Medical School (Pro 20170000357). Because of the retrospective nature of the research, the requirement for informed consent was waived.
Results
The hospital tumor registry identified 128 adult patients (age ≥21) with stage III colon cancer who underwent surgical resection at our institution. Forty-nine patients did not receive adjuvant chemotherapy (patient or physician choice, lost to follow-up, or >150 days from surgery). Therefore, 79 patients met our selection criteria and were included in final analysis. After surgery, the median time to chemotherapy for stage III colon cancer patients was 46 days (IQR, 36–61). The median time to pathology reporting after surgery was 5 days (IQR, 4–6). Data pertinent to central access for chemotherapy was documented in 49 patients with a median time of 39 days (IQR, 29–49) from surgery to central access established. In the 38 patients with data available, the median time to outpatient medical oncology appointment was 27 days (IQR, 18–40).
Sociodemographic characteristics of the study population and distribution of patients between various providers are shown in Table 1. In contrast with TTAC, age values were normally distributed and presented as mean values with the standard deviation. Proportions of males and females were equal. Whites, married patients, and those with Medicare and private insurance accounted for the majority of the study population. The proportion of patients treated in private medical oncology practices tended to be greater compared to the academic setting, and the majority of patients did not receive a consult by the medical oncologist during hospitalization. Among all patients, 11.4% developed intraoperative complications and postoperative complications occurred in 26.6% of cases.
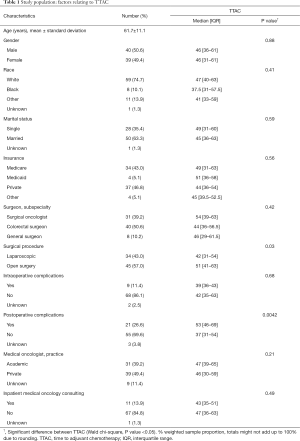
Full table
Overall, the median TTAC in the study population was 46 days (IQR, 36–61). A weak positive correlation between patient age and TTAC was found (r=0.23; P=0.04); only 5.3% of variation in TTAC may be explained by variation in age. The median hospital length of stay was 6 days (IQR, 4–10).
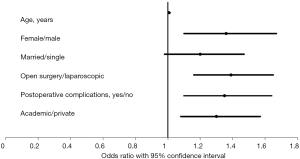
The previous nationally acknowledged differences in TTAC as well as factors acknowledged in this cohort for TTAC were confirmed with multivariable analysis adjusting for potential confounders (Figure 1). Each year of increased age increased delay to chemotherapy by 0.8% [odds ratio (OR), 1.01; 95% confidence interval (CI), 1.00–1.02]. TTAC was also longer if postoperative complications occurred (OR, 1.35; 95% CI, 1.10–1.64). Additionally, the results of the multivariable analysis revealed that TTAC in females was longer than in males (OR, 1.36; 95% CI, 1.10–1.67) and TTAC was delayed by medical oncologists in the academic settings compared to those in private practice (OR, 1.30; 95% CI, 1.08–1.57). TTAC in married patients tended to be longer than TTAC in single patients (OR, 1.20; 95% CI, 0.98–1.47).
There was no significant difference between colorectal surgeons (n=40), surgical oncologists (n=31), or general surgeons (n=8) for TTAC. Patients treated by surgical oncologists received postoperative adjuvant chemotherapy in an average of 51 days, colorectal surgeons 47.7 days, and general surgeons 53.4 days (Table 1).
There was a statistically significant difference between minimally invasive (laparoscopic or robotic) surgery, with a median of 42 days (IQR, 31–54), and open surgery with a median of 51 days (IQR, 41–63) on TTAC (Figure 1). There was no statistically significant difference between postoperative complications (31% open vs. 24% minimally invasive, P=0.61), age, BMI, insurance type, and race. There was a statistically significant difference between sex and EBL, (0.02 and 0.003, respectively) between operative types (Table 2).
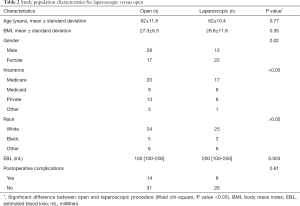
Full table
Discussion
The American Society of Clinical Oncology (ASCO) Quality initiative has identified adjuvant chemotherapy for colon cancer as a key measure in the delivery of quality cancer care (6). The timing of initiation of chemotherapy has been a focus of research that supports timely initiation of adjuvant chemotherapy. A meta-analysis of five studies compared delayed chemotherapy with standard treatment (7,8) and found that delaying chemotherapy more than 8 weeks was associated with worse OS [relative risk (RR), 1.20; 95% CI, 1.15–1.26] but not worse relapse-free survival. Hershman et al. examined the Surveillance, Epidemiology, and End Results (SEER) database to describe OS as a function of timing of initiation of adjuvant chemotherapy in a patient population age greater than 65 years. For OS, they described a significant increase in the hazard ratio (HR) when the initiation of chemotherapy after surgery for stage III colon cancer was delayed from 60 to 90 days. They found that disease-specific survival was significantly worse if TTAC was greater than 90 days (HR, 1.5; P<0.05) (9). A meta-analysis by Biagi et al. (3) demonstrated that a 30-day increase in time to initiation of adjuvant chemotherapy was associated with a significant decrease in both OS (HR, 1.14; 95% CI, 1.10–1.17) and disease-free survival (HR, 1.14; 95% CI, 1.10–1.18).
The median TTAC in our study of 46 days is within the current recommendations as based on adjuvant chemotherapy trials; the current recommendation is to initiate postoperative chemotherapy within 60 days of surgery. However, according to level I (data from a randomized trial) ASCO guidelines, the advantage and survival gains from adjuvant chemotherapy in stage III colon cancer patients occurs only if treatment is started within 5–6 weeks (35–42 days) after surgery (10). Examination of patients with stage III colon cancer at our hospital, which is affiliated with the Cancer Institute of New Jersey (CINJ), a National Cancer Institute (NCI) Designated Cancer Center, revealed that the majority of patients were not receiving adjuvant chemotherapy within a 6-week (42 days) postoperative interval as recommended. Our results compare favorably to a quality improvement study conducted at a community medical center, where researchers found that only 32% of patients commenced adjuvant chemotherapy within 56 days of surgery. Major delays identified in that study were attributed to a delay to request a consultation by a medical oncologist and to obtain access to central venous infusion devices (11). Other potential reasons for delay to see a medical oncologist were due to lack of referral, delay in patients getting timely appointments, surgeons not facilitating medical oncology appointments until after the initial postoperative visit, or because they weren’t seen in the hospital by medical oncologists. These are all potential areas of improvement to expedite TTAC.
Multiple studies have shown that patients who underwent a minimally invasive (laparoscopic or robotic) colon resection had significantly faster recovery compared to open colon resection, including less time to first flatus, less pain with lower analgesic use, and less hospital recovery time (4,12). Tong and Law found that a laparoscopic right hemicolectomy was associated with shorter hospital stays as well as earlier time to tolerating a regular diet (13). Multiple meta-analyses have been performed looking at TTAC (14-16). These analyses however, include both rectal and colon cancer cases, as well as both stage II and stage III, therefore we are unable to directly associate these results to our study results. The benefits of laparoscopy versus open surgery for TTAC has been looked at in a single meta-analysis, and was found to be helpful in preventing delays in TTAC (17). Overall, the open and laparoscopic groups were similar; the one major difference was EBL. EBL was found to be higher in the open group. This likely occurred due to the potential of a more difficult dissection and possibility of prior surgeries, requiring an open versus a laparoscopic approach. However, we did not observe any difference in postoperative complications. Could the increase in EBL, be related to the delay in TTAC, possibly, however, the postoperative complication rate was not found to be statistically significantly different, so an increase in EBL was unlikely to lead to the delay in TTAC. Our results contribute and add to the potential benefits of minimally invasive approaches to decreasing TTAC in stage III colon cancer, and we conclude that laparoscopy may be key to help preventing delays in TTAC without any worse OS or disease-free survival (18).
Previously published data on surgical subspecialty and colorectal cancer outcomes generally shows an improvement if specialty surgeons are involved in the patient’s care. Barbas described improved survival among patients treated at NCI-Designated Cancer Centers where there are higher proportions of specialty-trained surgeons (19). Specialization was found to be an independent predictor of survival based on a Cochrane database review in 2012 (20). The BMJ also reported improved 5-year survival in patients treated at high volume hospitals, high volume specialists, and colorectal trained surgeons. Moreover, the British Journal of Surgery reported improved colorectal cancer survival with surgical specialists (21). Oliphant also reported increased postoperative mortality in non-specialty trained surgeons, and increased 5-year survival among specialists (22). The results of this present study did not show a significant difference in specialty or subspecialty surgeon affecting TTAC, which may be related to local factors.
The impact of teaching affiliation was not significant in our study. This is congruent with prior studies, which have shown variable impact of teaching affiliation on TTAC. For example, one study found that care at a teaching institution was not associated with delayed initiation of adjuvant chemotherapy in patients with stage II and III rectal cancer (8,23). Another study found that patients treated at teaching hospitals were less likely to receive chemotherapy (24), while in contrast, a similar study found that patients who had surgery with a surgeon affiliated with a teaching hospital were more likely to have an evaluation by a medical oncologist (25,26).
Reducing postoperative and intraoperative complications is the focus of many quality improvement efforts nationally and worldwide. Our results give additional support to prior studies that have identified perioperative complications as an important contributor to delay of TTAC (13,26) Many institutions have moved towards implementing protocols for enhanced recovery after surgery (ERAS). Robust ERAS protocols have been shown to improve outcomes of surgical patients (27). Implementation may also therefore decrease TTAC by reducing postoperative complications and hospital length of stay.
Limitations of our study include incomplete data due to the multiple local and regional options for our patients to receive care. As a retrospective study, there are limits in the data that could be extracted, and risks of inaccurate data entry. Our study evaluated effects of timing at a single institution, which may not translate broadly to other institutions with different factors impacting TTAC. However, it is valuable to see the different steps in the process of getting patients to adjuvant chemotherapy and the potential timing pitfalls at different care points.
Our results are also limited in the number of patients studied, the percent lost to follow up, or those who never received chemotherapy. We also have not examined survival in this cohort; merely time to adjuvant therapy, due to the aims of this study and the limitations of our data. Our patients were all operated on at a high volume, NCI designated cancer center with ample resources for postoperative care and follow up. In that light, we did not see a difference in time to chemotherapy between different specialty trained surgeons.
Conclusions
Given the evidence supporting timely initiation of adjuvant chemotherapy and the numerous patient and institutional factors that may delay or improve the initiation of this important measure, timely access to adjuvant chemotherapy is an appropriate clinical quality indicator (23). While some delays may be unavoidable, implementation of ERAS protocols to decrease perioperative complications and the use of minimally invasive surgery as a technical approach to patients with colon cancer may improve TTAC. Quality improvement studies at other large institutions may be beneficial to determine if these results are applicable at a broader level and to determine if other factors may play a role in delayed TTAC.
Acknowledgments
We would like to thank Michael T. Scott for his help and expertise in final statistical analysis. We would also like to mention that the abstract was presented at the ASCO Quality Care Symposium, February 26–27, 2016 in Phoenix, Arizona.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/ales-20-95
Data Sharing Statement: Available at http://dx.doi.org/10.21037/ales-20-95
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales-20-95). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the Declaration of Helsinki (as revised in 2013). Institutional Review Board (IRB) approval for this study was obtained from Rutgers Robert Wood Johnson Medical School (Pro 20170000357). Because of the retrospective nature of the research, the requirement for informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sargent DJ, Goldberg RM, Jacobson SD, et al. A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med 2001;345:1091-7. [Crossref] [PubMed]
- André T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J. Clin Oncol 2009;27:3109-16. [Crossref] [PubMed]
- Biagi JJ, Raphael MJ, Mackillop WJ, et al. Association between time to initiation of adjuvant chemotherapy and survival in colorectal cancer: a systematic review and meta-analysis. JAMA 2011;305:2335-42. [Crossref] [PubMed]
- Clinical Outcomes of Surgical Therapy Study Group. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med 2004;350:2050-9. [Crossref] [PubMed]
- Porter GA, Soskolne CL, Yakimets WW, et al. Surgeon-related factors and outcome in rectal cancer. Ann Surg 1998;227:157-67. [Crossref] [PubMed]
- Schnipper LE, Smith TJ, Raghavan D, et al. American Society of Clinical Oncology identifies five key opportunities to improve care and reduce costs: the top five list for oncology. J Clin Oncol 2012;30:1715-24. [Crossref] [PubMed]
- Berglund Å, Cedermark B, Glimelius B. Is it deleterious to delay the start of adjuvant chemotherapy in colon cancer stage III? Ann Oncol 2008;19:400-2. [Crossref] [PubMed]
- Des Guetz G, Nicolas P, Perret GY, et al. Does delaying adjuvant chemotherapy after curative surgery for colorectal cancer impair survival? A meta-analysis. Eur J Cancer 2010;46:1049-55. [Crossref] [PubMed]
- Hershman D, Hall MJ, Wang X, et al. Timing of adjuvant chemotherapy initiation after surgery for stage III colon cancer. Cancer 2006;107:2581-8. [Crossref] [PubMed]
- Jeong WK, Shin JW, Baek SK. Oncologic outcomes of early adjuvant chemotherapy initiation in patients with stage III colon cancer. Ann Surg Treat Res 2015;89:124-30. [Crossref] [PubMed]
- Partridge A, Rother M, Enright K, et al. Time to adjuvant chemotherapy (AC) in stage III colon cancer at a community cancer center: a quality improvement initiative. J Clin Oncol 2013;31:e17503 [Crossref]
- Biondi A, Grosso G, Mistretta A, et al. Laparoscopic-assisted versus open surgery for colorectal cancer: short- and long-term outcomes comparison. J Laparoendosc Adv Surg Tech A 2013;23:1-7. [Crossref] [PubMed]
- Tong DK, Law WL. Laparoscopic versus open right hemicolectomy for carcinoma of the colon. JSLS 2007;11:76-80. [PubMed]
- Alexander M, Blum R, Burbury K, et al. Timely initiation of chemotherapy: a systematic literature review of six priority cancers–results and recommendations for clinical practice. Intern Med J 2017;47:16-34. [Crossref] [PubMed]
- Santos MD, Silva C, Oliveira J, et al. Extensive colectomy in colorectal cancer and hereditary nonpolyposis colorectal cancer-long-term results. JCOL 2019;39:223-30. [Crossref]
- Kim IY, Kim BR, Kim YW. Factors affecting use and delay (≥8 weeks) of adjuvant chemotherapy after colorectal cancer surgery and the impact of chemotherapy-use and delay on oncologic outcomes. PLoS One 2015;10:e0138720 [Crossref] [PubMed]
- Malietzis G, Mughal A, Currie AC, et al. Factors implicated for delay of adjuvant chemotherapy in colorectal cancer: a meta-analysis of observational studies. Ann Surg Oncol 2015;22:3793-802. [Crossref] [PubMed]
- Fleshman J, Sargent DJ, Green E, et al. Laparoscopic colectomy for cancer is not inferior to open surgery based on 5-year data from the COST Study Group trial. Ann Surg 2007;246:655-62; discussion 662-4. [Crossref] [PubMed]
- Barbas AS, Turley RS, Mantyh CR, et al. Effect of surgeon specialization on long-term survival following colon cancer resection at an NCI-designated cancer center. J Surg Oncol 2012;106:219-23. [Crossref] [PubMed]
- Archampong D, Borowski D, Wille-Jørgensen P, et al. Workload and surgeon s specialty for outcome after colorectal cancer surgery. Cochrane Database Syst Rev 2012;CD005391 [Crossref] [PubMed]
- Vallance AE, Fearnhead NS, Kuryba A, et al. Effect of public reporting of surgeons’ outcomes on patient selection, “gaming,” and mortality in colorectal cancer surgery in England: population based cohort study. BMJ 2018;361:k1581. [PubMed]
- Oliphant R, Nicholson GA, Horgan PG, et al. Contribution of surgical specialization to improved colorectal cancer survival. Br J Surg 2013;100:1388-95. [Crossref] [PubMed]
- Richardson LC, Tian L, Voti L, et al. The roles of teaching hospitals, insurance status, and race/ethnicity in receipt of adjuvant therapy for regional-stage breast cancer in Florida. Am J Public Health 2006;96:160-6. [Crossref] [PubMed]
- Hendren S, Birkmeyer JD, Yin H, et al. Surgical complications are associated with omission of chemotherapy for stage III colorectal cancer. Dis Colon Rectum 2010;53:1587-93. [Crossref] [PubMed]
- Chen LM, Wilk AS, Thumma JR, et al. Use of medical consultants for hospitalized surgical patients: an observational cohort study. JAMA Intern Med 2014;174:1470-7. [Crossref] [PubMed]
- Duvalko KM, Sherar M, Sawka C. Creating a system for performance improvement in cancer care: Cancer Care Ontario’s clinical governance framework. Cancer Control 2009;16:293-302. [Crossref] [PubMed]
- Ljungqvist O, Scott M, Fearon KC. Enhanced recovery after surgery: a review. JAMA Surg 2017;152:292-8. [Crossref] [PubMed]
Cite this article as: Donohue K, NeMoyer RE, Dombrovskiy V, Brown T, Patella S, Rezac C, Moss R, Patel NM. A minimally invasive approach to colon cancer resection improves time to adjuvant chemotherapy. Ann Laparosc Endosc Surg 2021;6:16.


