The role of indocyanine green performing a minimally invasive right colectomy
Introduction
Compared with open surgery, the minimally invasive technique for treatment of right colon cancer presents several advantages that were extensively demonstrated (1). Laparoscopy can achieve the same oncological outcomes of traditional surgery so it is widely diffused (2). However, as every surgery, this technique presents a percentage of complications like anastomotic leakage and visceral injuries. Moreover, some aspects of the technique, as the extension of the lymphadenectomy, are still debated.
In recent years, indocyanine green (ICG)-enhanced fluorescence was introduced in laparoscopic surgery to improve the view and provide detailed anatomical information during surgery (3). Indocyanine-green is a sterile, anionic, water soluble but relatively hydrophobic, tricarbocyanine molecule, which was approved for clinical use in 1959 by the Food and Drug Administration (FDA) (4). After intravenous injection, ICG rapidly bounds to plasma protein, especially lipoproteins, with minimal leakage into the interstitium. This dye has no known metabolites. It is rapidly extracted by the liver without modifications and excreted in unconjugated form in the bile about eight minutes after injection, depending on liver functionality and vascularization (4,5). When injected outside blood vessels (e.g., into the normal tissue close to tumors), ICG binds to proteins and is found into the lymph, reaching the nearest lymph node usually within 15 minutes. After one-two hours it binds to the regional lymph nodes, deposited into macrophages (6-8).
The standard dose commonly administered in clinical practice is 0.1–0.5 mg/mL/kg, which is well below the toxicity level and has practically no adverse effects (4).
ICG becomes fluorescent when excited either by a laser beam (9), or by near-infrared light at about 820 nm and longer wave lengths (10). The fluorescence released by ICG can be detected using specifically designated scopes and cameras.
ICG fluorescence imaging has found several different applications in colorectal surgery aiming in reducing complications, such as anastomotic leakage, clarifying anatomic landmarks and as a guidance for lymphadenectomy. To date, the clinical and experimental applications of ICG are: ICG-fluorescence guided angiography for bowel perfusion control, lymphography for sentinel nodes detection and lymphatic mapping, intraoperative ureter identification, intraoperative tumor identification.
We present the following article in accordance with the Narrative Review reporting checklist (available at http://dx.doi.org/10.21037/ales-20-60).
Methods
A literature search was performed using PubMed, Embase and Cochrane Library database to identify relevant articles on ICG fluorescence guided surgery and laparoscopic right colectomy. with searching keywords of “indocyanine green”, “ICG”, “fluorescence”, “fluorescent angiography”, “anastomotic leak/age”, “bowel perfusion”, “perfusion assessment”, “colorectal surgery”, “right colectomy”, “right colon”, “lymphadenectomy”, “fluorescence lymphography”, “lymphatic mapping”, “ureter visualization”, “tumor identification” and using the Boolean operator “OR”, “AND” for each keyword.
Discussion
ICG-fluorescence guided angiography for bowel perfusion control
Anastomotic leakage represents the most concerning postoperative complication in colorectal surgery. The reported average leak rate is 1–8% for ileo-colic anastomosis and up to 10–30% for low colorectal anastomosis with adverse impact on post-operative outcomes (11-13). The rate of patients who need surgical revision ranges from 10% to 35% (14), with a mortality rate of 6–22% (15). All these consequences reflect an increase of costs: it was estimated that a significant leakage, or a severe complication, increases the cost by a factor of five (16,17).
Even though multiple conditions have been identified as responsible for anastomotic leakage (e.g., patient’s risk factors, suture materials, surgical devices, inadequate anastomotic blood supply), the pathogenesis is still unclear (18). A common determining factor of viability is adequate arterial perfusion to ensure sufficient local tissue oxygenation (19). Consequently, detection of bowel ischemia or hypoperfusion intraoperatively may reduce the risk of anastomotic leakage.
The most widely used method to evaluate tissue perfusion is surgeon intraoperative judgment based on clinical findings such as serosal-mucosal color, bleeding edges of resected margins, pulsation of marginal vessels, temperature and peristalsis. All these methods present limitations because they are based on totally subjective evaluation and intraoperative ischemic bowel demarcation may require extended time. Since it has been demonstrated that surgeon’s ability to predict anastomotic leakage is inaccurate, with a sensitivity of 61.3% and a specificity of 88.5% (20), more objective evaluation of vascular supply is required. Other techniques to determine anastomotic perfusion are Doppler-flowmetry and pH-reading in the colon, but are time consuming and not very convincing (21).
Due to the ability of ICG to become fluorescent once excited, this dye can be injected intravenously to obtain a real-time angiography in order to evaluate vascular anatomy and bowel perfusion. As regards laparoscopic right colectomy, three are the possible applications of ICG real-time intraoperative angiography:
- Check bowel perfusion before, and eventually after, performing ileo-colic anastomosis;
- Clarify vascular anatomy during dissection. Some anatomical regions, like hepatic flexure that is on the border between two vascular districts, can present an unclear and variable vascular anatomy. Therefore, the vascular dissection and pedicle ligation can be performed on the guide of a real-time angiography of tumor area (22). ICG can be injected in small boluses of 3–5 mL each (0.4 mg/mL/kg), recording the real-time fluorescence (23);
- Assessment of ileo-colic anastomotic perfusion in case of surgical revision due to anastomotic leakage. It has been demonstrated that re-laparoscopy is a safe and effective method to confirm diagnosis and treat early post-operative complications following colorectal surgery, in selected patients (24,25). Therefore, since inadequate bowel perfusion is not always responsible for anastomotic leakage, ICG fluorescence angiography can be used to assess the perfusion and help the surgeon in choosing the most appropriate treatment (e.g., a primary repair of enterotomy or staple line defect can be performed in case of adequate bowel perfusion, in selected patients).
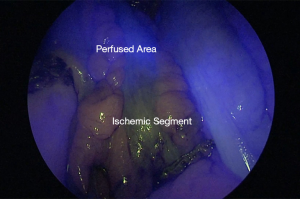
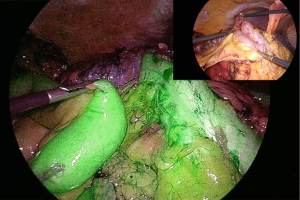
As concerns technique, in order to evaluate bowel perfusion, diluted ICG is injected intravenously using a bolus of 3 mL at a concentration of 0.2 mg/kg. The bolus is given prior to bowel anastomosis to confirm adequate vascularization of the proximal and distal stumps (Figure 1). In case of an ischemic area, this allows a further resection of the stump prior to performing the anastomosis (Figure 2). It is recommendable to test the perfusion with the bowel segments in their definitive position after anastomosis, to be sure that the perfusion does not change due to any tension or anatomic factors occurring after anastomosis. If extracorporeal bowel division is to be performed, adequate visualization is feasible only with the operating room lighting turned off, because room lighting might interfere with fluorescence detection sensitivity of the near-infrared camera (22,26).
According to literature, ICG-fluorescence angiography is considered safe, feasible and does not require extensive learning curve to be applied. To date, no complications attributable to the use of the technique were recorded, so it is easily applicable. The mean added surgical time is on average less than 5 minutes to the total duration of the surgical procedure (27).
Two systematic review have analyzed the efficacy of ICG fluorescence imaging in reducing the anastomotic leakage rate after colorectal surgery (27,28).
The review of van de Bos et al. included 10 studies and compared a group of 894 patients who underwent surgery with ICG fluorescence angiography with a group of 434 patients without it. Fluorescence imaging changed the surgical plan in 10.8% of all ICG cases. Anastomotic leakage was significantly less prevalent in ICG group (3.5%) than in the control group (7.4%). This may be considered an indication of the benefit of the technique, improving surgeons’ ability to detect areas of poor blood supply. The ability of fluorescence imaging to change the surgical plan with positive outcome in operative results reflects its value. An anastomotic leak rate of 8.9% was found after revision of the surgical plan, compared to 2.8% in the patients with initial good fluorescence signal. This could mean that a good fluorescence signal is predicting a good outcome, while a lesser fluorescent signal could mean a higher risk of anastomotic leakage, whether or not the transection line is moved (27).
The review of Blanco-Colino et al. included five studies comparing 555 patients in the ICG group with 747 patients in the control group. Taking into account both benign and malignant cases, there was no significant difference in anastomotic leakage rate. While, taking into account 956 cancer patients, the anastomotic leakage was significantly less prevalent in ICG group than in the control group (28).
A review of the literature was carried out in order to identify right colectomy performed with the use of ICG fluorescence angiography and their results. We selected only full text and English language papers in which the right colectomy results were set apart from other colorectal procedures. Table 1 summarizes the results.

Full table
In a retrospective study, Boni et al. included eight right colectomies in a group of 38 colorectal resections. The fluorescence imaging was always obtained and no anastomotic leakages were recorded (23).
In another retrospective study, the same group included 40 right colectomies in a series of 107 colorectal patients. Even in this case ICG-enhanced fluorescence was detected in 100% of the cases and, according to a subjective qualitative evaluation of the perfusion intensity, no modifications of the surgical plan were needed. One patient underwent re-operation for anastomotic leakage after right colectomy due to incomplete closure of the enterotomy used for stapler introduction, while ICG fluorescence angiography during re-intervention confirmed an adequate anastomotic perfusion (29).
Ris et al. included six right colectomies in a prospective study. The ICG-fluorescence imaging of the anastomosis was achieved in every patient except one, but it was not reported if it was a right colectomy or not. There was no need to redo the anastomosis in any patient based on the fluorescence angiography findings. None of the patients developed anastomotic leakage (13).
In a large retrospective cohort study, Dinallo et al. compared a group of 234 colorectal resections with ICG fluorescence angiography with an historical group of 320 colorectal resections without it. The right colectomies included were 64 in the ICG group and 84 in the non-ICG group. The perfusion was evaluated in a quantitative manner, using the SPY Elite Intraoperative Perfusion Assessment System (Novadaq Technologies Inc, Bonita Springs, Fl). This software provides an absolute numerical value on a 0–256 grey scale representing ICG fluorescence intensity, thus providing an objective measurement. No alterations of the planned anastomosis site were made in both groups of right colectomies. The anastomotic leakage rate in these two subgroups was not reported. In the whole study the anastomotic leakage rate was 1.3% in both ICG and non-ICG groups. The absolute value of perfusion assessment for all colon resections ranged from 34 to 253, while that of patient with anastomotic leak was from 50 to 100 (30).
Santi et al. recently published a prospective study, including 22 right colectomies. ICG fluorescence angiography was detected in all patients, according to a subjective evaluation of perfusion intensity. In this group no changes of the surgical plan were needed and one patient underwent reoperation due to anastomotic leakage caused by a mechanical problem of the stapler (22).
Taking into account small sample size, missing data, absence of subgroup analysis and heterogeneity of these studies it was not possible to carry out a statistical analysis in order to identify the incidence of anastomotic leakage rate in right colectomies with ICG fluorescence imaging and to compare it with the rate in standard right colectomies in literature. All the studies agree that ICG fluorescence angiography is a feasible and safe technique, according to previous literature. Large multicentre randomized control trials are needed to establish if ICG fluorescence imaging in right colectomy could be associated with a lower rate of anastomotic leakage.
Currently, one of the limits of ICG-enhanced angiography is that the fluorescent intensity is mainly assessed in a qualitative subjective manner in clinical practice and consequently in literature reports. Quantitative blood flow analysis is necessary to measure changes in colon microcirculation to predict bowel viability (31). Nevertheless, until now there have been many limitations in clinically applying the quantitative analysis of ICG fluorescence. Signal quantification could make the technique an even more reliable tool to real-time assessment of bowel perfusion.
According with this, animal experiments using fluorescent imaging demonstrated that decreased microfiltration causes intestinal necrosis (32,33). Subsequently, a quantitative assessment of the bowel perfusion was attempted and described in some clinical studies, correlating the intensity of the fluorescence with bowel perfusion (34-36).
Son et al. carried out a quantitative evaluation of bowel perfusion in a series of 86 colorectal cancer resections, showing that anastomotic complications were distributed among patients with slow perfusion (low time from first fluorescence increase to half of maximum) (37). Hayami et al. published a quantitative assessment of bowel perfusion in 23 colorectal resections in a prospective study. The authors demonstrated that there was a significantly longer time between ICG injection and beginning of fluorescence in anastomotic leakage group than in non-anastomotic leakage group (38).
Therefore, according to literature there have been many reports and some systematic reviews on the effectiveness of ICG fluorescence imaging in colorectal surgery. Indeed, the technique is associated with a lower anastomotic leakage rate and allows a revision of the surgical plan when necessary. The power of the studies published to date is limited, so large multicentre trials are necessary before stating the superiority of the technique when compared with standard procedures.
Furthermore, a quantitative evaluation of the bowel perfusion seems to be more precise than the qualitative one, identifying some factors which are significantly associated with a lower incidence of anastomotic leakage.
ICG fluorescence lymphography for sentinel nodes detection and lymphatic mapping
It is established that an adequate lymphadenectomy in colorectal oncologic surgery is fundamental both for a therapeutic aim and for accurate disease staging, defining the need for adjuvant therapy. The European Society of Medical Oncology (ESMO) and the National Comprehensive Cancer Network (NCCN) guidelines still recommend the removal of a minimum number of 12 regional lymph nodes around the arterial arcades for adequate pathological staging, as it was proposed by the Working Party Report to the World Congress of Gastroenterology in 1990 (39-42). Nevertheless, this number was only partially supported by evidence, as afterwards several authors proposed a different minimum number to be harvested in right colectomy (43).
In 2009, Hohenberger et al. described the complete mesocolic excision (CME) with central vascular ligation (CVL) as a technique for right colectomy with extended lymphadenectomy along with the embryological anatomical planes. The aim of this procedure is to increase the number of harvested lymph nodes (median number of 32 lymph nodes per patient) in order to improve the staging and the oncological outcomes: they reported a reduction of local recurrences from 6.5% to 3.6% and 5-year cancer-related survival rate of 85%. However, CME technique is technically demanding and it is associated with a higher rate of complications (19.7%) compared to standard right colectomy and a postoperative mortality rate of 3.1% (44).
In contrast with European and American guidelines, the Japanese Society for Cancer of the Colon and Rectum (JSCCR) introduced the nomenclature “D”, differentiating the extension of lymphadenectomy according to tumor wall invasion and evidence of suspect lymph node metastases on preoperative staging. In relation to TNM staging, these guidelines suggest the following lymphadenectomy:
- D0 and D1: cTis;
- D2: cT1, cT2;
- D3: cT2, cT3, cT4 and/or cN+ (45).
It has been demonstrated that in 19% of patients the lymph node metastases do not always follow a linear pattern but a “jump” one. Thus, it is possible to find metastatic lymph nodes far from the tumor (e.g., station 3) but not next to it (e.g., station 1 and/or 2). These metastases are defined as skip metastases (46,47).
In addition, it is possible to find lymph node metastases beyond the tumor related mesocolic excision: these are defined as aberrant metastases. In a 2016 review, Bertelsen et al. reported a prevalence of aberrant metastases from 2% to 19%, even if the definition varies according to different authors (48). True aberrant metastases outside the resection margins of CME have been reported, but the prevalence in colon cancer will stay unknown, because resection of extramesocolic lymph nodes would be need without any benefit for the patients (49,50).
Lately this contrasting overview of the literature, the differences in clinical practice between Western and Eastern countries and the absence of clear data have leaded to an open discussion about the technique and the extension of lymphadenectomy in right colectomy. Indeed, the risks are, on the one hand, performing an unnecessary wide lymphadenectomy for small tumors without lymph node metastasis and, on the other, to leave in place metastatic lymph nodes for advanced tumors, if the technique is not defined.
In order to clarify this issue, several studies examined the role of ICG fluorescence imaging in guiding the lymphadenectomy in right colectomy. The main applications of ICG lymphography are sentinel lymph nodes (SLn) detection and lymphatic mapping. Unfortunately, literature data on this topic always embrace colon or colorectal cancers, without a subgroup analysis focused on right colectomies.
The concept of SLn was first described by Gould et al. in 1960, defining the first order of lymph nodes draining the tumor area (51). It is a well-established technique in breast cancer, melanoma, gastric cancer, but it currently has a limited impact on colon cancer and it is not routinely used in clinical practice (52,53). As a matter of fact, literature data on this topic are not satisfying, probably because some studies included patients with locally advanced disease (54,55) or used different tracers (56,57). Years ago, ICG has already been proposed as a potentially effective lymphatic tracer in colon cancer (58).
The rationale of SLn in colon cancer is identifying lymph node metastases after local resection of early colon cancers, in order to select patients who needs resection with extended lymphadenectomy. The sentinel lymph node mapping allows a detailed analysis (immunohistochemistry or reverse transcription polymerase chain reaction) to detect micrometastatic disease (<2 mm) and improve nodal staging (59). If feasible, this procedure could prevent the risks of morbidity and mortality of colectomy with lymphadenectomy in patients with early colon cancer without nodal involvement (60).
Several studies have been published showing the use of sentinel lymph node imaging in early colon cancer.
Nagata et al. included 8 right colectomies in a series of 48 laparoscopic colorectal resections. The ICG (total volume 5 mL) was administered inside the wall proximal and distal to the tumor during laparoscopy. Sentinel lymph nodes were detected in 47 patients, 11 patients of which had lymphatic metastases. Five out of these 11 cases had false negative sentinel lymph node (all with T3 cancer). Of the four patients with T1 and T2 cancer with lymphatic metastases, all had positive sentinel lymph nodes (58).
Ankersmit et al. carried out a feasibility study in 20 colorectal cancer patients. Depending on tumor size, ICG diluted in albumin was injected in subserosal layer during laparoscopic surgery. Sentinel lymph nodes were identified in the first 10 patients and were negative for metastases. In four of these 10 cases, a non-sentinel lymph node was found positive for metastases. In three out of these four patients, the tumor was larger than 60 mm. The authors do not report the results about the remaining 10 patients included in the study (59).
Cahill et al. included 4 right colectomies in a series of 18 consecutive laparoscopic colorectal resection. Multiple boluses of ICG (mean total volume 2.8 mL) were injected in the submucosal layer around the tumor through peri-operative colonoscopy. From one to five fluorescent sentinel lymph nodes were detected approximately 10 minutes after injection in all cases. Sentinel lymph nodes were along the main arterial vessel in all cases, but in 14 patients these were found at the origin of the vessel. Three patients had mesocolic nodal metastases, with a metastatic sentinel lymph node in every case (61).
Andersen et al. compared the in vivo sentinel node mapping with ICG with the Ex vivo sentinel node mapping with methylene blue in 29 laparoscopic colon resections. Taking into account only the in vivo results, SLn were identified in 19 patients. Lymph node metastases were found in 10 patients and only 2 had metastases in SLn. Sentinel lymph nodes were located in each zone: D1, D2 and D3 (62).
In a case series of 30 patients, including 13 right colectomies, Currie et al. described technique and results of SLn detection with ICG in T1 and T2 colon cancer. ICG was injected in the submucosal layer around the tumor through an intraoperative colonoscopy. From 1 to 4 sentinel lymph nodes were identified in 27 out of 30 patients within 20 minutes after injection. These lymph nodes were located in D1 area in nine patients, in D2 area in eight patients, in D1 and D2 areas in 10 patients. Of the 27 patients with SLn mapping, 9 had lymph node metastases. Only 2 out of 9 patients had positive nodes identified by sentinel node mapping. Of the remaining seven lymph node metastases: one patient had positive sentinel lymph nodes after immunohistochemistry evaluation (with negative hematoxylin eosin evaluation), six patients had false negative sentinel lymph nodes. In 5 out of 6 false negative patients, tumors were larger than 35 mm, with four being T3/T4 on final hystopatological examination. This study demonstrated that the technique is feasible in T1/T2 colon cancer. The relatively high false negative rate of sentinel lymph nodes can be explained by the small sample size and the large diameter of the tumor in 17 out of 30 patients. One of the limits of this study, indeed, is that 11 out of 30 patients had T3/T4 cancer. It seems that sentinel lymph node mapping is more accurate in smaller and non-advanced tumors, because T3/T4 cancer could obstruct lymphatic pathways causing a higher rate of false negative (60).
It is clear from above data that the lymphography technique has to be standardized before it could be applied routinely in clinical practice. The cases in which sentinel lymph nodes could not be retrieved can be explained by injection technique problems, which are more frequent in the first cases performed, or by tumor features. Spillage of ICG results in a spread of fluorescence in the abdominal cavity, so it is impossible to detect the sentinel lymph nodes. The high number of false negative sentinel lymph nodes can be explained by the tumor size and T staging, as the technique seems to be reliable in small early colon cancer (Tis, T1, T2). Patient BMI and mesocolic fat are also important as indeed is surgeon and centre experience (57).
Therefore, in order to maximize the results of sentinel lymph node mapping, it seems better to apply the technique in early stage tumor without previous ink tattoo (63). Large and advanced stage tumor, likewise ink tattoo, can block efferent channels and nodes (a factor associated with increased false-negative rates). Furthermore, it has been described that the injection of the dye precisely close around the tumor allows all the lymphatic channels that drain the cancer to take it up. According to this, another limit of large tumors is that this technique does not allow central area mapping (61). Moreover, at least three sentinel lymph nodes need to be found in order to maximize technique utility (64).
As previously disclosed, the second rationale ICG fluorescent lymphography is to obtain a lymphatic mapping in order to collect all lymph nodes draining from the tumor both for disease clearance and to target delivery of adjuvant therapy (65) (Figures 3,4). Ex vivo studies have defined that aberrant metastases lying outside the usual mesenteric resection can occur in up to 10% of patients (66).
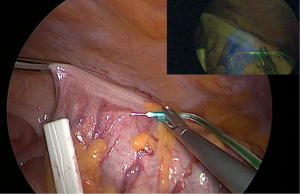

In the abovementioned study of Cahill et al., 4 out of 18 patients had fluorescent lymph nodes outside the intended conventional field of resection. Nevertheless, in this series, all of these nodes were clear of metastases (61).
Chand et al. reported additional lymph nodes located outside of the proposed resection margins in 2 out of 10 cases. In those 2 patients, nodes were metastatic (67).
Recently, Park et al. published an interesting 1:2 matched case-control study comparing ICG lymphography with standard white light technique (25 vs. 50 patients) in performing extended lymphadenectomy for T3–T4 right colon cancers. Their results showed that numbers of central lymph nodes and total harvested lymph nodes were significantly higher in the fluorescence group than in the conventional group. In the multivariate analysis, the use of ICG fluorescence imaging was an independently related factor for the retrieval of higher numbers of overall and central lymph nodes (68).
The relevance of detecting draining lymph nodes beyond the mesenteric resection margins, in case of lymphatic metastases, can be considered and assessed only in larger series.
On this topic a systematic review and meta-analysis of Emile et al. selected 12 prospective case series, comprising 248 patients, evaluating the capability of ICG fluorescence lymphography to detect metastatic lymph nodes in colorectal cancer. After injection (submucosal or subserosal or both) of ICG (different concentrations) fluorescent lymph nodes were detected after 5–30 minutes. Nine authors reported the total number of harvested lymph nodes to be 3,351, 1,175 (35.1%) of which were metastatic. Of the 1,175 lymph node metastases, 859 (73%) were fluorescent positive and 316 (27%) fluorescent negative. A total of 1759 lymph nodes were fluorescent, 859 (74%) of which were malignant, whereas 300 (26%) were negative. The pooled sensitivity of ICG in detecting malignant lymph nodes was 71% and the pooled specificity 84.6%. In this review is was notable that the best results were achieved when the ICG was administered in a concentration depending on the body weight. Furthermore, combined submucosal and subserosal injection achieved the highest sensitivity, specificity and accuracy (69).
Limitations of the technique are the fluorophore, which is currently non-specific to cancerous tissue, and the limited depth of penetration of the imaging system.
Nevertheless, prior to any routine clinical application, further validation is necessary to define if the lymph nodes identified with ICG fluorescence imaging are reflective of patient’s lymphatic basin status. Subsequently, on the one hand studies including selectively early colon cancers are required to define the incidence of false negative sentinel lymph nodes, to standardize technique and to define if minimally invasive sentinel lymph node mapping can be associated to a local resection of the tumor. On the other hand, further data are necessary to determine the effective utility in clinical practice of ICG fluorescence lymph mapping of aberrant lymph nodes.
Intraoperative ureter identification
Despite ureteral injury is an uncommon complication during laparoscopic right colectomy, in some situations (e.g., advanced stage tumors, abscessualization) the neoplastic lesion can be tightly attached to right ureter, making its recognition more difficult. Intraoperative ureter identification is achieved by visual inspection and palpation. Both can be more challenging during laparoscopic procedures, translating into a higher risk of injuries (70,71).
As ICG is completely excreted in the bile, ICG-fluorescence imaging of the ureter can be obtained with retrograde injection of the dye into the ureter through a ureteral catheter. The catheter is clamped after injection allowing ureter visualization, without background noise. Already reported by Siddighi et al. and Lee et al. during gynaecologic and urologic procedures respectively, the technique was applied by Santi et al. during a right colectomy (22,72,73). This technique is reported to be safe and effective. The disadvantage is that it requires catheter positioning and additional surgical time (74).
The ideal dye for ureter visualization would include exclusively renal clearance, with low background noise from surrounding tissues. Therefore, some experimental dyes have been tested to identify the best one for clinical practice (74,75).
Intraoperative tumor identification
Unlike laparotomy, during which lesions can be identified by palpation, small lesion, especially when located at the right flexure, are more difficult to find during minimally invasive surgery due to lack of tactile sensation. Several methods have been described to localize tumors, like India ink tattooing, intraoperative colonoscopy, endoscopic clips. All these methods have drawbacks, including inaccurate localization caused by faint or diffused tattoo, severe adhesions, peritonitis, bowel distension due to air insufflation or clips invisibility (76-78). Recently, ICG fluorescence has been used for tumor localization in vivo (79,80).
Miyoshi et al. reported a case series of 40 laparoscopic or open colorectal resections after ICG endoscopic marking (5 mL). The dye was reliably identified in the operations (29 cases) performed within eight days after endoscopic injection (81).
Nagata et al. compared India ink and IGC endoscopic tattooing (<96 hours before surgery) in a case series of 24 laparoscopic colorectal resection, including 6 right colectomies. ICG tattoo was identified in all 24 cases, while India ink in 14 cases. In six cases India ink had spilled inside the peritoneal cavity (82).
Hyun et al. reported tumor localization through a fluorescent rubber band, made of ICG and a liquid rubber solution mixture. The fluorescent rubber bands, previously endoscopically positioned in the stomach and in the colon of two pigs, were successfully identified with laparoscopy. However, the study is not able to define how long the band are maintained in the target site (83).
These preliminary results show that the technique is safe and feasible with some advantages, but authors agree that ICG endoscopic marking has to be performed within few days before surgery.
Conclusions
ICG fluorescence imaging seems to be a promising tool in minimally invasive right colectomy. ICG fluorescence angiography is a safe technique that allows to assess bowel perfusion before performing ileo-colic anastomosis. Two reviews on colorectal resection studies stated that ICG fluorescence imaging is associated with a significant lower rate of anastomotic leakage and allows a change of surgical plane in about 10% of cases.
It has been demonstrated the feasibility of ICG fluorescence lymphography in order to perform the sentinel lymph node mapping, which could be associated to local excision of early stage colorectal cancer, and lymphatic mapping to identify aberrant metastasis.
Furthermore, intraoperative ureter and tumor identification are interesting potential application of fluorescence guided surgery.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Marco Milone and Ugo Elmore) for the series “Right Colectomy 2.0” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at http://dx.doi.org/10.21037/ales-20-60
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/). The series “Right Colectomy 2.0” was commissioned by the editorial office without any funding or sponsorship. Dr. Luigi Boni serves as an unpaid editorial board member of Annals of Laparoscopic and Endoscopic Surgery from Jun 2016 to May 2021. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Abraham NS, Young JM, Solomon MJ, et al. Metaanalysis of short term outcomes after laparoscopic resection for colorectal cancer. Br J Surg 2004;91:1111-24. [Crossref] [PubMed]
- Liang Y, Li G, Chen P, et al. Laparoscopic versus open colorectal resection for cancer: a meta-analysis of results of randomized controlled trial on recurrence. Eur J Surg Oncol 2008;34:1217-24. [Crossref] [PubMed]
- Schaafsma BE, Mieog JS. The clinical use of indocyanine green as a near-infrared fluorescent contrast agent for image guided oncologic. J Surg Oncol 2011;104:323-32. [Crossref] [PubMed]
- Alander JT, Kaartinen I, Laakso A, et al. A review of indocyanine green fluorescent imaging in surgery. Int J Biomed Imaging 2012;2012:940585 [Crossref] [PubMed]
- Ishizawa T, Fukushima N, Shibahara J, et al. Real-time identification of liver cancers by using indocyanine green fluorescent imaging. Cancer 2009;115:2491-504. [Crossref] [PubMed]
- Tajima Y, Murakami M, Yamazaki K, et al. Sentiel node mapping guided by indocyanine green fluorescence imaging during laparoscpic surgery in gastric cancer. Ann Surg Oncol 2010;17:1787-93. [Crossref] [PubMed]
- Korn JM, Tellez-Diaz A, Bartz-Kurycki M, et al. Indocyanine green SPY elite-assisted sentinel lymph node biopsy in cutaneous melanoma. Plast Reconstr Surg 2014;133:914-22. [Crossref] [PubMed]
- Tanaka E, Choi HS, Fujii H, et al. Image-guided oncologic surgery using invisible light: completed preclinical development for sentinel lymph node mapping. Ann Surg Oncol 2006;13:1671-81. [Crossref] [PubMed]
- Daskalaki D, Fernandes E, Wang X, et al. Indocianine green (icg) fluorescent cholangiography during robotic cholecystectomy: results of 184 consecutive cases in a single institution. Surg Innov 2014;21:615-21. [Crossref] [PubMed]
- Luo S, Zhang E, Su Y, et al. A review of NIR dyes in cancer targeting and imaging. Biomaterials 2011;32:7127-38. [Crossref] [PubMed]
- . European Society of Coloproctology collaborating group. The relationship between method of anastomosis and anastomotic failure after right colectomy and ileo-caecal resections: an international snapshot audit. Clorectal Dis 2017;38:42-9.
- Cong ZJ, Hu LH, Bian ZQ, et al. Systematic review of anastomotic leakage rate according to an international graging system following anterior resection for rectal cancer. PLoS One 2013;8:e75519 [Crossref] [PubMed]
- Ris F, Hompes R, Cunningham C, et al. Near-infrared (NIR) perfusion angiography in minimally invasive colorectal surgery. Surg Endosc 2014;28:2221-6. [Crossref] [PubMed]
- Alves A, Panis Y, Pocard M, et al. Management of anastomotic leakage after nondiverted large bowel resection. J Am Coll Surg 1999;189:554-9. [Crossref] [PubMed]
- Buchs NC, Gervaz P, Secic M, et al. Incidence, consequences, and risk factors for anastomotic dehiscence after colorectal surgery: a prospective monocentric study. Int J Colorectal Dis 2008;23:265-70. [Crossref] [PubMed]
- Koperna T. Cost effectiveness of defunctioning stomas in low anterior resection for rectal cancer: a call for bench marking. Arch Surg 2003;138:1334-8. [Crossref] [PubMed]
- Vonlanthen R, Slankamenac K, Breitenstein S, et al. The impact of complications on costs of major surgical procedures: a cost analysis of 1200 patients. Ann Surg 2011;254:907-13. [Crossref] [PubMed]
- Shogan BD, Carlisle EM, Alverdy JC, et al. Do we really know why colorectal anastomosis leak? J Gastrointest Surg 2013;17:1698-707. [Crossref] [PubMed]
- Allison AS, Bloor C, Faux W, et al. The angiography anatomy of the small arteries and their collaterals in colorectal resections: some insights into anastomotic perfusion. Ann Surg 2010;251:1092-7. [Crossref] [PubMed]
- Karliczek A, Harlaar NJ, Zeebregts CJ, et al. Surgeons lack predictive accuracy for anastomotic leakage in gastrointestinal surgery. Int J Colorectal Dis 2009;24:569-76. [Crossref] [PubMed]
- Nachiappan S, Askari A, Currie A, et al. Intraoperative assessment of colorectal anastomotic integrity: a systematic review. Surg Endosc 2014;28:2513-30. [Crossref] [PubMed]
- Santi C, Casali L, Franzini C, et al. Applications of indocyanine greenenhanced fluorescence in laparoscopic colorectal resections. Updates Surg 2019;71:83-8. [Crossref] [PubMed]
- Boni L, David G, Mangano A, et al. Clinical applications of indocyanine green (ICG) enhanced fluorescence in laparoscopic surgery. Surg Endosc 2015;29:2046-55. [Crossref] [PubMed]
- Chang KH, Bourke MG, Kavanagh DO, et al. A systematic review of the role of re-laparoscopy in the management of complications following laparoscopic colorectal surgery. Surgeon 2016;14:287-93. [Crossref] [PubMed]
- Wright DB, Koh CE, Solomon MJ. Systematic review of the feasibility of laparoscopic reoperation for early postoperative complications following colorectal surgery. Br J Surg 2017;104:337-46. [Crossref] [PubMed]
- Boni L, Macina S, David G, et al. ICG-enhanced fluorescence-guided laparoscopic surgery-2nd Edition. Tuttlingen: Endo Press, 2019.
- van den Bos J, Al-Taher M, Schols RM, et al. Near-Infrared Fluorescence Imaging for Real-Time Intraoperative Guidance in Anastomotic Colorectal Surgery: A Systematic Review of Literature. J Laparoendosc Adv Surg Tech A 2018;28:157-67. [Crossref] [PubMed]
- Blanco-Colino R, Espin-Basany E. Intraoperative use of ICG fluorescence imaging to reduce the risk of anastomotic leakage in colorectal surgery: a systematic review and. Tech Coloproctol 2018;22:15-23. [Crossref] [PubMed]
- Boni L, David G, Dionigi G, et al. Indocyanine green-enhanced fluorescence to assess bowel perfusion during laparoscopic colorectal resection. Surg Endosc 2016;30:2736-42. [Crossref] [PubMed]
- Dinallo AM, Kolarsick P, Boyan WP, et al. Does routine use of indocyanine green fluorescence angiography prevent anastomotic leaks? A retrospective cohort analysis. Am J Surg 2019;218:136-9. [Crossref] [PubMed]
- Gröne J, Koch D, Kreis ME, et al. Impact of intraoperative microperfusion assessment with Pinpoint Perfusion Imaging on surgical management of laparoscopic low rectal and anorectal anastomoses. Colorectal Dis 2015;17:22-8. [Crossref] [PubMed]
- Diana M, Noll E, Diemunsch P, et al. Enhanced-reality video fluorescence: a real-time assessment of intestinal viability. Ann Surg 2014;259:700-7. [Crossref] [PubMed]
- Ashitate Y, Vooght CS, Hutteman M, et al. Simultaneous assessment of luminal integrity and vascular perfusion of the gastrointestinal tract using dual-channel near-infrared fluorescence. Mol Imaging 2012;11:301-8. [Crossref] [PubMed]
- Kamiya K, Unno N, Miyazaki S, et al. Quantitative assessment of the free jejunal graft perfusion. J Surg Res 2015;194:394-99. [Crossref] [PubMed]
- Protyniak B, Dinallo AM, Boyan WP Jr, et al. Intraoperative indocyanine green fluorescence angiography: an objective evaluation of anastomotic perfusion in colorectal surgery. Am Surg 2015;81:580-4. [Crossref] [PubMed]
- Wada T, Kawada K, Takahashi R, et al. ICG fluorescence imaging for quantitative evaluation of colonic perfusion in laparoscopic colorectal surgery. Surg Endosc 2017;31:4184-93. [Crossref] [PubMed]
- Son GM, Kwon MS, Kim Y, et al. Quantitative analysis of colon perfusion pattern using indocyanine green (ICG) angiography in laparoscopic colorectal surgery. Surg Endosc 2019;33:1640-9. [Crossref] [PubMed]
- Hayami S, Matsuda K, Iwamoto H, et al. Visualization and quantification of anastomotic perfusion in colorectal surgery using nearinfrared fluorescence. Tech Coloproctol 2019;23:973-80. [Crossref] [PubMed]
- Schmoll HJ, Van Cutsem E, Stein A, et al. Consensus Guidelines for management of patients with colon and rectal cancer. a personalized approach to clinical decision making. Ann Oncol 2012;23:2479-516. [Crossref] [PubMed]
- National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Guideline Colon Cancer V.1.2019. Available online: https://www.nccn.org/professionals/physician_gls/default.aspx#site.
- Pellino G, Warren O, Mills S, et al. Comparison of Western and Asian guidelines concerning the management of colon cancer. Dis Colon Rectum 2018;61:250-9. [Crossref] [PubMed]
- Fielding LP, Arsenault PA, Chapuis PH, et al. Clinicopathological staging for colorectal cancer: an International Documentation System (IDS) and an International Comprehensive Anatomical Terminology (ICAT). J Gastroenterol Hepatol 1991;6:325-44. [Crossref] [PubMed]
- Joseph NE, Sigurdson ER, Hanlon AL, et al. Accuracy of determining nodal negativity in colorectal cancer on the basis of the number of nodes retrieved on resection. Ann Surg Oncol 2003;10:213-8. [Crossref] [PubMed]
- Hohenberger W, Weber K, Matzel K, et al. Standardized surgery for colonic cancer: complete mesocolic excision and central ligation - technical notes and outcome. Colorectal Dis 2009;11:354-64. [Crossref] [PubMed]
- Watanabe T, Muro K, Ajioka Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. Int J Clin Oncol 2018;23:1-34. [Crossref] [PubMed]
- Liang JT, Lai HS, Huang J, et al. Long-term oncologic results of laparoscopic D3 lymphadenectomy with complete mesocolic excision for right-sided colon cancer with clinically positive lymph nodes. Surg Endosc 2015;29:2394-401. [Crossref] [PubMed]
- Søndenaa K, Quirke P, Hohenberger W, et al. The rationale behind complete mesocolic excision (CME) and a central vascular ligation for colon cancer in open and laparoscopic surgery. Int J Colorectal Dis 2014;29:419-28. [Crossref] [PubMed]
- Bertelsen CA, Kirkegaard-Klitbo A, Nielsen M, et al. Pattern of Colon Cancer Lymph Node Metastases in Patients Undergoing Central Mesocolic Lymph Node Excision: A Systematic Review. Dis Colon Rectum 2016;59:1209-21. [Crossref] [PubMed]
- Suzuki S, Shigaki N, Yokoyama S, et al. A resected case of gastric regional lymph node metastasis from ascending colon cancer. Gan To Kagaku Ryoho 2011;38:473-5. [PubMed]
- Wood TF, Nora DT, Morton DL, et al. One hundred consecutive cases of sentinel lymph node mapping in early colorectal carcinoma: detection of missed micrometastases. J Gastrointest Surg 2002;6:322-9. [Crossref] [PubMed]
- Gould EA, Winship T, Philbin PHKH. Observations on a “sentinel node” in cancer of the parotid. Cancer 1960;13:77-8. [Crossref] [PubMed]
- Saikawa Y, Otani Y, Kitagawa Y, et al. Interim results of sentinel node biopsy during laparoscopic gastrectomy: possible role in function-preserving surgery for early cancer. World J Surg 2006;30:1962-8. [Crossref] [PubMed]
- Kelder W, Nimura H, Takahashi N, et al. Sentinel node mapping with indocyanine green (ICG) and infrared ray detection in early gastric cancer: an accurate method that enables a limited lymphadenectomy. Eur J Surg Oncol 2010;36:552-8. [Crossref] [PubMed]
- Des Guetz G, Uzzan B, Nicholas P, et al. Is sentinel lymph node mapping in colorectal cancer a future prognostic factor? A meta-analysis. World J Surg 2007;31:1304-12. [Crossref] [PubMed]
- Cahill RA, Leroy J, Marescaux J. Could lymphatic mapping and sentinel node biopsy provide oncological providence for local resectional techniques for colon cancer? A review of the literature. BMC Surg 2008;8:17. [Crossref] [PubMed]
- Stojadinovic A, Nissan A, Protic M, et al. Prospective randomized study comparing sentinel lymph node evaluation with standard pathologic evaluation for the staging of colon carcinoma results from the United States Military Cancer Institute Clinical Trials Group Study GI-01. Ann Surg 2007;245:846-57. [Crossref] [PubMed]
- Bembenek AE, Rosenberg R, Wagler E, et al. Sentinel lymph node biopsy in colon cancer: a prospective multicenter trial. Ann Surg 2007;245:858-63. [Crossref] [PubMed]
- Nagata K, Endo S, Hidaka E, et al. Laparoscopic sentinel node mapping for colorectal cancer using infrared ray laparoscopy. Anticancer Res 2006;26:2307-11. [PubMed]
- Ankersmit M, van der Pas MH, van Dam DA, et al. Near infrared fluorescence lymphatic laparoscopy of the colon and mesocolon. Colorectal Dis 2011;13:70-3. [Crossref] [PubMed]
- Currie AC, Brigic A, Thomas-Gibson S, et al. A pilot study to assess near infrared laparoscopy with indocyanine green (ICG) for intraoperative sentinel lymph node mapping in early colon cancer. Eur J Surg Oncol 2017;43:2044-51. [Crossref] [PubMed]
- Cahill RA, Anderson M, Wang LM, et al. Near-infrared (NIR) laparoscopy for intraoperative lymphatic road-mapping and sentinel node identification during definitive surgical resection of early-stage colorectal neoplasia. Surg Endosc 2012;26:197-204. [Crossref] [PubMed]
- Andersen HS, Bennedsen ALB, Burgdorf SK, et al. In vivo and Ex vivo sentinel node mapping does not identify the same lymph nodes in colon cancer. Int J Colorectal Dis 2017;32:983-90. [Crossref] [PubMed]
- Cahill RA, Bembenek A, Sirop S, et al. Sentinel node biopsy for the individualization of surgical strategy for cure of early-stage colon cancer. Ann Surg Oncol 2009;16:2170-80. [Crossref] [PubMed]
- Nissan A, Protic M, Bilchik A, et al. Predictive model of outcome of targeted nodal assessment in colorectal cancer. Ann Surg 2010;251:265-74. [Crossref] [PubMed]
- Currie AC. Intraoperative Sentinel Node Mapping in the Colon: Potential and Pitfalls. Eur Surg Res 2019;60:45-52. [Crossref] [PubMed]
- van der Pas MH, Meijer S, Hoekstra OS, et al. Sentinel-lymph-node procedure in colon and rectal cancer: a systematic review and meta-analysis. Lancet Oncol 2011;12:540-50. [Crossref] [PubMed]
- Chand M, Keller DS, Joshi HM, et al. Feasibility of fluorescence lymph node imaging in colon cancer: FLICC. Tech Coloproctol 2018;22:271-7. [Crossref] [PubMed]
- Park SY, Park JS, Kim HJ, et al. Indocyanine green fluorescence imaging-guided laparoscopic surgery could achieve radical D3 dissection in patients with advanced right-sided colon cancer. Dis Colon Rectum 2020;63:441-9. [Crossref] [PubMed]
- Emile SH, Elfeki H, Shalaby M, et al. Sensitivity and specificity of indocyanine green near-infrared fluorescence imaging in detection of metastatic lymph nodes in colorectal cancer: Systematic review and meta-analysis. J Surg Oncol 2017;116:730-40. [Crossref] [PubMed]
- Palaniappa NC, Telem DA, Ranasinghe NE, et al. Incidence of iatrogenic ureteral injury after laparoscopic colectomy. Arch Surg 2012;147:267-71. [Crossref] [PubMed]
- Packiam VT, Cohen AJ, Pariser JJ, et al. The Impact of Minimally Invasive Surgery on Major Iatrogenic Ureteral Injury and Subsequent Ureteral Repair During Hysterectomy: A National Analysis of Risk Factors and Outcomes. Urology 2016;98:183-8. [Crossref] [PubMed]
- Siddighi S, Yune JJ, Hardesty J, et al. Indocyanine green for intraoperative localization of ureter. Am J Obstet Gynecol 2014;211:436.e1-e2. [Crossref] [PubMed]
- Lee Z, Moore B, Giusto L, et al. Use of indocyanine green during robot-assisted ureteral reconstructions. Eur Urol 2015;67:291-8. [Crossref] [PubMed]
- Slooter MD, Janssen A, Bemelman WA, et al. Currently available and experimental dyes for intraoperative nearinfrared fluorescence imaging of the ureters: a systematic review. Tech Coloproctol 2019;23:305-13. [Crossref] [PubMed]
- van den Bos J, Al-Taher M, Bouvy ND, et al. Near-infrared fluorescence laparoscopy of the ureter with three preclinical dyes in a pig model. Surg Endosc 2019;33:986-91. [Crossref] [PubMed]
- Park JW, Sohn DK, Hong CW, et al. The usefulness of preoperative colonoscopic tattooing using a saline test injection method with prepackaged sterile India ink for localization in laparoscopic colorectal surgery. Surg Endosc 2008;22:501-5. [Crossref] [PubMed]
- Cho YB, Lee WY, Yun HR, et al. Tumor localization for laparoscopic colorectal surgery. World J Surg 2007;31:1491-5. [Crossref] [PubMed]
- Montorsi M, Opocher E, Santambrogio R, et al. Original technique for small colorectal tumor localization during laparoscopic surgery. Dis Colon Rectum 1999;42:819-22. [Crossref] [PubMed]
- Lee JG, Low AH, Leung JW. Randomized comparative study of indocyanine green and India ink for colonic tattooing: an animal survival study. J Clin Gastroenterol 2000;31:233-6. [Crossref] [PubMed]
- Watanabe M, Tsunoda A, Narita K, et al. Colonic tattooing using fluorescence imaging with light-emitting diode-activated indocyanine green: a feasibility study. Surg Today 2009;39:214-8. [Crossref] [PubMed]
- Miyoshi N, Ohue M, Noura S, et al. Surgical usefulness of indocyanine green as an alternative to India ink for endoscopic marking. Surg Endosc 2009;23:347-51. [Crossref] [PubMed]
- Nagata J, Fukunaga Y, Akiyoshi T, et al. Colonic Marking With Near-Infrared, Light-Emitting, Diode-Activated Indocyanine Green for Laparoscopic Colorectal Surgery. Dis Colon Rectum 2016;59:e14-8. [Crossref] [PubMed]
- Hyun JH, Kim S, Kim KG, et al. A novel endoscopic fluorescent band ligation method for tumor localization. Surg Endosc 2016;30:4659-63. [Crossref] [PubMed]
Cite this article as: Cassinotti E, Boni L, Della Porta M, Baldari L. The role of indocyanine green performing a minimally invasive right colectomy. Ann Laparosc Endosc Surg 2021;6:30.