
Development of surgical concepts in rectal cancer resection and challenges in minimally invasive surgical proctectomy
Introduction
Surgery continues to remain the cornerstone for curative intent in patients with rectal cancer. Despite reporting significant oncological improvements with multimodal therapies such as chemoradiotherapy in the neoadjuvant and adjuvant setting, all patients, except for complete responders, continue to undergo some form of operative intervention for definitive management (1,2). Such operative intervention ranges from local excision to a restorative or non-restorative proctectomy with total mesorectal excision (TME). The history and evolution of rectal cancer surgery is an incredibly interesting one that spans throughout the last century with a change of focus from palliation to curative intent with improved patient and long-term oncological outcomes. Miles performed the first rectal resection with radicality for curative intent in 1907 in which he performed an abdominoperineal resection (APR) in a patient with a rectal malignancy. Miles APR became the gold standard for many decades (3). Earlier reports however do exist by Czerny [1884] and Mayo [1904] highlighting the use of a combined abdominal and perineal approach to facilitate proctectomy (4,5). During this time period it was assumed that rectal malignancy metastasised both proximally and distally in a tubular fashion and therefore distal control in the form of a complete proctectomy and end colostomy formation was necessary in all patients (6).
Overtime, with the development of surgical adjuncts such as leg stirrups, pelvic retractors, surgical stapling devices in tandem with a greater understanding of rectal cancer biology there has been dramatic changes in both the surgical approach and management of patients with rectal cancer. After Miles had noted recurrence of tumor in the pelvic mesocolon in the 1890’s, Sir Berkeley George Andrew Moynihan postulated in the 1920s that surgical resection of a malignant organ should not be confined to the organ, however, should encompass the synchronous resection of the surrounding lymphatic system (7). Further changes in surgical approaches were observed in France in 1921 when Henri Albert Hartmann performed an anterior resection for proximal rectal tumours with preservation of the sphincter complex and thus reducing the morbidity and mortality associated with a perineal resection (8). In the post World WarII era, further advances were achieved in performing restorative resections. Dixon in 1948 reported the first series demonstrating favourable outcomes from the Mayo Clinic in patients undergoing anterior resection with restoration of GI continuity (9). Anterior rectal resections significantly increased with improved development and broader accessibility to surgical stapling devices. The capabilities of sphincter preserving resection with low anastomosis was further aided in 1983, when Pollett et al. demonstrated that a 2 cm distal resection margin did not alter long-term survival compared to the previous proposed 5 cm “safety margin” (10). Throughout the 1980’s multiple studies were published, documenting the ability to achieve restoration with adequate functional outcomes in distal rectal cancers by undertaking a coloanal or intersphincteric anastomosis with or without pouch formation (11).
Of late, one of the greatest advances in modern rectal cancer management has been the introduction of TME by Heald in 1982. TME is now recognised throughout the literature as standard of care for the surgical approach in patients with rectal tumours (12). TME, involves meticulous dissection along the non-vascular TME plane of embryological origin resulting in en bloc resection of the rectum with the surrounding mesorectum (draining blood vessels and lymphatics, Figure 1) (13). Prior to this technique, anterior resections were typically performed with blunt dissection of the rectal tube in the mid and distal one third resulting in high local recurrence rates (>20%) and overall 5-year survival less than 50% (14).
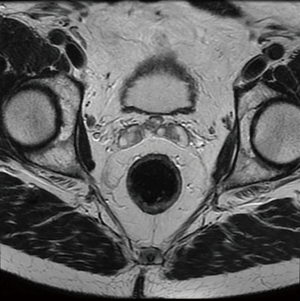
Principles of rectal cancer surgery
The basic principles for rectal cancer surgery are the division of the major vascular pedicle (inferior mesenteric artery, IMA) suppling the rectum along with its lymphatics, obtaining a tumour free circumferential resection margin (CRM) and en-bloc resection of any contiguous organs or structures attached to the tumour. This should be performed meticulously, preserving the autonomic nerve plexus where not involved (Figure 2). Additionally, macroscopic evaluation should demonstrate a smooth mesorectum without defects. Complete TME is associated with lower rates of local recurrence (15-17). Despite maximum efforts, TME is technically difficult due to myriad of patient, tumour, and treatment factors, resulting in sub-optimal resection. Factors such as a narrow male pelvis, obesity, locally advanced mid, distal and anterior rectal tumours and post-radiotherapy treatment are well established as adversely affecting margin clearance. This has led to the development and application of a modified transanal approach to rectal cancers in high volume centres (18-23). During initial studies examining pathological margin clearance, distal and proximal margins were initially reported rather than CRM. After initial concepts of proximal and distal tumour extension were clarified, Golligher et al. reported data highlighting that local tumour dissemination did not exceed 2 cm form the distal margin in 98% of 1,500 analysed rectal specimens (24). Two decades later it has been further demonstrated a distal tumour margin of >1 cm confers no oncologic superiority (11,25). More recently, authors have documented that patients undergoing sphincter preserving surgery with intersphincteric dissection and neoadjuvant chemoradiation with margins of less than 1 cm are not compromised from a local recurrence or overall disease-free survival (26).
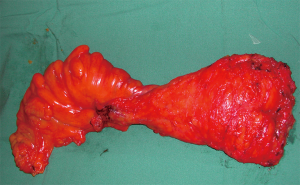
Obtaining a complete mesorectal excision confers significantly improved local recurrence rates. High recurrence rates are seen in patients with an incomplete mesorectal excision and this was previously reported as high as 25% for stage 2 and 3 rectal adenocarcinomas managed with surgery alone (27). By resecting the mesorectum, this theoretically encapsulates the distal extramural spread and CRM of excision thus allowing for an R0 resection. Within the Dutch mesorectal trial, local recurrence was reduced to 8.2% by performing a complete surgical TME alone (28).
It has now been widely recognised that radial or CRM is of significant prognostic benefit. CRM is defined as the non-peritonealised surface of a resected specimen created by the dissection of the subperitoneal aspect at surgery (29). An involved CRM has been shown to be associated with increased rates of both local and distant disease and is typically a marker of the quality of surgery performed (30-33). The pathological assessment of the mesorectal plane is also an important measure of the quality of the surgical resection. Most studies consider a CRM as positive when it is <1 mm from the tumour margin (34,35). Patients undergoing APR compared to low anterior resections have been documented to have increased rates of positive CRM with approximately 16% of patients developing local recurrence within two years of surgery (36). Moreover, patients with a CRM of greater than 2 mm had a significantly lower local recurrence rate. When examining distant metastatic disease, patients with a positive CRM developed metastatic disease in 37.6% of cases as opposed to 12.7% in those with a CRM >1 cm.
Other key principles in proctectomy is restoration of gut continuity and autonomic nerve function preservation while achieving oncological cure where possible. Surgical exposure and precise dissection of the TME plane with effort to correctly identify and preserve pelvic autonomic nerves is paramount in proctectomy. Multiple factors including poor visualisation, bulky tumours, post neoadjuvant therapy and pelvic morphology can hinder precise dissection and result in increased incidence of urinary and sexual dysfunction (37). Anatomically the rectum descends concavely along the sacrum and is confined within a rigid fixed space bounded by the ischial tuberosity and iliac wings laterally and pubic symphysis anteriorly. In a select group of patients with a narrow or deep pelvis adherence to the principles of proctectomy and TME surgery can be very challenging even in the hands of experience colorectal surgeons. A study analysing sexual dysfunction following rectal cancer surgery in both male and female patients using a survey style questionnaire reported that 76% of patients experienced moderate to severe sexual dysfunction after rectal cancer treatment (38). Similarly, Dulskas et al. reported that erectile dysfunction rates in males increased from 41.7% in the pre-operative setting to 63.9% post-operatively with sexual dysfunction increasing from 83.3% to 94% post-operatively in females (39). Moreover, urinary dysfunction significantly increased from 80.1% to 88.9% in male patients. With greater understanding of pelvic anatomy and increased survivorship in patients undergoing proctectomy is it critical that every adjunct possible is utilised to improved rectal dissection and nerve preservation. A recent study by Attaallah et al. in 2018 highlighted a significantly lower rate of sexual dysfunction in both male and female patients undergoing laparoscopic proctectomy compared to open proctectomy (40). Oncology safety in conjunction with well described patient benefits have translated to increasing rates of minimally invasive surgical (MIS) approaches to rectal cancer in the last two decades (41-45).
Benefits of MIS
To date, numerous studies have demonstrated clear advantages of laparoscopic over open surgical colorectal resection (46,47). Advances in laparoscopic surgery since the 1990s has been one of the greatest milestones in the technical evolution of medicine. Notes dating as far back as Hippocrates (460–375 B.C.) documented the use of placing instruments into natural human orifices to inspect internal anatomy. Major advances in laparoscopy occurred after the invention of the lightbulb by Edison in 1879 which saw the development of a rigid endoscopic device with an inbuilt light source (48). Kelling and Ott have been credited with performing the first true laparoscopic procedure by utilising an incision in the posterior vaginal fornix to advance a “ventroscope” to examine the pelvic cavity (49). With the development of new equipment and the use of carbon dioxide for insufflation a German surgeon known as Kurt Semm performed the first appendectomy in 1982 and Erich Muehe undertook the first successful laparoscopic cholecystectomy in 1985 (50). Prior to 1991 all laparoscopic surgeries were confined to a single quadrant until Jacobs et al. performed the first multiquadrant laparoscopic assisted colectomy (51). Despite initial concerns surrounding high rates port site metastases which were subsequently refuted, cases of laparoscopic surgery excelled and a large number of trials were undertaken to examine oncological outcomes in laparoscopic colorectal resection. Multiple randomised controlled trials (RCTs) such as COST 2004, MRC CLASiCC 2005, COLOR 2009, and ALCCAS 2010 in laparoscopic colonic surgery have consistently demonstrated the safety and oncological equivalence to open resection (45,52-56). The COST study reported outcomes on 872 randomised cases from 1994–1999 across 48 institutions by video credentialed surgeons with a 4.4 years mean follow-up. No significance difference in survival between patients undergoing open versus laparoscopic colectomy for stage 1–3 disease was seen. Following a decade of multicentre trials and a large number of publish meta-analyses, laparoscopic colonic resection has now been widely accepted by the colorectal community as equivalent in long-term oncological outcomes with significant short-term benefits over open surgery (57). Most notably laparoscopic interventions have been shown to be associated with reduced blood loss, early feeding, faster recovery, lower morbidity, shorter length of hospital stay and decreased hernia rates (46,47). With such positive early clinical and long-term oncological outcomes laparoscopic intervention for malignancy was translated to rectal cancer resection (58).
MIS proctectomy
Technological advances in minimally invasive proctectomy has significantly progressed in the last decade. The development of 3D laparoscopes with higher degrees of instrument versatility and greatly improved adjuncts such as laparoscopic fluid suction devices, retractors and staplers has led to an increased number of centres now performing proctectomy for both benign and malignant diseases laparoscopically. This has and undoubtedly continues to divide colorectal surgeon opinion on best approach to proctectomy particularly in the setting of rectal malignancy. Supporters of open proctectomy state that regardless of the short-term benefits, a laparoscopic approach may not allow the same oncological clearance and adequate TME resection. Multiple publications including multicentre trials have demonstrated Laparoscopic TME to be equivalent or superior to open TME (Table 1) (59,60). To date there have been some inherent weaknesses to the published studies surrounding MIS proctectomy. A large proportion of data comes from non-randomised comparative studies commonly reporting outcomes on both colonic and rectal cancers. Therefore, a large degree of heterogeneity exists in earlier MIS proctectomy studies. Early experience by Morino et al. in 2003 reported outcomes in 100 consecutive resections for mid/low rectal cancers (average 6.1 cm from anal verge) (61). Mean operative time was 250 minutes with a 12% conversion rate and 12-day hospital stays. Local recurrence and survival at four years was 4.2% and 82% respectively. Since then numerous studies have reported favourable outcomes in MIS proctectomy (Table 1).
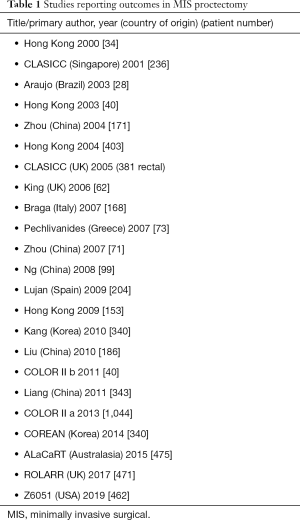
Full table
A large multicenter randomized controlled trial, COLOR II, analysed 3-year locoregional recurrence and survival in 1,044 patients undergoing laparoscopic and open resection for rectal cancer (62). Their findings reported equivalent locoregional recurrence and disease free and overall survival in patients undergoing MIS proctectomy compared to open. Most recently in 2019 the ACOSOG Z6051 study from the United States reported disease free survival and recurrence in 462 patients undergoing open and laparoscopic rectal resection for cancer (63). They concluded that there was no significant difference in oncological outcomes in both groups. Finally, a study from japan concentrating on low rectal tumours examined laparoscopy compared to open resection in advanced low rectal tumours in 1,500 patients across 69 institutions (64). Hida et al. reported significantly less complications in the laparoscopic group with no difference in recurrence-free survival and overall survival at three years. Despite the majority of studies reporting oncological equivalence in MIS proctectomy compare to open, concerns do exist regarding a lack of pathological equivalence (Z6051 & ALaCaRT) and distal rectal cancer resection remains technically challenging.
Challenges of MIS rectal resection
Despite improvement in minimally invasive techniques to proctectomy, access to distal 1/3 tumors, especially in male, obese patients, remains technically challenging. Neoadjuvant chemoradiotherapy, in conjunction with local tumor edema or fibrosis, can further complicate plane identification and dissection. This results in an increased risk of margin positivity, reduced distal resection margin and an incomplete (grade 1) mesorectal specimen, associated with increased local recurrence rates and suboptimal long-term disease free and overall survival. Warrier et al. reported that low rectal cancers were an independent risk factor for CRM positivity (65). Moreover Daniel et al. documented significantly reduced survival rates in rectal cancer patients with obesity (66). Interestingly however a meta-analysis in 2016 focusing on surgical outcomes in obese and non-obese patients highlighted that obese patients had significantly increased rates of conversion to open and post-operative morbidity without significant influence on pathological results (67). Despite this, the importance of achieving a good quality TME with negative margins is paramount to patient outcome. Due to tapering of the rectal wall and mesorectal fascia as the low rectum descends towards the anus the range for error reduces and risk of a positive margin increases. Due to fixed trocar placement and reduced angulation in a fixed bony pelvis visualisation and determination of the distal margin can often be imprecise leading to high conversion rates of up to 34% (45). A pioneering approach to overcome these non-modifiable factors has been developed and currently utilised globally. Transanal TME (taTME), or “bottom up” approach to rectal cancer overcomes many of the described hindrances to access and precise dissection of the distal rectum, translating to improved pathologic specimens in early global registries (68).
Conclusions
The management of rectal cancer has seen dramatic changes over the last century. Multimodal treatment is now the mainstay in managing such patients. Minimally invasive surgery has continued to evolve and has now revolutionised our approach to patients with rectal cancer. MIS proctectomy has clearly become a core technique in the armamentarium of colorectal surgeons performing TME surgery. This inclusion was initially due to promising data from multicentre randomised controlled trials in MIS colonic resection for malignancy. In the last decade numerous studies have reported oncological equivalence in MIS proctectomy compared to open resection with significant short-term patient benefits. Despite this MIS proctectomy for distal rectal tumours can be challenging in obese male patients with large bulky distal tumours in a fixed narrow bony pelvis. The literature has continuously highlighted that such patients are at increased risk of margin positivity with subsequent increase local recurrence and poor overall survival rates. A pioneering approach called taTME has been developed to overcome these non-modifiable factors by performing a “bottom-up” TME dissection, with promising early results.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Laparoscopic and Endoscopic Surgery for the series “taTME”. The article has undergone external peer review.
Peer Review File: Available at http://dx.doi.org/10.21037/ales-20-74
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales-20-74). The series “taTME” was commissioned by the editorial office without any funding or sponsorship. AGH served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wiegering A, Isbert C, Dietz UA, et al. Multimodal therapy in treatment of rectal cancer is associated with improved survival and reduced local recurrence - a retrospective analysis over two decades. BMC Cancer 2014;14:816. [Crossref] [PubMed]
- Thiels CA, Bergquist JR, Meyers AJ, et al. Outcomes with multimodal therapy for elderly patients with rectal cancer. Br J Surg 2016;103:e106-14. [Crossref] [PubMed]
- Miles WE. A method of performing abdomino-perineal excision for carcinoma of the rectum and of the terminal portion of the pelvic colon (1908). CA Cancer J Clin 1971;21:361-4. [Crossref] [PubMed]
- Basu S, Srivastava V, Shukla VK. Recent advances in the management of carcinoma of the rectum. Clin Exp Gastroenterol 2009;2:49-60. [Crossref] [PubMed]
- Mayo CH. Cancer of the large bowel. Med Sentinel 1904;12:466-73.
- Miles WE. Technique of the radical operation for cancer of the rectum. Br J Surg 1914;2:292-305. [Crossref]
- Moynihan BGA. The surgical treatment of cancer of the sigmoid flexure and rectum. Surg Gynecol Obstet 1908;6:463-8.
- Ronel DN, Hardy M. Henri Albert Hartmann: Labor and discipline. Curr Surg 2002;59:59-64. [Crossref] [PubMed]
- Dixon CF. Anterior resection for malignant lesions of the upper part of the rectum and lower part of the sigmoid. Ann Surg 1948;128:425-42. [Crossref] [PubMed]
- Pollett WG, Nicholls RJ. The relationship between the extent of distal clearance and survival and local recurrence rates after curative anterior resection for carcinoma of the rectum. Ann Surg 1983;198:159-63. [Crossref] [PubMed]
- Reguero JL, Longo WE. The evolving treatment of rectal cancer. In: Longo WEMReddy V, Audisio RA Eds., Modern management of cancer of the rectum. New York: Springer, 2015:1-12.
- Heald RJ, Husband EM, Ryall RD. The mesorectum in rectal cancer surgery-the clue to pelvic recurrence? Br J Surg 1982;69:613-6. [Crossref] [PubMed]
- Heald RJ. The 'Holy Plane' of rectal surgery. J R Soc Med 1988;81:503-8. [Crossref] [PubMed]
- Ludwig KA. Sphincter-sparing resection for rectal cancer. Clin Colon Rectal Surg 2007;20:203-12. [Crossref] [PubMed]
- Garlipp B, Ptok H, Schmidt U, et al. Factors influencing the quality of total mesorectal excision. Br J Surg 2012;99:714-20. [Crossref] [PubMed]
- Quirke P, Steele R, Monson J, et al. Effect of the plane of surgery achieved on local recurrence in patients with operable rectal cancer: a prospective study using data from the MRC CR07 and NCIC-CTG CO16 randomised clinical trial. Lancet 2009;373:821-8. [Crossref] [PubMed]
- Veltcamp Helbach M, Deijen CL, Velthuis S, et al. Transanal total mesorectal excision for rectal carcinoma: short-term outcomes and experience after 80 cases. Surg Endosc 2016;30:464-70. [Crossref] [PubMed]
- Buchs NC, Nicholson GA, Ris F, et al. Transanal total mesorectal excision: A valid option for rectal cancer? World J Gastroenterol 2015;21:11700-8. [Crossref] [PubMed]
- Fernández-Hevia M, Delgado S, Castells A, et al. Transanal Total Mesorectal Excision in Rectal Cancer: Short-term Outcomes in Comparison With Laparoscopic Surgery. Ann Surg 2015;261:221-7. [Crossref] [PubMed]
- Ma Bin, Gao P, Song Y, et al. Transanal total mesorectal excision (taTME) for rectal cancer: a systematic review and meta-analysis of oncological and perioperative outcomes compared with laparoscopic total mesorectal excision. BMC Cancer 2016;16:380. [Crossref] [PubMed]
- Simillis C, Hompes R, Penna M, et al. A systematic review of transanal total mesorectal excision: is this the future of rectal cancer surgery? Colorectal Dis 2016;18:19-36. [Crossref] [PubMed]
- deSouza AL, Prasad LM, Marecik SJ, et al. Total Mesorectal Excision for Rectal Cancer: The Potential Advantage of Robotic Assistance. Diseases of the Colon & Rectum 2010;53:1611-7. [Crossref] [PubMed]
- Atallah S, Martin-Perez B, Albert M, et al. Transanal minimally invasive surgery for total mesorectal excision (TAMIS–TME): results and experience with the first 20 patients undergoing curative-intent rectal cancer surgery at a single institution. Tech Coloproctol 2014;18:473-80. [Crossref] [PubMed]
- Goligher JC, Dukes CE, Bussey HJR. Local recurrences after sphincter saving excisions for carcinoma of the rectum and rectosigmoid. Br J Surg 1951;39:199-211. [Crossref] [PubMed]
- Lange MM, Rutten HJ, van de Velde CJ. One hundred years of curative surgery for rectal cancer: 1908-2008. Eur J Surg Oncol 2009;35:456-63. [Crossref] [PubMed]
- Kuvshinoff B, Maghfoor I, Miedema B, et al. Distal margin requirements after preoperative chemoradiotherapy for distal rectal carcinomas: are < or = 1 cm distal margins sufficient? Ann Surg Oncol 2001;8:163-9. [PubMed]
- Rich T, Gunderson LL, Lew R, et al. Patterns of recurrence of rectal cancer after potentially resectable curative surgery. Cancer 1983;52:1317-29. [Crossref] [PubMed]
- Kapiteijn E, Marijnen CAM, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med 2001;345:638-46. [Crossref] [PubMed]
- Hermanek P, Junginger T. The circumferential resection margin in rectal carcinoma surgery. Tech Coloproctol 2005;9:193-9. [Crossref] [PubMed]
- Birbeck KF, Macklin CP, Tiffin NJ, et al. Rates of circumferential resection margin involvement vary between surgeons and predict outcomes in rectal cancer surgery. Ann Surg 2002;235:449-57. [Crossref] [PubMed]
- Baik SH, Kim NK, Lee YC, et al. Prognostic significance of circumferential resection margin following total mesorectal excision and adjuvant chemoradiotherapy in patients with rectal cancer. Ann Surg Oncol 2007;14:462-9. [Crossref] [PubMed]
- Wibe A, Syse A, Andersen ENorwegian Rectal Cancer Group, et al. Oncological outcomes after total mesorectal excision for cure for cancer of the lower rectum: anterior vs. abdominoperineal resection. Dis Colon Rectum 2004;47:48-58. [Crossref] [PubMed]
- Kang J, Kim H, Hur H, et al. Circumferential resection margin involvement in stage III rectal cancer patients treated with curative resection followed by chemoradiotherapy: a surrogate marker for local recurrence? Yonsei Med J 2013;54:131-8. [Crossref] [PubMed]
- Trakarnsanga A, Gonen M, Shia J, et al. What is the significance of the circumferential margin in locally advanced rectal cancer after neoadjuvant chemoradiotherapy? Ann Surg Oncol 2013;20:1179-84. [Crossref] [PubMed]
- Park JS, Huh JW, Park YA, et al. A circumferential resection margin of 1 mm is a negative prognostic factor in rectal cancer patients with and without neoadjuvant chemoradiotherapy. Dis Colon Rectum 2014;57:933-40. [Crossref] [PubMed]
- Nagtegaal ID, Marijnen CAM, Kranenbarg EK, et al. Circumferential margin involvement is still an important predictor of local recurrence in rectal carcinoma: not one millimeter but two millimeters is the limit. Am J Surg Pathol 2002;26:350-7. [Crossref] [PubMed]
- Kim NK, Kim YW, Cho MS. Total mesorectal excision for rectal cancer with emphasis on pelvic autonomic nerve preservation: Expert technical tips for robotic surgery. Surg Oncol 2015;24:172-80. [Crossref] [PubMed]
- Attaallah W, Ertekin C, Tinay I, et al. High rate of sexual dysfunction following surgery for rectal cancer. Ann Coloproctol 2014;30:210-5. [Crossref] [PubMed]
- Dulskas A, Samalavicius NE. A prospective study of sexual and urinary function before and after total mesorectal excision. Int J Colorectal Dis 2016;31:1125-30. [Crossref] [PubMed]
- Attaallah W, Ertekin C, Yegen C. Prospective study of sexual dysfunction after proctectomy for rectal cancer Asian J Surg 2018;41:454-61. [Crossref] [PubMed]
- Hohenberger W, Weber K, Matzel K, et al. Standardized surgery for colonic cancer: Complete mesocolic excision and central ligation–technical notes and outcome. Colorectal Dis 2009;11:354-64. [Crossref] [PubMed]
- Yozgatli TK, Aytac E, Ozben V, et al. Robotic Complete Mesocolic Excision Versus Conventional Laparoscopic Hemicolectomy for Right-Sided Colon Cancer. J Laparoendosc Adv Surg Tech A 2019;29:671-6. [Crossref] [PubMed]
- Miller PE, Dao H, Paluvoi N, et al. Comparison of 30-day postoperative outcomes after laparoscopic vs. robotic colectomy. J Am Coll Surg 2016;223:369-73. [Crossref] [PubMed]
- de’Angelis N, Lizzi V, Azoulay D, et al. Robotic versus laparoscopic right colectomy for colon cancer: Analysis of the initial simultaneous learning curve of a surgical fellow. J Laparoendosc Adv Surg Tech A 2016;26:882-92. [Crossref] [PubMed]
- Guillou PJ, Quirke P, Thorpe H, et al. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASSICC trial): Multicenter, randomised controlled trial. Lancet 2005;365:1718-26. [Crossref] [PubMed]
- Green BL, Marshall HC, Collinson F, et al. Long-term follow-up of the Medical Research Council CLASICC trial of conventional versus laparoscopically assisted resection in colorectal cancer. Br J Surg 2013;100:75-82. [Crossref] [PubMed]
- Schwenk W, Haase O, Neudecker J, et al. Short term benefits for laparoscopic colorectal resection. Cochrane Database Syst Rev 2005;CD003145 [Crossref] [PubMed]
- Kaiser AM. Evolution and future of laparoscopic colorectal surgery. World J Gastroenterol 2014;20:15119-24. [Crossref] [PubMed]
- Kelling G. Ueber Oesophagoskopie, Gastroskopie und Kölioskopie. Munch Med Wochenschr 1902;1:21-4.
- Muhe E. Die erste Cholecystectomie durch das Laparoskop. Langenbecks Arch Chir 1986;369:804. [Crossref]
- Jacobs M, Verdeja JC, Goldstein HS. Minimally invasive colon resection (laparoscopic colectomy). Surg Laparosc Endosc 1991;1:144-50. [PubMed]
- Veldkamp R, Kuhry E, Hop WC, et al. COlon cancer Laparoscopic or Open Resection Study Group (COLOR) Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomised trial. Lancet Oncol 2005;6:477-84. [Crossref] [PubMed]
- Leung KL, Kwok SP, Lam SC, et al. Laparoscopic resection of rectosigmoid carcinoma: prospective randomised trial. Lancet 2004;363:1187-92. [Crossref] [PubMed]
- Nelson H, Sargent DJ, Wieand HS, et al. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med 2004;350:2050-9. [Crossref] [PubMed]
- Weeks JC, Nelson H, Gelber S, et al. Short-term quality-of-life outcomes following laparoscopic-assisted colectomy vs open colectomy for colon cancer: a randomized trial. JAMA 2002;287:321-8. [Crossref] [PubMed]
- Lacy AM, García-Valdecasas JC, Delgado S, et al. Laparoscopy-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer: a randomised trial. Lancet 2002;359:2224-9. [Crossref] [PubMed]
- Kuhry E, Schwenk WF, Gaupset R, et al. Long-term results of laparoscopic colorectal cancer resection. Cochrane Database Syst Rev 2008;CD003432 [Crossref] [PubMed]
- Bonjer HJ, Hop WC, Nelson H, et al. Laparoscopically assisted vs open colectomy for colon cancer: a meta-analysis. Arch Surg 2007;142:298-303. [Crossref] [PubMed]
- van der Pas MH, Haglind E, Cuesta MA, et al. Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol 2013;14:210-8. [Crossref] [PubMed]
- Kang SB, Park JW, Jeong SY, et al. Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): short-term outcomes of an open-label randomised controlled trial. Lancet Oncol 2010;11:637-45. [Crossref] [PubMed]
- Morino M, Parini U, Giraudo G, et al. Laparoscopic total mesorectal excision: a consecutive series of 100 patients. Ann Surg 2003;237:335-42. [Crossref] [PubMed]
- Bonjer HJ, Deijen CL, Haglind E, et al. A randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med 2015;373:194. [Crossref] [PubMed]
- Fleshman J, Branda ME, Sargent DJ, et al. Disease-free Survival and Local Recurrence for Laparoscopic Resection Compared With Open Resection of Stage II to III Rectal Cancer: Follow-up Results of the ACOSOG Z6051 Randomized Controlled Trial. Ann Surg 2019;269:589-95. [Crossref] [PubMed]
- Hida K, Okamura R, Sakai Y, et al. Japan Society of Laparoscopic Colorectal Surgery. Open versus Laparoscopic Surgery for Advanced Low Rectal Cancer: A Large, Multicenter, Propensity Score Matched Cohort Study in Japan. Ann Surg 2018;268:318-24. [Crossref] [PubMed]
- Warrier SK, Kong JC, Guerra GR, et al. Risk Factors Associated With Circumferential Resection Margin Positivity in Rectal Cancer: A Binational Registry Study. Dis Colon Rectum 2018;61:433-40. [Crossref] [PubMed]
- Daniel CR, Shu X, Ye Y, et al. Severe obesity prior to diagnosis limits survival in colorectal cancer patients evaluated at a large cancer centre. Br J Cancer 2016;114:103-9. [Crossref] [PubMed]
- Qiu Y, Liu Q, Chen G, et al. Outcome of rectal cancer surgery in obese and nonobese patients: a meta-analysis. World J Surg Oncol 2016;14:23. [Crossref] [PubMed]
- Roodbeen SX, Penna M, Mackenzie H, et al. Transanal total mesorectal excision (TaTME) versus laparoscopic TME for MRI-defined low rectal cancer: a propensity score-matched analysis of oncological outcomes. Surg Endosc 2019;33:2459-67. [Crossref] [PubMed]
Cite this article as: Waters PS, Heriot AG. Development of surgical concepts in rectal cancer resection and challenges in minimally invasive surgical proctectomy. Ann Laparosc Endosc Surg 2021;6:18.


