Alternatives to hiatal hernia repair for the high-risk patient
Introduction
Hiatal hernia is a relatively common disorder affecting a large percentage of the population worldwide. It is defined by herniation of elements of the abdominal cavity other than the esophagus into the thoracic cavity through the hiatus of the diaphragm. Most commonly, hiatal hernia can be categorized into four types. Type I refers to a sliding type hiatal hernia, where the gastroesophageal (GE) junction is displaced above the diaphragm. Type II refers to hernias where a portion of the gastric fundus herniates through the hiatus while the GE junction remains in its normal anatomic position. Type III hernia is a combination of types I and II when both the GE junction and fundus herniate through the hiatus. Type IV hiatal hernia is characterized by presence of organs other than the stomach (e.g., omentum, colon, small intestine) within the hernia sac. Type I is most common, accounting for over 95%. Type II–IV are referred to collectively as paraesophageal hernias, of which 90% are type III hernias (1). Although less common than Type I hernias, paraesophageal hernias tend to occur in elderly patients with significant pre-existing medical comorbidities (2).
There is a wide range of symptoms associated with hiatal hernias including reflux, dyspepsia, dysphagia, chest pain, shortness of breath, etc. Most patients with hiatal hernias have only mild symptoms and are diagnosed incidentally on imaging studies. However, paraesophageal hernias can lead to serious complications including bleeding, aspiration pneumonia, gastric volvulus, obstruction, and perforation with a large body of literature to help guide the management hiatal hernias. While the mainstay of treatment for Type I hiatal hernias involve management of reflux, the Society of American Gastrointestinal and Endoscopic Surgery (SAGES) recommended that all symptomatic paraesophageal hiatal hernias (Type II–IV) be repaired especially in patients with acute obstruction or history of volvulus (1).
Traditionally, the approach for paraesophageal hernia repair include transabdominal (laparoscopic or open) and transthoracic via open thoracotomy, and both have been shown to be effective. However, despite the advantage of better esophageal mobilization, thoracotomy is rarely performed routinely given high morbidity and prolonged recovery. The standard for hiatal hernia repair is laparoscopic transabdominal repair (1). Currently, the definitive repair of hiatal hernia involves key steps of dissection and reduction of hiatal hernia, mobilization of GE junction, hiatal defect repair with crural closure, and anti-reflux fundoplication with or without gastropexy (1,3,4). Depending on the surgical approach, the operative time for definitive repair can range from 1.5–5.5 hours which may not be suitable for elderly patients with many comorbidities who cannot tolerate prolonged anesthesia and pneumoperitoneum (3).
There are limited recommendations regarding alternatives to hiatal hernia repair particularly in older patients as well as patients with significant comorbidities and increased perioperative risks. While SAGES recommendation states that hernia reduction along with gastropexy may be a safe alternative in high-risk patients, there is a wide range of endoscopic and operative interventions that can be explored (1). In this article, we review the literature and provide an overview and technical considerations of different alternatives to managing hiatal hernia and its associated complications.
High risk considerations
As previously mentioned, paraesophageal hernia is found predominantly in the elderly patient population with inherent age-related comorbidities (2). While age alone should not be a barrier to hernia repair in these patients since the laparoscopic approach has been shown to be safe, the morbidity significantly increases with advanced age and comorbidities (2,5). A study by Gangopadhyay et al. examined the impact of age and comorbidities on complications and outcomes of laparoscopic paraesophageal hernia repairs and found that older patients (>75 years) had larger crural defect size and higher postoperative complications rates up to 29.8%. Moreover, five of the six patients who underwent early reoperation for complications in this series were 74 or older (5).
In a large population-based study by Larusson et al., they found that patients older than 70 years of age had higher postoperative morbidity and mortality and that high-risk patients (ASA 3 and 4) had significantly higher morbidity (OR 2.3). They concluded that age, ASA score, and type of operation significantly influence postoperative morbidity and mortality, and that indication for surgery in high-risk patients should be carefully considered (2).
Non-operative management of hiatal hernia
Until recent decades, many have advocated for the surgical correction of paraesophageal hernias irrespective of symptoms based on the idea of preventing life-threatening complications such as strangulation and avoiding the morbidity and mortality associated with an emergency operation. Additionally, the practice of laparoscopic technique offering less morbid approach has been used in support of elective repair of paraesophageal hernias (6,7). However, more recent literature would suggest that the incidence of life-threatening complications is less than was previously thought.
Stylopoulos et al. used a Markov Monte Carlo model based on five studies to predict clinical outcomes related to elective laparoscopic hernia repair and watchful waiting. They found the annual probability of developing acute complications that require emergency operation to be 1.1% in the watchful waiting group. They also demonstrated the mortality associated with emergency surgery has been over-estimated in the past (17% vs. 5.4%). In fact, their model showed that elective repair in asymptomatic patients actually decreases the qualify-adjusted life expectancy for patients 65 years or older (6).
A more thoughtful approach to paraesophageal hernia treatment should be based on patient symptoms and considerations of patient risk factors. For a limited group of patients with asymptomatic or minimally symptomatic hiatal hernias, non-operative management is certainly a reasonable option to consider. That being said, when a patient is symptomatic or presents with acute symptoms of obstruction or strangulation, most often the result of gastric volvulus, operative intervention remains the mainstay of management.
It is worth noting that the majority of patients with paraesophageal hernias are in their 70s and 80s with significant comorbidities which make operative intervention high risk. In fact, sometimes primary care physicians do not refer their elderly comorbid patients for surgical evaluation due to the patients’ poor overall health. However, these are the patients who would benefit from a surgical consult and a formal anesthetic risk assessment to make a more informed decision (8).
Interventions for small type I hiatal hernias
As previously discussed, the mainstay of treatment for type I hiatal hernia is management of reflux. GE reflux has a complex and multifactorial pathophysiology so symptoms are not always well controlled with medical management alone, not to mention long-term PPI therapy can have negative consequences (9,10). Traditionally, the gold standard operative intervention is laparoscopic fundoplication which is not always well tolerated or preferred in the high-risk patient populations. Recently, a number of endoscopic alternatives to trans-abdominal operations have been described. In this section, we describe two endoscopic alternatives in management of high-risk patients with small Type I hiatal hernias.
One of the endoscopic interventions for reflux that has been shown to have long-term efficacy in reflux control is transoral incisionless fundoplication (TIF) (11-13). First introduced in 2005, it is an endoscopic procedure that aims to create a full thickness esophagogastric fundoplication. While minor variations exist, it is usually performed with the goal of constructing a 3–5 cm omega-shaped valve in a 250 to 300 degrees circumferential pattern around the GE junction to create a one-way GE valve (14,15). Notably, the TIF procedure includes the reduction of hiatal hernias less than 2cm. This is achieved by gripping the esophagus with the tissue invaginator and advancing the device caudally and applying suction before closure of the tissue mold prior to creating the plications. In a meta-analysis published in 2018, 65.6% of patients had evidence of a hiatal hernia prior to TIF, and hiatal hernia reduction or complete resolution was achieved in 91% of those patients (11). With increasing evidence of favorable long-term outcomes of patients who underwent TIF, this procedure should certainly be considered as an option in high-risk patients with a small type I hiatal hernia.
Another endoscopic anti-reflux procedure that can be performed in patients with less than 2cm hiatal hernias is anti-reflux mucosectomy (ARMS). First described in 2003, the ARMS procedure utilizes cap endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD) to perform a hemi-circumferential EMR of the gastric cardia around the GE junction, causing contraction and scarring which is thought to tighten the GE junction (16,17). While the true anti-reflux mechanism of ARMS has not been studied, small series with short-term outcomes have reported good reflux control on select patients in the absence of hiatal hernias or very small (<2 cm) hiatal hernias (16). Another proposed mechanism of reflux control from ARMS is disruption of the neuropathway that contributes to transient lower esophageal sphincter (LES) relaxations. The pathophysiology of reflux is multifactorial including crural tightness, LES dysfunction, and intraabdominal pressure in addition to the presence of a hiatal hernia. A small hiatal hernia alone may not be the main factor driving the patient’s symptoms. Therefore, it would be reasonable to consider a simple endoscopic procedure such as ARMS to offer to high-risk patients to control reflux symptoms without addressing the small hiatal hernia.
Hiatal hernia and gastric volvulus
One of the absolute indications for operative repair of hiatal hernia in high risk patients is gastric volvulus with obstruction or strangulation. Since the majority of existing literature describing alternative management of hiatal hernia repair are in the setting of acute presentations of gastric volvulus, we will focus on the management strategies related to gastric volvulus in this section.
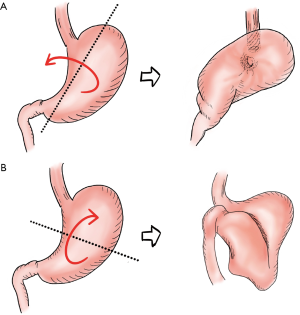
Gastric volvulus, first reported in 1866 by Berti, and later on described by Borchards in 1904, occurs when the stomach rotates along its longitudinal axis (organoaxial volvulus), or axis joining the mid-lesser and greater curves (mesenteroaxial volvulus) as shown in Figure 1. Organoaxial volvulus is the most common type. Primary gastric volvulus, caused by a lax gastrocolic ligament accounts for one third of gastric volvulus while secondary gastric volvulus account for the rest, usually occurring in association with a paraesophageal hernia, acquired diaphragmatic defect, or abdominal adhesions (1,18-20). Gastric volvulus is more frequently diagnosed in elderly patients and presenting symptoms usually include the classic Borchards’s triad of severe epigastric pain, nonproductive retching, and inability to place nasogastric tube.
Clinically, gastric volvulus can present as an acute abdominal emergency or a chronic recurrent problem. Acute gastric volvulus is considered a surgical emergency and if not treated in a timely fashion, may lead to obstruction, strangulation, ischemia, hemorrhage, perforation, and full-thickness necrosis. Mortality rates of acute gastric volvulus has been reported to be as high as 20% while those for chronic gastric volvulus range from 0–13% (19,20).
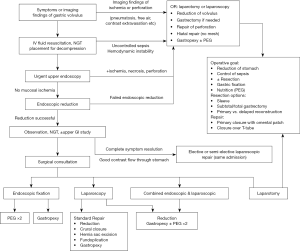
Traditional treatment of gastric volvulus consists of laparotomy, gastric detorsion, fixation, and when present, repair of associated diaphragmatic hernia with fundoplication. However, various endoscopic, laparoscopic, and combined approaches have been described with good short-term outcomes (3,21-23). A proposed management algorithm utilizing existing literature is shown in Figure 2 (1,24-26).
The initial management of patients presenting with symptoms concerning for acute gastric volvulus includes appropriate IV fluid resuscitation, correction of electrolyte abnormalities, and imaging studies to confirm diagnosis. Computed tomography (CT) scan of the chest and abdomen with water soluble contrast should be performed early given its high sensitivity and ability to clearly visualize the abnormal position and torsion of the stomach as well as any signs of obstruction (1,24,26). Once diagnosis is confirmed, a nasogastric tube should be placed if possible for decompression (27). Since CT is not able to assess the degree of mucosal ischemia until there is gastric necrosis, urgent upper endoscopy is also required to assess the presence of mucosal ischemia. There is agreement among the literature that with evidence of gastric ischemia, necrosis, or perforation, urgent exploratory laparotomy should be performed to address the problem such as limited resection in case of gastric necrosis, which sometimes can be done laparoscopically depending on patient condition (24). Of note, when there is evidence or high suspicion of gastric necrosis or perforation, broad spectrum IV antibiotics should also be initiated as part of the initial management.
Endoscopic interventions
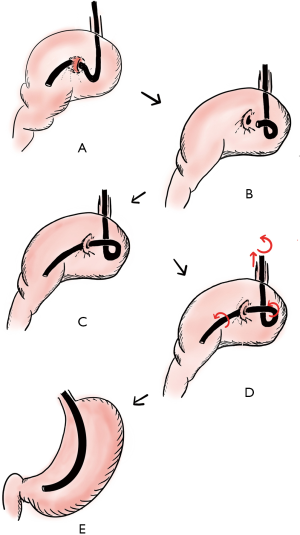
Once the diagnosis of gastric volvulus has been made and resuscitation initiated, urgent upper endoscopy should be pursued as it is crucial to fully evaluate the gastric mucosa for any signs of ischemia. Moreover, endoscopy can often help decompress and reduce the volvulus, while noting endoscopic reduction should only be attempted if there is no significant evidence of ischemia due to risk of perforation. Several techniques have been reported for endoscopic reduction including the J-shape maneuver, extended J-shape maneuver, alpha-loop maneuver, and nonspecific rotational maneuvers (27). Tsang et al. described the alpha-loop maneuver and was successful in reducing gastric volvulus in 7 of 8 patients in the series, all of whom had associated hiatal hernias (27).
The alpha-loop maneuver is performed by first advancing the endoscope through the narrowed lumen from the twisted gastric fold into the distal body and antrum, while using a J-turn maneuver to confirm passage through the gastric volvulus. Then withdraw the endoscope to form an alpha-loop before re-advancing through the volvulus. Finally, pull back the endoscope while torqueing clockwise to allow uncoiling of the alpha-loop and reduction of the volvulus. If the volvulus does not reduce with clockwise rotation of the endoscope, it could be a result of a less common posteriorly rotated volvulus and the same maneuver with counterclockwise rotation could be attempted. Of note, when possible, this procedure should be done under fluoroscopic guidance to confirm reduction and avoid twisting the stomach further (27). Figure 3 depicts the sequential steps of the alpha-loop maneuver, adapted from original publication by Tsang et al. (27).
While endoscopic reduction can be successful in some cases, without addressing the underlying etiology, the volvulus can recur. Therefore, following endoscopic reduction, the patient should be closely monitored and offered more definitive endoscopic or surgical interventions. For example, Eckhauser and Ferron first described the use of dual percutaneous gastrostomy tubes to anchor the stomach in patients who were poor surgical candidates, providing two areas of fixation far apart along the greater curve to prevent recurrence (28). The use of gastrostomy tubes as gastropexy is now widely accepted and practiced with variations in number of gastrostomy tubes fixation sites, ranging from one to three (28,29). In patients who have difficulty with oral intake, placement of gastrostomy tubes is particularly helpful with postoperative management to help maintain nutritional intake and also provide decompression as needed. In addition to a gastrostomy tube, there are case reports of using other newer endoscopic devices such as the Funada-type gastropexy device in the management of gastric volvulus (24).
Surgical interventions
When endoscopic reduction is unsuccessful or definitive endoscopic gastrostomy or gastropexy are technically challenging, surgical intervention may still be required. Laparoscopic repair is the preferred surgical intervention for gastric volvulus associated with paraesophageal hernias and is generally well tolerated with less morbidity compared to open repair in the elective setting. However, the operation usually requires several hours of general anesthesia and significant technical skills on the part of the surgeon (19,21). When presented with an elderly patient with high ASA class and significant comorbidities, one should consider alternative procedures to the traditional surgical management mentioned previously.
In the interest of minimizing time of general anesthesia and pneumoperitoneum, the focus of the operative intervention should be on reduction of the gastric volvulus and paraesophageal hernia with gastropexy. In a case report by Naim et al., a 92 years old patient underwent emergent laparoscopic exploration after only partial decompression by endoscopy, after reduction of the volvulus and hiatal hernia, an anterior gastropexy was performed where the greater curvature of the stomach was sutured to the fascia of the anterior abdominal wall using a suture passer. The hernia sac was left intact and a fundoplication was not performed given the patient’s high-risk profile and emergent indication (19). Furthermore, many controversies still exist when it comes to the optimal surgical technique in paraesophageal hernia repair, one of which is the necessity to include an antireflux procedure at the time of repair (22,30). This further supports the idea of a limited operation in high-risk patients.
On the other hand, in a series by Channer et al., four patients with gastric volvulus and hiatal hernias underwent standard laparoscopic repair including reduction of the volvulus, excision of the hernia sac, re-approximation of the diaphragmatic crura, fundoplication, and anterior abdominal wall gastropexy with a gastrostomy tube. Here the authors reported only one patient with postoperative dysrhythmia and no other major complications (31). However, two of the four patients were younger than 65 years of age without significant medical comorbidities.
Combining the limited case reports of laparoscopic intervention for gastric volvulus, laparoscopic intervention may be safe in the management of gastric volvulus with paraesophageal hernias and limiting the surgical intervention to reduction of the volvulus with anterior gastropexy alone can potentially minimize the morbidity in high-risk and elderly patients.
Combined endoscopic and laparoscopic interventions
As discussed above, endoscopic reduction with percutaneous endoscopic gastrostomy (PEG) gastropexy, by providing 2-point fixation of stomach to the abdominal wall, can be a safe and effective alternative to surgery in treating gastric volvulus and hiatal hernia in patients who are poor surgical candidates. However, successful endoscopic reduction and safe placement of PEGs are not always possible due to potential anatomic problems such as other organs overlying the stomach or conditions affecting transillumination (22,23). There are a number of case series and case reports that describe the successful management of hiatal hernia and gastric volvulus using a combine laparoscopic and endoscopic approach in the high-risk patient population (3,4,20,21,23,29).
Most recently, Shehzad et al. reported a case series of five high-risk elderly patients (median age 78 and four out of five with ASA class four) with emergency paraesophageal hernias who underwent combined laparoscopic and PEG gastropexy (23). After a full exploratory laparoscopy, the stomach was reduced with atraumatic graspers and held close to the anterior abdominal wall, under direct visualization making sure there were no adhesions or organs overlying the stomach, an endoscope was placed in the stomach and the first site of the PEG selected in the antrum along the greater curvature and a PEG tube placed using the usual Ponsky pull method. The second PEG site was then placed 5–10 cm proximal to the first along the greater curve in the same technique. The procedure was successful in all five patients without major complications and the PEG tubes were removed in four out of five patients at six weeks while one patient required long-term enteral feeding (23).
A number of other series and case reports have described similar techniques with different variations (4,20,29,32). Kercher et al. summarized the management of 11 elderly patients with severe cardiopulmonary disease and symptomatic paraesophageal hernia (3). They described using endoscopy and continuous insufflation of air from the endoscope just before the volvulized portion of the stomach to reduce the stomach back into the abdominal cavity. If successful, two PEG tubes were placed along the greater curvature, one at the body and the other in the gastric antrum 12 cm or more from the first. When the stomach is not easily reducible endoscopically, patients proceeded to the operating room where the hernia was reduced laparoscopically and gastrostomy tubes placed under direct visualization. In addition, an intracorporeal anterior gastropexy was performed using two to four 2-0 silk sutures, providing additional intra-abdominal fixation of the stomach (see Figure 4). Of note, the average operative time in this series was one hour for all cases (3). There were also no documented postoperative mortality or documented recurrence at a mean follow up of 4.1 months.
Ho et al. performed a similar procedure in a case report but they placed a jejunostomy tube over the second PEG tube to temporary nutritional support (4). In another series that included 13 high-risk patients, 4 of them underwent laparoscopic repair in the emergent setting similar to above that also included an anterior cruroplasty with extracorporeal suturing but without fundoplication (22). The gastropexy was carried out using 4 T-fasteners and a gastrostomy tube was placed was in the center of the 4 points of fixations by the T-fasteners. There were no intraoperative complications or mortalities reported (22). In another paper by Halka et al., the PEG tube was reinforced with 2-0 Vicryl ties via a Trocar closure suture grasper at 4 sites around the gastrostomy tube site to avoid the possible complications associated with T-fasteners such as erosion into the anterior abdominal wall (33).
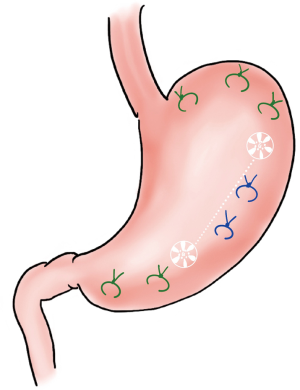
In a series by Yates et al., 11 high risk patients with obstructive gastric volvulus were managed successfully with laparoscopic reduction and anterior abdominal sutured gastropexy with and without gastrostomy (21). The laparoscopic anterior gastropexy in this study was done using 2-0 silk sutures placed every 3 cm along the greater curvature starting from the left crus and moving towards the antrum (see Figure 5). A PEG tube was placed in the majority of patients in this series. There were no intraoperative complications but two patients required reoperation for prematurely displaced gastrostomy tubes. Yates suggested that PEG tube placement in the setting of laparoscopic gastropexy should be limited to patients with concerns for inadequate oral intake postoperatively (21).
Though current literature mostly consists of case reports and limited series. The combined endoscopic and laparoscopic approach to managing high-risk patients with symptomatic hiatal hernias and associated complications such as gastric volvulus seems to be safe and well-tolerated. While different variations of technique exist, the general principle of performing limited operation focusing on hernia reduction and gastric fixation without complete excision of hernia sac, crural closure, or fundoplication is agreed upon for this specific patient population. Although currently lacking, further long-term outcomes including hernia recurrence and quality of life data would be helpful in guiding any future recommendations for the most appropriate intervention and technique.
Conclusions
Hiatal hernia is a common disorder in the elderly high-risk patients with many comorbidities that results in increased morbidity when undergoing the traditional standard operative repair. However, alternative minimally invasive interventions exist that can help minimize the perioperative morbidity in these patients. Practitioners should be familiar with these minimally invasive interventions in order to better take care of our aging population.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Lee L. Swanstrom and Steven G. Leeds) for the series “Hiatal Hernia” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Conflicts of interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales-19-256). The series “Hiatal Hernia” was commissioned by the editorial office without any funding or sponsorship. MBU reports other from Boston Scientific, personal fees from Gore, personal fees from Olympus, personal fees from Cook, personal fees from Medtronic, Erbe, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kohn GP, Price RR, DeMeester SR, et al. Guidelines for the management of hiatal hernia. Surg Endosc 2013;27:4409-28. [Crossref] [PubMed]
- Larusson HJ, Zingg U, Hahnloser D, et al. Predictive Factors for Morbidity and Mortality in Patients Undergoing Laparoscopic Paraesophageal Hernia Repair: Age, ASA Score and Operation Type Influence Morbidity. World J Surg 2009;33:980-5. [Crossref] [PubMed]
- Kercher KW, Matthews BD, Ponsky JL, et al. Minimally invasive management of paraesophageal herniation in the high-risk surgical patient. Am J Surg 2001;182:510-4. [Crossref] [PubMed]
- Ho CKM, Cheung FKY, Yien RLC, et al. Minimally-invasive approach to paraoesophageal hernia in high surgical-risk patients. Surg Pract 2014;18:87-9. [Crossref]
- Gangopadhyay N, Perrone JM, Soper NJ, et al. Outcomes of laparoscopic paraesophageal hernia repair in elderly and high-risk patients. Surgery 2006;140:491-8. [Crossref] [PubMed]
- Stylopoulos N, Gazelle GS, Rattner DW. Paraesophageal Hernias: Operation or Observation? Ann Surg 2002;236:492-500. [Crossref] [PubMed]
- Wirsching A, El Lakis MA, Mohiuddin K, et al. Acute Vs. Elective Paraesophageal Hernia Repair: Endoscopic Gastric Decompression Allows Semi-Elective Surgery in a Majority of Acute Patients. J Gastrointest Surg 2018;22:194-202. [Crossref] [PubMed]
- Dellaportas D, Papaconstantinou I, Nastos C, et al. Large Paraesophageal Hiatus Hernia: Is Surgery Mandatory?. Chirurgia (Bucur) 2018;113:765-71. [Crossref] [PubMed]
- Menezes MA, Herbella FAM. Pathophysiology of Gastroesophageal Reflux Disease. World J Surg 2017;41:1666-71. [Crossref] [PubMed]
- Reimer C. Safety of long-term PPI therapy. Best Pract Res Clin Gastroenterol 2013;27:443-54. [Crossref] [PubMed]
- McCarty TR, Itidiare M, Njei B, et al. Efficacy of transoral incisionless fundoplication for refractory gastroesophageal reflux disease: a systematic review and meta-analysis. Endoscopy 2018;50:708-25. [Crossref] [PubMed]
- Testoni PA, Testoni S. Transoral fundoplication for gastroesophageal reflux disease. Ann Esophagus 2018;1:7. [Crossref]
- Testoni PA, Testoni S, Distefano G, et al. Transoral incisionless fundoplication with EsophyX for gastroesophageal reflux disease: clinical efficacy is maintained up to 10 years. Endosc Int Open 2019;7:E647-54. [Crossref] [PubMed]
- Bell RCW, Cadière G-B. Transoral rotational esophagogastric fundoplication: technical, anatomical, and safety considerations. Surg Endosc 2011;25:2387-99. [Crossref] [PubMed]
- Cadière GB, Rajan A, Germay O, et al. Endoluminal fundoplication by a transoral device for the treatment of GERD: A feasibility study. Surg Endosc 2008;22:333-42. [Crossref] [PubMed]
- Hedberg HM, Kuchta K, Ujiki MB. First Experience with Banded Anti-reflux Mucosectomy (ARMS) for GERD: Feasibility, Safety, and Technique (with Video). J Gastrointest Surg 2019;23:1274-8. [Crossref] [PubMed]
- Inoue H, Ito H, Ikeda H, et al. Anti-reflux mucosectomy for gastroesophageal reflux disease in the absence of hiatus hernia: a pilot study. Ann Gastroenterol 2014;27:346-51. [PubMed]
- Wasselle JA, Norman J. Acute gastric volvulus: pathogenesis, diagnosis, and treatment. Am J Gastroenterol 1993;88:1780-4. [PubMed]
- Naim HJ, Smith R, Gorecki P. Emergent Laparoscopic Reduction of Acute Gastric Volvulus With Anterior Gastropexy. Surg Laparosc Endosc Percutan Tech 2003;13:389-91. [Crossref] [PubMed]
- Jeong SH, Ha CY, Lee YJ, et al. Acute gastric volvulus treated with laparoscopic reduction and percutaneous endoscopic gastrostomy. J Korean Surg Soc 2013;85:47-50. [Crossref] [PubMed]
- Yates RB, Hinojosa MW, Wright AS, et al. Laparoscopic gastropexy relieves symptoms of obstructed gastric volvulus in highoperative risk patients. Am J Surg 2015;209:875-80. [Crossref] [PubMed]
- Arevalo G, Wilkerson J, Saxe J. Acute Paraesophageal Hernia: Laparoscopic Repair With Adjunct T-Fastener Gastropexy for the High Operative Risk Patient. Surg Laparosc Endosc Percutan Tech 2018;28:123-7. [Crossref] [PubMed]
- Shehzad K, Askari A, Slesser AAP, et al. A Safe and Effective Technique of Paraesophageal Hernia Reduction Using Combined Laparoscopy and Nonsutured PEG Gastropexy in High-Risk Patients. JSLS 2019;23:e2019.00041.
- Zuiki T, Hosoya Y, Lefor AK, et al. The management of gastric volvulus in elderly patients. Int J Surg Case Rep 2016;29:88-93. [Crossref] [PubMed]
- Light D, Links D, Griffin M. The threatened stomach: management of the acute gastric volvulus. Surg Endosc 2016;30:1847-52. [Crossref] [PubMed]
- Bawahab M, Mitchell P, Church N, et al. Management of acute paraesophageal hernia. Surg Endosc 2009;23:255-9. [Crossref] [PubMed]
- Tsang TK, Walker R, Yu DJ. Endoscopic reduction of gastric volvulus: The alpha-loop maneuver. Gastrointest Endosc 1995;42:244-8. [Crossref] [PubMed]
- Eckhauser ML, Ferron JP. The use of dual percutaneous endoscopic gastrostomy (DPEG) in the management of chronic intermittent gastric volvulus. Gastrointest Endosc 1985;31:340-2. [Crossref] [PubMed]
- Xenos ES. Percutaneous Endoscopic Gastrostomy in a Patient with a Large Hiatal Hernia Using Laparoscopy. JSLS 2000;4:231-3. [PubMed]
- Draaisma WA, Gooszen HG, Tournoij E, et al. Controversies in paraesophageal hernia repair; a review of literature. Surg Endosc 2005;19:1300-8. [Crossref] [PubMed]
- Channer LT, Squires GT, Price PD. Laparoscopic Repair of Gastric Volvulus. JSLS 2000;4:225-30. [PubMed]
- Lee HY, Park JH, Kim SG. Chronic Gastric Volvulus with Laparoscopic Gastropexy after Endoscopic Reduction: A Case Report. J Gastric Cancer 2015;15:147-50. [Crossref] [PubMed]
- Halka JT, Yee D, Angus A, et al. Alexis St. Martin Gastropexy: A Novel Technique for Gastropexy During Percutaneous Endoscopic Gastrostomy Tube Placement. Surg Laparosc Endosc Percutan Tech 2019;29:e20-3. [Crossref] [PubMed]
Cite this article as: Wong HJ, Ujiki MB. Alternatives to hiatal hernia repair for the high-risk patient. Ann Laparosc Endosc Surg 2021;6:32.