
To mesh or not to mesh for hiatal hernias: what does the evidence say
A brief history
The laparoscopic approach for paraesophageal hernia repair (LPEHR) began in the 1990s but was plagued by a recurrence rate as high as 33–42% (1,2). Prior to this, the laparoscopic approach was less frequently performed, and outcomes were more heavily focused on the technique and morbidity of repair rather than on anatomic recurrence (2-5). Once it was clear that laparoscopic repair was associated with lower morbidity, the focus shifted toward long-term anatomic success.
Large hiatal hernias have an intrinsically high recurrence rate attributed to tension at the hiatus, either axial along the length of esophagus or laterally at the crural repair (6,7). Sources of tension at the hiatus include esophageal shortening, the natural pressure gradient between the abdomen and thorax, large hiatal defects, attenuated crural tissue, and the dynamic function of the diaphragm, which is in constant motion from respiration and also subject to sudden increased tension from coughing, laughing, sneezing, or straining (2,6-9). Factors that have been proposed to contribute to the higher rates or recurrence after laparoscopic repair include inaccurate assessment of intraabdominal esophageal length due to elevation of the diaphragm by pneumoperitoneum, decreased adhesions, less tactile feedback to determine tension of the crural closure, and lack of deep bites at the crura when using laparoscopic suturing devices (7,10).
To address the high recurrence rates of LPEHR, surgeons began to examine the use of mesh to reinforce the hiatal repair given the success of using mesh for inguinal and ventral hernias (8). In the late 1990s to early 2000s, several randomized controlled trials (RCTs) compared recurrence rates after simple suture repair versus suture repair with mesh reinforcement of the hiatus during laparoscopic repair (9,11,12). All three RCTs found decreased short-term recurrence rates with the use of mesh reinforcement for hiatal hernia repair.
Still, there was concern with the use of mesh at the hiatus, including complications such as erosion, dense fibrosis, and esophageal stenosis (8,13-16). These complications have resulted in serious morbidity including esophageal perforation and need for reoperation, including major esophageal or gastric resection (16,17). Concern for complications from synthetic mesh has since led to the investigation of other types of intraperitoneal mesh for reinforcement of the hiatus, but to this day, the practice of whether to use mesh and what kind of mesh to use remains highly variable among surgeons (18).
In this article, we will review the evidence for outcomes from synthetic, biologic, and absorbable synthetic mesh reinforcement of the hiatus for paraesophageal hernias (PEH). We will briefly discuss mesh repair of type I hiatal hernias and the difficult hiatus, and then conclude with directions for future research.
Synthetic mesh
Early reports of using synthetic mesh for LPEHR involved the use of polypropylene mesh, which was associated with visceral adhesions to the mesh, prompting the search for another option for intraperitoneal mesh (8). One of the first reports of using polytetrafluoroethylene (PTFE) mesh to reinforce the crural closure in hiatal hernias was published by Frantzides and Carlson in 1997 (19). In their report, the authors described their experience using a PTFE mesh to circumferentially reinforce the posterior cruroplasty in a “keyhole” fashion in three patients with a large (≥8 cm) hiatal hernia. There were no complications or recurrences within the first 11 months of follow-up, prompting the authors to perform a preliminary comparison between 16 cases of primary cruroplasty to 15 cases of cruroplasty with PTFE mesh reinforcement (20). At follow up of 12–36 months, they found an 18.8% recurrence in the primary repair group versus no recurrence in the mesh group (P=0.08), suggesting that there may be some benefit to PTFE mesh reinforcement of the crural repair in large hiatal hernias.
Therefore, Frantzides et al. published a subsequent RCT that enrolled over twice as many patients with a hiatal hernia defect ≥8 cm undergoing laparoscopic repair (9). In their analysis of 36 patients undergoing suture repair versus 36 patients undergoing suture repair with PTFE mesh reinforcement, patients were followed with an upper endoscopy and esophagram 3 months postoperatively and every 6 months thereafter to document any anatomic recurrence. The authors reported no recurrences in the mesh group compared to 22% recurrence in the non-mesh group at a median of 2.5 years (range, 6 months to 6 years) of follow-up (P<0.006). There were no differences in complications between the two groups and specifically no mesh-related complications.
The association between synthetic mesh reinforcement and decreased recurrence rates was affirmed by a subsequent RCT by Granderath et al. comparing 50 patients who had polypropylene mesh reinforcement of the hiatus versus 50 patients who underwent suture cruroplasty without mesh (11). In this study, patients with gastroesophageal reflux disease undergoing laparoscopic Nissen fundoplication were randomized to either hiatal suture repair versus suture repair with a 1×3 cm2 polypropylene mesh sutured across the posterior crural repair. The authors found a statistically significant decrease in the rate of recurrence in the mesh group at one-year follow-up (26% versus 8%; P<0.001). Of note, patients with mesh reinforcement also experienced a statistically significant higher rate of postoperative dysphagia at 3-month follow-up (12% versus 4%; P<0.05), but this difference disappeared over time with 4% residual dysphagia in both groups by 1-year follow-up. Similar to the results published by Frantzides et al. above, there were no reported mesh-related complications in this study, which the authors attributed to keeping the mesh away from the esophagus, buffered by the fundoplication.
The most recent RCT comparing synthetic mesh use in LPEHR was published by Oor et al., who examined reinforcement for hiatal defects >5 cm. The authors compared 36 patients undergoing laparoscopic hiatal repair with mesh versus 36 patients undergoing repair with suture alone (21). In contrast to the previously mentioned RCTs, the authors of this study found no statistically significant difference in rates of recurrence between the two groups at 6-month follow-up with endoscopy and/or esophagram (25% versus 19.5%, respectively; P=0.581). A possible explanation for the different outcomes may be the type of fundoplication used in Oor et al.’s study compared to the other two RCTs. In Oor et al.’s study, although patients were randomized, statistically significantly more patients underwent a posterior partial fundoplication (Toupet) in the mesh versus non-mesh group (83.3% versus 16.7%, respectively; P<0.001), and the rest of the patients underwent an anterior 180-degree partial (Dor) fundoplication. Choice of fundoplication was left to the surgeon’s discretion and was thus unblinded to the use of mesh. No patients underwent a full, 360-degree (Nissen) fundoplication, whereas in both Frantzides et al.’s (9) and Granderath et al.’s (11) studies, all patients underwent a complete Nissen fundoplication. It is unclear how the type of fundoplication affects recurrence rates for PEHs in combination with or without mesh reinforcement, as such a study has yet to be published. However, the nonrandomized use of Toupet fundoplication in 83.3% of mesh cases versus only 16.7% of non-mesh cases should certainly be regarded as a significant confounder in Oor et al.’s study, and results of the study should be interpreted with caution.
Despite level 1 evidence from two RCTs demonstrating that hiatal reinforcement with synthetic mesh reduced recurrence rates after LPEHR with no difference in rates of complications or postoperative symptoms, routine adoption of the technique was limited by reports of significant complications resulting from placement of synthetic mesh at the hiatus such as erosion, stricture, and significant dysphagia (8,13-15). More recent evidence has reinforced these concerns. In Müller-Stich et al.’s systematic review of 124 studies on mesh use in LPEHR, the authors found an overall 0.9–1.9% rate of mesh-associated complications, the majority (71.5%) of which involved synthetic mesh, either polypropylene or PTFE, with a synthetic mesh-related complication rate of 0.8–2.5% (22).
Although mesh-related complications are rare, they can have disastrous consequences. In their report of 28 hiatal hernia mesh-related complications, Stadlhuber et al. described 21 complications specifically related to use of synthetic mesh, including 16 cases of mesh erosion, three cases of dense fibrosis, and one case of esophageal stenosis (16). Major resection (partial or total esophagectomy or gastrectomy) was required in 45% of the reoperations for synthetic mesh complications and in 32% of the mesh complications overall. This is consistent with the finding that 30% of patients undergoing revisional foregut surgery end up requiring major resection if they have a history of mesh placement at the hiatus, compared to only 4% incidence of major resection without mesh (23). As a result, most foregut surgeons avoid using synthetic mesh during hiatal hernia repair with rare exception, as demonstrated in a Society of American Gastrointestinal and Endoscopic Surgeons survey reporting that 65% of surgeons use biologic material when mesh is used for crural reinforcement during hiatal hernia repair (18).
Biologic mesh
In an effort to avoid the complications related to synthetic mesh at the hiatus, when biologic mesh materials hit the market, surgeons began using them as an alternative to synthetic mesh. The theoretic advantage of a biologic prosthesis was that provides a temporary collagen matrix to allow native tissue ingrowth at the hiatal repair with a resulting repair that is stronger than native tissue (24). Since the biologic scaffold dissolves over time as it is incorporated by the body, the thought was that the mesh could be used to support the hiatal repair during healing while also avoiding complications related to having a permanent foreign body at the hiatus.
In the only published RCT focused exclusively on biologic mesh use in LPEHR, we compared outcomes of patients with PEH who had their hiatal repair reinforced with mesh made from porcine small intestinal submucosa (n=51) versus primary repair without mesh (n=57) (12). We found a statistically significant decrease in anatomic recurrence with the use of mesh reinforcement at six months postoperatively (9% versus 24%; P=0.04), which encouraged the use of biologic mesh reinforcement among the surgical community. However, long-term follow-up at five years demonstrated no statistically significant difference in recurrence rates between the two groups (54% versus 59%, respectively; P=0.7) (25). Nevertheless, there were no mesh-related complications and no reoperations in the mesh group throughout the long-term follow-up of median 58 months (range, 42–78 months). There were also no statistically significant differences in postoperative symptom or quality-of-life scores between the two groups either at short-term or long-term follow-up. In other words, although there appeared to be no downside to biologic mesh, the short-term reduction in recurrence rate did not appear durable.
A more recent RCT by Watson et al. compared outcomes between patients undergoing LPEHR with mesh reinforced by either synthetic (n=42) or biologic (n=41) mesh versus patients with no mesh reinforcement (n=43), and found no difference in the 6-month recurrence rates between mesh reinforcement (either synthetic or biologic) versus no mesh (21.8% versus 23.1%, respectively; P=1) (26). The authors’ definition of recurrence was any intrathoracic stomach as determined by esophagram or endoscopy, whereas our study limited recurrence to a hiatal hernia >2 cm on upper gastrointestinal series. However, even when Watson et al. limited their analysis of recurrence to cases in which the hiatal hernia was ≥2 cm, there was still no statistically significant difference between the mesh versus no-mesh groups (2.6% versus 7.7%, respectively; P=0.329). There were no reported cases of mesh-related erosion or stricture. At one-year follow-up, there were no differences in dysphagia rates or satisfaction scores among the three groups, but biologic mesh was associated with increased nausea (27.5% versus 15% no mesh and 4.9% synthetic mesh; P=0.0197) and deceased ability to relieve bloating (72.5% versus 92.5% no mesh and 97.5% synthetic mesh; P=0.0017). The authors point out that the magnitude of these differences was small and unlikely to be clinically significant. They further reiterated that the overall outcomes and adverse effects in all groups were similar, and that long-term analysis with more extended follow-up is needed.
The natural next step was to compare recurrence rates between synthetic versus biologic mesh for LPEHR, however the lack of comparative studies renders making any comparison very difficult. The only randomized trial published to date that compares biologic to synthetic mesh for hiatal hernia repair is Watson et al.’s study discussed above (26). Recurrence of any degree of intrathoracic stomach was 30.8% in the absorbable mesh group versus 12.8% in the synthetic mesh group, and recurrence limited to a hiatal hernia ≥2 cm was 5.9% versus 0%, respectively. While these differences were not statistically significant, the results suggest that synthetic mesh may decrease recurrence rates more than biologic mesh, but perhaps the study groups were too small to reach statistical significance.
However, a recently published systematic review of nonrandomized studies by Castelijns et al. did find a statistically significant decrease in the rate of recurrence with the use of synthetic mesh reinforcement compared to biologic mesh (27). The authors pooled 16 studies on the use of mesh in laparoscopic hiatal hernia repair to compare 704 patients who received synthetic mesh with 385 patients who received biologic mesh and found a recurrence rate of 6.8% in the synthetic mesh group versus 16.1% in the biologic mesh group (P<0.05). However, given the heterogeneity of the included studies, the authors highlighted the need for more RCTs to directly compare outcomes from biologic and synthetic meshes. Fortunately, there is an ongoing RCT that will compare recurrence rates and symptomatic/quality-of-life assessments between synthetic and biologic mesh reinforcement of LPEHR, but the study completion date is scheduled for August 2020 (ClinicalTrials.gov Identifier NCT02242526) and thus results are still unavailable.
While there are a number of meta-analyses of case series and clinical trials on the use of mesh for LPEHR and the comparison of synthetic versus biologic mesh, interpretation of the results is limited by the significant diversity of the included studies (22,28-33). These studies compared cases in which there were variable indications for hernia repair, different types/sizes of hiatal hernias, various types/shapes/positions of mesh, diverse methods of securing mesh, and different types of fundoplication. In addition, some studies included cases that involved a lengthening procedure (e.g., Collis gastroplasty) and/or relaxing incisions, whereas these cases were excluded in other studies. Furthermore, the definition of recurrence (e.g., any intrathoracic stomach versus only >2 cm intrathoracic stomach) and duration of follow-up were highly variable among studies. The extreme heterogeneity among the studies used in these meta-analyses limits the reliability of any of the conclusions drawn from the analyses.
However, even if synthetic mesh is ultimately found to have lower recurrence rates compared to biologic mesh, observational studies seem to suggest that there are fewer mesh-related complications from biologic mesh compared to synthetic mesh. In a paper summarizing mesh-related complications after hiatal hernia repair, synthetic mesh was associated with 75% of the reported complications and all 16 cases of erosion related to a specific type of mesh (16). There was one additional case of mesh erosion associated with biologic mesh, but it was unclear whether the biologic mesh or the coexisting synthetic mesh was the underlying cause of the complication. Li and Cheng likewise reported that the vast majority of mesh-related erosion at the hiatus was related to synthetic mesh, with only 2% of the 50 reported cases occurring after a biologic mesh (34). The only case of erosion related to biologic mesh in their study was the same one that was combined with a synthetic mesh as reported by Stadlhuber et al. Of particular concern is that 26% of the patients with mesh-related erosion at the hiatus ultimately require major resection including distal esophagectomy or partial/total gastrectomy (34). In contrast, long-term follow-up out to a median of 4–5 years after biologic mesh placement has revealed no mesh-related complications (25,35). Thus, it is important to consider whether the potential decreased recurrence rate offered by synthetic mesh reinforcement is worth the added risk of significant morbidity. While the short-term benefit of biologic mesh reinforcement does not appear to be durable at 5 years, there are also limited if any long-term complications from the use of biologic mesh at the hiatus.
Absorbable synthetic mesh
Given the outcomes reported from biologic mesh use in LPEHR, the absorbable properties that make biologic mesh appealing for reducing mesh-related complications may also impair its ability to provide a durable hiatal hernia repair. In addition, the relatively high cost of biologic mesh material has limited its routine use for even abdominal wall hernias (36). Thus, absorbable synthetic mesh material was developed as a potentially more cost-effective option for hernia repair (37). Although data are still lacking, the use of absorbable synthetic mesh in LPEHR may also be a promising option that combines the more resilient crural reinforcement provided by synthetic mesh with the absorptive properties of biologic mesh. Indeed, comparison of an absorbable synthetic versus biologic mesh for ventral hernia repair in a rat model demonstrated that the absorbable synthetic mesh is rapidly incorporated with less mesh shrinkage compared to biologic mesh, and the repair was stronger than native tissue at 60 days (38). These biophysical properties of absorbable synthetic mesh are encouraging, but whether the results translate to clinical outcomes remains to be seen. However, there are a few published case series that suggest positive results when absorbable synthetic mesh is used for reinforcement during hiatal hernia repair (39-42).
In a retrospective cohort study, Asti et al. compared 43 patients who had LPEHR with no mesh reinforcement to 41 patients who had hiatal reinforcement with Bio-A mesh (polyglycolic acid/trimethylene carbonate) (40). At a median 24-month (minimum 1-year) follow-up, there was a 9.7% recurrence rate in the mesh group versus an 18.6% recurrence rate in the non-mesh group, although this result was not statistically significant. There were no differences in complications and no reoperations in either group. Asti et al. later reported outcomes of their first 100 cases of any hiatal hernia repair (90% PEHs) with absorbable synthetic mesh and reported a recurrence rate of only 9% at a median 30 months of follow-up (41). This recurrence rate was similar to the 9.5% recurrence at median follow-up of 14 months (range, 11–34 months) reported by Zehetner et al. in their review of 35 patients undergoing LPEHR with Vicryl (polyglactin) absorbable synthetic mesh reinforcement (39).
In a recent report of 50 consecutive cases of LPEHR with Phasix-ST absorbable synthetic mesh, Abdelmoaty et al. reported an 8% recurrence rate at 1-year follow-up with no reported mesh complications (42). Phasix-ST mesh is composed of poly-4-hydroxybutyrate with a Sepra-Technology coating that allows for placement next to viscera, with slow resorption over 12–18 months (43,44). While the results appear promising, the authors noted that they were unable to determine whether the relatively low recurrence rate was due to the mesh itself, due to the use of Collis gastroplasty (used in 42% of cases) or relaxing incisions (4% of cases), or due to a combination of these techniques.
Currently there have been no reported cases of mesh-related complications from the use of absorbable mesh during LPEHR, but the meshes are relatively new and long-term outcomes have yet to be published.
Type I hiatal hernias
The discussion thus far has focused on repair of PEHs, with typically an irreducible portion of intrathoracic stomach and/or with a hiatal defect of >5 cm. However, given a recurrent hiatal hernia is one of the most common reasons for symptomatic failure after a fundoplication for antireflux surgery (45-47), some surgeons have extrapolated the potential benefit of mesh reinforcement in PEH to type I (sliding) hiatal hernias (45,46,48-50).
There are no RCTs evaluating the effect of mesh reinforcement of the hiatus during repair for exclusively type I hiatal hernias. Although Granderath et al.’s (11) study likely included a number of patients with type I hiatal hernias, each group had about 60% of patients with a defect >5 cm and thus likely included a majority of PEHs.
There are limited published case series comparing the effect of mesh reinforcement of the hiatus for type I hiatal hernias. Kamolz et al. examined the outcomes of 100 patients with synthetic mesh reinforcement of the crural repair during laparoscopic antireflux surgery and compared them to outcomes of 100 patients without mesh reinforcement (45). At 1-year follow-up, there were no recurrences in the mesh group compared to 9% in the non-mesh group based on endoscopy. The mesh group was associated with increased rates of dysphagia at 3 months postoperatively (35.4% versus 20.8%), but there were no differences by 1 year after surgery (4.8% versus 5.3%). Similarly, Jacobs et al. found decreased recurrence rates in patients who underwent laparoscopic antireflux surgery with biologic mesh reinforcement of the hiatus compared to repair without mesh (3.3% versus 20%, respectively) (48). Note that neither of these studies specified the size or type of hiatal hernias repaired, and it is possible that some PEHs were included with variable frequency in either group.
Turkcapar et al. specifically excluded all PEHs in their case series examining 511 patients who underwent laparoscopic fundoplication with synthetic mesh (n=176) versus without mesh (n=335) reinforcement of the crural repair (46). The authors found a recurrence rate of 1.8% in the mesh group versus 6% in the group without mesh based on endoscopy and/or esophagram at a follow-up of a least 2 years and a mean follow-up of 75 months (P=0.036). There were no differences in dysphagia between the two groups and no reported mesh-related complications.
Schmidt et al. examined the effect of biologic mesh reinforcement on recurrence rates for small hiatal defects, defined as measuring 1–5 cm on either preoperative esophagram or endoscopy (50). The authors compared laparoscopic fundoplication with mesh (n=38) versus without mesh reinforcement and found that at 1-year follow-up with esophagram and/or endoscopy, the rate of recurrence in the mesh group was 0% compared to 16% in the group without mesh (P=0.017).
Of note, none of these studies were randomized and all represented case series in which mesh reinforcement was introduced and implemented as standard practice after a specific time point. Thus, the non-mesh group comprises patients operated on earlier in the series, whereas patients in the mesh group were operated on later in the surgeons’ experience. The sequential collection of data for one group followed by the comparison group can confound the results given that recurrence rates have been observed to decrease over time as surgeons overcome their learning curve (51).
There is currently no high-quality evidence that mesh reinforcement of the hiatus reduces recurrence rates in type I hiatal hernias, although results from a few case series suggest a possible benefit. Nonetheless, few surgeons believe mesh should be used for type I hiatal hernias until a benefit is clearly shown for larger PEHs.
The difficult hiatus
When the crura cannot be approximated at all or will only do so under excessive tension, a diaphragmatic relaxing incision with mesh reinforcement is recommended to decrease tension at the hiatus (6,52,53) (Figure 1A,B). Diaphragmatic relaxing incisions can be made either to the left or the right of the hiatus, or even bilaterally when needed to allow approximation of the crural pillars and primary closure of the hiatal defect.
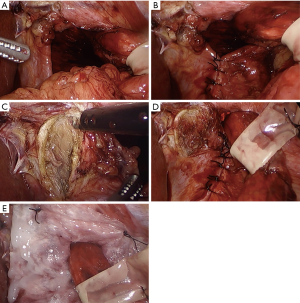
Typically, a right-sided diaphragmatic relaxing incision is preferred as the first maneuver to close a difficult hiatus (Figure 1C,D,E), and can reduce average tension at the hiatus by 46% (52). The right-sided relaxing incision is preferred as it typically involves just a small incision parallel to the right crus. In addition, the caudate and left lateral segment of the liver may help cover the defect to minimize the occurrence of a diaphragmatic hernia. In contrast, a left diaphragmatic relaxing incision is not only more exposed, but it requires a large lateral incision to avoid injury to the pericardium and phrenic nerve (6). However, if the right crus is excessively attenuated, fibrotic, or too close to the inferior vena cava, a left-sided diaphragmatic relaxing incision can certainly be used as the initial maneuver to reduce radial tension at the hiatus (6,53). On rare occasions, bilateral relaxing incisions may be needed to decrease the radial tension enough to approximate the crus. Some authors also advocate intentional entry into the pleural space to improve pliability of the diaphragm and reduce any tension from an adhered lung (6,7,52).
Any diaphragmatic defect from a relaxing incision should be covered with mesh to prevent herniation of intraabdominal contents (Figure 1E). On the right side, it is debatable whether to use synthetic or biologic mesh. There are no studies specifically comparing synthetic versus biologic mesh for a right-sided diaphragmatic relaxing incision. Those who advocate for synthetic mesh repair believe biologic materials should never be used to bridge a gap, while biologic proponents point to liver protection and lack of published evidence on right crural hernias. Regardless, the type of mesh at this location is unlikely to have great significance since the mesh is placed away from the esophagus and is protected from the viscera by the caudate and left lateral lobe of the liver. For a left-sided relaxing incision, we recommend the use of synthetic mesh only, since absorbable mesh can result in a diaphragmatic hernia once the mesh absorbs in this exposed position (6,53).
Conclusions
The literature to date supports the potential of mesh to decrease recurrence after LPEHR compared to primary suture cruroplasty. The evidence is great for synthetic materials, but these are also associated with devastating, albeit rare, complications when placed at the hiatus. On the other hand, there are fewer reports of complications related to biologic mesh, but little evidence for preventing long-term recurrence 5 years and beyond. Newer absorbable synthetic mesh may reduce the cost associated with biologic materials but data on efficacy compared to synthetic or biologic mesh are lacking.
There is no strong evidence to either support or oppose the use of mesh reinforcement at the hiatus, although mesh should certainly be used to cover a diaphragmatic defect resulting from a relaxing incision for a difficult hiatus. Unfortunately, there is a lack of high-quality comparative data between different types of mesh for LPEHR, so when mesh is needed, there is little evidence to guide which type to use within the main three categories of mesh.
Future research should focus on RCTs to determine long-term outcomes for different types of mesh compared to each other, as well as compared to non-mesh LPEHR. This topic has many unanswered questions deserving of further investigation.
Acknowledgements
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Lee L. Swanstrom and Steven G. Leeds) for the series “Hiatal Hernia” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales-19-249). The series “Hiatal Hernia” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study did not require approval by an ethics board as there was no involvement of patients or patient data.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mattar SG, Bowers SP, Galloway KD, et al. Long-term outcome of laparoscopic repair of paraesophageal hernia. Surg Endosc 2002;16:745-9. [Crossref] [PubMed]
- Hashemi M, Peters JH, DeMeester TR, et al. Laparoscopic repair of large type III hiatal hernia: objective follow up reveals high recurrence rate. J Am Coll Surg 2000;190:553-60. [Crossref] [PubMed]
- Schauer PR, Ikramuddin S, McLaughlin RH, et al. Comparison of laparoscopic versus open repair of paraesophageal hernia. Am J Surg 1998;176:659-65. [Crossref] [PubMed]
- Gantert WA, Patti MG, Arcerito M, et al. Laparoscopic repair of paraesophageal hiatal hernias. J Am Coll Surg 1998;186:428-32. [Crossref] [PubMed]
- Horgan S, Eubanks TR, Jacobsen G, et al. Repair of paraesophageal hernias. Am J Surg 1999;177:354-8. [Crossref] [PubMed]
- Greene CL, DeMeester SR, Zehetner J, et al. Diaphragmatic relaxing inci-sions during laparoscopic paraesophageal hernia repair. Surg Endosc 2013;27:4532-8. [Crossref] [PubMed]
- Alicuben ET, Worrell SG, DeMeester SR. Impact of crural relaxing incisions, Collis gastroplasty, and non-cross-linked human dermal mesh crural reinforcement on early hiatal hernia recurrence rates. J Am Coll Surg 2014;219:988-92. [Crossref] [PubMed]
- Paul MG, DeRosa RP, Petrucci PE, et al. Laparoscopic tension-free repair of large paraesophageal hernias. Surg Endosc 1997;11:303-7. [Crossref] [PubMed]
- Frantzides CT, Madan AK, Carlson MA, et al. A prospective, randomized trial of laparoscopic polytetrafluoroethylene (PTFE) patch repair vs simple cruroplasty for large hiatal hernia. Arch Surg 2002;137:649-52. [Crossref] [PubMed]
- Rochefort M, Wee JO. Management of the difficult hiatal hernia. Thorac Surg Clin 2018;28:533-9. [Crossref] [PubMed]
- Granderath FA, Schweiger UM, Kamolz T, et al. Laparoscopic Nissen fundoplication with prosthetic hiatal closure reduces postoperative intrathoracic wrap herniation: preliminary results of a prospective randomized functional and clinical study. Arch Surg 2005;140:40-8. [Crossref] [PubMed]
- Oelschlager BK, Pellegrini CA, Hunter J, et al. Biologic prosthesis reduces recurrence after laparoscopic paraesophageal hernia repair: a multicenter, prospective, randomized trial. Ann Surg 2006;244:481-90. [PubMed]
- Carlson MA, Condon RE, Ludwig KA, et al. Management of intrathoracic stomach with polypropylene mesh prosthesis reinforced transabdominal hiatus hernia repair. J Am Coll Surg 1998;187:227-30. [Crossref] [PubMed]
- Coluccio G, Ponzio S, Ambu V, et al. Dislocation into the cardial lumen of a PTFE prosthesis used in the treatment of voluminous hiatal sliding hernia: a case report. Minerva Chir 2000;55:341-5. [PubMed]
- Targarona EM, Bendahan G, Balague C, et al. Mesh in the hiatus: a controversial issue. Arch Surg 2004;139:1286-96. [Crossref] [PubMed]
- Stadlhuber RJ, Sherif AE, Mittal SK, et al. Mesh complications after prosthetic reinforcement of hiatal closure: a 28-case series. Surg Endosc 2009;23:1219-26. [Crossref] [PubMed]
- Tatum RP, Shalhub S, Oelschlager BK, et al. Complications of PTFE mesh at the diaphragmatic hiatus. J Gastrointest Surg 2008;12:953-7. [Crossref] [PubMed]
- Pfluke JM, Parker M, Bowers SP, et al. Use of mesh for hiatal hernia repair: a survey of SAGES members. Surg Endosc 2012;26:1843-8. [Crossref] [PubMed]
- Frantzides CT, Carlson MA. Prosthetic reinforcement of posterior cruroplasty during laparoscopic hiatal herniorrhaphy. Surg Endosc 1997;11:769-71. [Crossref] [PubMed]
- Carlson MA, Richards CG, Frantzides CT. Laparoscopic prosthetic reinforcement of hiatal herniorrhaphy. Dig Surg 1999;16:407-10. [Crossref] [PubMed]
- Oor JE, Roks DJ, Koetje JH, et al. Randomized clinical trial comparing laparoscopic hiatal hernia repair using sutures versus sutures reinforced with non-absorbable mesh. Surg Endosc 2018;32:4579-89. [Crossref] [PubMed]
- Müller-Stich BP, Kenngott HG, Gondan M, et al. Use of mesh in laparoscopic paraesophageal hernia repair: a meta-analysis and risk-benefit analysis. PLoS One 2017;12:e0171865 [Crossref] [PubMed]
- Parker M, Bowers SP, Bray JM, et al. Hiatal mesh is associated with major resection at revisional operation. Surg Endosc 2010;24:3095-101. [Crossref] [PubMed]
- Badylak S, Kokini K, Tullius B, et al. Strength over time of a resorbable bioscaffold for body wall repair in a dog model. J Surg Res 2001;99:282-7. [Crossref] [PubMed]
- Oelschlager BK, Pellegrini CA, Hunter JG, et al. Biologic prosthesis to prevent recurrence after laparoscopic paraesophageal hernia repair: long-term follow-up from a multicenter, prospective, randomized trial. J Am Coll Surg 2011;213:461-8. [Crossref] [PubMed]
- Watson DI, Thompson SK, Devitt PG, et al. Laparoscopic repair of very large hiatus hernia with sutures versus absorbable mesh versus nonabsorbable mesh: a randomized controlled trial. Ann Surg 2015;261:282-9. [Crossref] [PubMed]
- Castelijns PSS, Ponten JEH, van de Poll MCG, et al. A collective review of biological versus synthetic mesh-reinforced cruroplasty during laparoscopic Nissen fundoplication. J Minim Access Surg 2018;14:87-94. [Crossref] [PubMed]
- Antoniou SA, Antoniou GA, Koch OO, et al. Lower recurrence rates after mesh-reinforced versus simple hiatal hernia repair: a meta-analysis of randomized trials. Surg Laparosc Endosc Percutan Tech 2012;22:498-502. [Crossref] [PubMed]
- Huddy JR, Markar SR, Ni MZ, et al. Laparoscopic repair of hiatus hernia: does mesh type influence outcome? A meta-analysis and European survey study. Surg Endosc 2016;30:5209-21. [Crossref] [PubMed]
- Memon MA, Memon B, Yunus RM, et al. Suture cruroplasty versus prosthetic hiatal herniorrhaphy for large hiatal hernia: a meta-analysis and systematic review of randomized controlled trials. Ann Surg 2016;263:258-66. [Crossref] [PubMed]
- Tam V, Winger DG, Nason KS. A systematic review and meta-analysis of mesh vs suture cruroplasty in laparoscopic large hiatal hernia repair. Am J Surg 2016;211:226-38. [Crossref] [PubMed]
- Sathasivam R, Bussa G, Viswanath Y, et al. 'Mesh hiatal hernioplasty' versus 'suture cruroplasty' in laparoscopic para-oesophageal hernia surgery; a systematic review and meta-analysis. Asian J Surg 2019;42:53-60. [Crossref] [PubMed]
- Memon MA, Siddaiah-Subramanya M, Yunus RM, et al. Suture cruroplasty versus mesh hiatal herniorrhaphy for large hiatal hernias (HHs): an updated meta-analysis and systematic review of randomized controlled trials. Surg Laparosc Endosc Percutan Tech 2019;29:221-32. [Crossref] [PubMed]
- Li J, Cheng T. Mesh erosion after hiatal hernia repair: the tip of the iceberg? Hernia 2019;23:1243-52. [Crossref] [PubMed]
- Wassenaar EB, Mier F, Sinan H, et al. The safety of biologic mesh for laparoscopic repair of large, complicated hiatal hernia. Surg Endosc 2012;26:1390-6. [Crossref] [PubMed]
- Reynolds D, Davenport DL, Korosec RL, et al. Financial implications of ventral hernia repair: a hospital cost analysis. J Gastrointest Surg 2013;17:159-66. [Crossref] [PubMed]
- Köckerling F, Alam NN, Antoniou SA, et al. What is the evidence for the use of biologic or biosynthetic meshes in abdominal wall reconstruction? Hernia 2018;22:249-69. [Crossref] [PubMed]
- Gruber-Blum S, Brand J, Keibl C, et al. Abdominal wall reinforcement: biologic vs. degradable synthetic devices. Hernia 2017;21:305-15. [Crossref] [PubMed]
- Zehetner J, Lipham JC, Ayazi S, et al. A simplified technique for intrathoracic stomach repair: laparoscopic fundoplication with Vicryl mesh and BioGlue crural reinforcement. Surg Endosc 2010;24:675-9. [Crossref] [PubMed]
- Asti E, Lovece A, Bonavina L, et al. Laparoscopic management of large hiatus hernia: five-year cohort study and comparison of mesh-augmented versus standard crura repair. Surg Endosc 2016;30:5404-9. [Crossref] [PubMed]
- Asti E, Sironi A, Bonitta G, et al. Crura augmentation with Bio-A mesh for laparoscopic repair of hiatal hernia: single-institution experience with 100 consecutive patients. Hernia 2017;21:623-8. [Crossref] [PubMed]
- Abdelmoaty WF, Dunst CM, Filicori F, et al. Combination of surgical technique and bioresorbable mesh reinforcement of the crural repair leads to low early hernia recurrence rates with laparoscopic paraesophageal hernia repair. J Gastrointest Surg 2020;24:1477-81. [Crossref] [PubMed]
- Deeken CR, Matthews BD. Characterization of the mechanical strength, re-sorption properties, and histologic characteristics of a fully absorbable material (poly-4-hydroxybutyrate-PHASIX mesh) in a porcine model of hernia repair. ISRN Surg 2013;2013:238067 [Crossref] [PubMed]
- Biondo-Simões MLP, Pessini VCA, Porto PHC, et al. Adhesions on polypropylene versus Sepramesh meshes: an experimental study in rats. Rev Col Bras Cir 2018;45:e2040 [PubMed]
- Kamolz T, Granderath FA, Bammer T, et al. Dysphagia and quality of life after laparoscopic Nissen fundoplication in patients with and without prosthetic reinforcement of the hiatal crura. Surg Endosc 2002;16:572-7. [Crossref] [PubMed]
- Turkcapar A, Kepenekci I, Mahmoud H, et al. Laparoscopic fundoplication with prosthetic hiatal closure. World J Surg 2007;31:2169-76. [Crossref] [PubMed]
- Müller-Stich BP, Koninger J, Muller-Stich BH, et al. Laparoscopic mesh-augmented hiatoplasty as a method to treat gastroesophageal reflux without fundoplication: single-center experience with 306 consecutive patients. Am J Surg 2009;198:17-24. [Crossref] [PubMed]
- Jacobs M, Gomez E, Plasencia G, et al. Use of surgisis mesh in laparoscopic repair of hiatal hernias. Surg Laparosc Endosc Percutan Tech 2007;17:365-8. [Crossref] [PubMed]
- Parsak CK, Erel S, Seydaoglu G, et al. Laparoscopic antireflux surgery with polyglactin (vicryl) mesh. Surg Laparosc Endosc Percutan Tech 2011;21:443-9. [Crossref] [PubMed]
- Schmidt E, Shaligram A, Reynoso JF, et al. Hiatal hernia repair with biologic mesh reinforcement reduces recurrence rate in small hiatal hernias. Dis Esophagus 2014;27:13-7. [Crossref] [PubMed]
- Soper NJ, Dunnegan D. Anatomic fundoplication failure after laparoscopic antireflux surgery. Ann Surg 1999;229:669-76. [Crossref] [PubMed]
- Bradley DD, Louie BE, Farivar AS, et al. Assessment and reduction of diaphragmatic tension during hiatal hernia repair. Surg Endosc 2015;29:796-804. [Crossref] [PubMed]
- Crespin OM, Yates RB, Martin AV, et al. The use of crural relaxing incisions with biologic mesh reinforcement during laparoscopic repair of complex hiatal hernias. Surg Endosc 2016;30:2179-85. [Crossref] [PubMed]
Cite this article as: Inaba CS, Oelschlager BK. To mesh or not to mesh for hiatal hernias: what does the evidence say. Ann Laparosc Endosc Surg 2021;6:40.


