Hiatal hernia, lower esophageal sphincter and their combined effect on the natural history of gastroesophageal reflux disease: implications for surgical therapy
Introduction
Gastroesophageal reflux disease (GERD) is the most common foregut disorder and affects millions of persons worldwide. Epidemiological studies show that the prevalence increases with age and is similar in males and females. In the general population, the prevalence of typical reflux symptoms is up to 30%, and there is an increasing incidence worldwide by 30% every 10 years (1-3). Impairment of quality of life in GERD is relevant, and is largely due to incomplete response to proton-pump inhibitors in patients with high symptom load and night-time reflux causing sleep disturbances (4). Moreover, it is estimated that progression to Barrett’s esophagus, the pre-malignant lesion causing esophageal adenocarcinoma, occurs in 10% of patients under routine medical care over a 5-year follow-up (5).
Historically, in the 1950s, hiatal hernia (HH) was considered a necessary pre-condition for GERD. As a surgeon of this era, Allison devoted his efforts to repairing the diaphragm to treat reflux symptoms and esophagitis at a time when no effective pharmacologic therapy was available (6). Subsequent manometric studies conducted at Mayo Clinic demonstrated that a high-pressure zone, namely the lower esophageal sphincter (LES), was also a key factor in preventing gastroesophageal reflux (7). This finding was later corroborated by anatomical studies demonstrating a muscular equivalent of the manometric LES at the esophago-gastric junction (EGJ) (8).
However, it was not until the 1980s that the “two-sphincter hypothesis” started to emerge (9,10). According to this theory, the antireflux barrier consists of an extrinsic sphincter (the crura) and an intrinsic sphincter (the LES), both playing a crucial role in the maintenance of an efficient EGJ. This implies that failure of one of these two components may facilitate GERD, but it is still debated whether the crura causes LES failure or viceversa. Over the past three decades, the pathophysiology of HH and GERD has been revisited in an effort to clarify the appropriate indications and the role of antireflux surgical therapy in the management of these patients.
Anatomy of the esophagogastric junction
The esophageal hiatus is an elliptically-shaped opening, with a surface area of about 10 cm2, most commonly originating from the right crus of the diaphragm (11). The phreno-esophageal ligament, also known as Laimer membrane, arises from the subdiaphragmatic and endothoracic fascia and attaches the esophagus to the diaphragm. It consists of two sheaths, one enveloping the distal 2–4 cm of the esophagus and inserting into the submucosa, and the other extending inferiorly across the cardia and blending into the gastric serosa, dorsal mesentery and gastrohepatic ligament. The phreno-esophageal ligament confers stability to the EGJ through elastic recoil, allowing the distal esophagus to remain in its natural intra-abdominal position and resist challenges resulting from swallowing, breathing, or abdominal straining. The EGJ is difficult to identify radiologically because of its intrinsic mobility and absence of precise anatomical landmarks. Using endoscopic criteria, the EGJ is the squamocolumnar junction or Z-line or the proximal tip of gastric mucosal folds. Using histologic criteria, the EGJ is the proximal extent of gastric oxyntic epithelium or the point where no submucosal esophageal glands are present. From a surgical standpoint, the EGJ is marked by the peritoneal reflection on the stomach and the junction with the tubular esophagus.
Classification, etiology, and natural history of HH
HHs are heterogeneous anatomical and clinical entities, whose incidence in the general population is not well defined since many patients are asymptomatic or complain of minimal non-specific symptoms: in fact, even large HH may be discovered incidentally on chest radiography. HHs are typically classified into four subtypes. Type I, the most common and best known as “sliding hernia”, results from widening of the hiatal passage and circumferential laxity of the phreno-esophageal ligament that permits dynamic upward migration of the EGJ into the mediastinum. Para-esophageal hernias are less common entities that add the presence of a true peritoneal sac. Type II hernia occurs as a result of an anterior defect in the diaphragmatic hiatus allowing migration of the gastric fundus into the chest with the EGJ remaining in the intra-abdominal position. With progressive enlargement of the hiatus, a type III mixed paraesophageal and sliding hernia occurs. This can evolve into a complete intrathoracic stomach with the pylorus lying aside the gastric cardia and with a variable degree of rotation along the longitudinal (organo-axial volvulus) or transverse (meso-axial volvulus) gastric axis. When the diaphragmatic defect is large enough, it can even allow the transverse colon, small bowel or other abdominal contents into the hernia sac (type IV hernia) (12,13). Some have referenced a type V hernia which is a post surgical herniation of a wrapped LES (Herniated fundoplication).
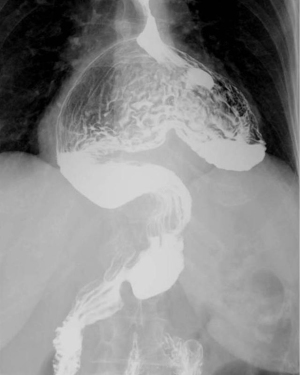
Risk factors for HH are ageing, kyphosis, obesity, thoraco-abdominal trauma, and previous hiatal surgery (14-17). The main factors implicated in the pathogenesis of HH are the thoraco-abdominal pressure gradient, the esophageal shortening secondary to reflux-induced fibrosis, and the hiatal enlargement due to tissue deterioration (18). The esophageal hiatus represents a locus minoris resistentiae of the diaphragm which is subjected to continuous mechanical stress. The esophagus does not completely fit the diaphragmatic hiatus, allowing abdominal contents to potentially herniate in the chest depending on the size and shape of defect, radial tension, longitudinal esophageal tension, and the elasticity of the phreno-esophageal ligament. Progressive slippage of the stomach into the chest cavity leads to GERD and/or cardio-respiratory symptoms, Cameron’s ulcers and occult anemia, intrathoracic gastric volvulus, gastric ischemia and perforation (19,20) (Figures 1,2).
HH: idiopathic or connective tissue disease?
Tissue factors causing impairment of diaphragmatic crural muscle, diaphragmatic central tendon, and phreno-esophageal ligament can determine hiatal enlargement and subsequent primary or recurrent HH (21). The collagen is composed by three polypeptides chains embraced together in a left-handed helical structure. To confer more stability and strength, the whole molecule itself is twisted in the opposite way into a right-handed super helix. Type I mature collagen is mainly responsible for tensile strength. Type III immature collagen consists of thinner fibers and provides a temporary scaffold for tissue remodeling. A change in the collagen ratio toward immature type III collagen may result in loss of tensile strength. The amorphous extracellular matrix, containing elastin, glycosaminoglycans, proteoglycans and metalloproteinases, regulates the network of these macromolecules that are deposited by fibroblasts and modified by the matrix metalloproteinases. The elastin allows the connective tissue to stretch and return to the natural state. Elastin degradation by specific metalloproteinases is likely to determine loss of the recoil properties of elastic fibers and to lead to deterioration of the phreno-esophageal ligament (22).
Asling et al. showed that patients with HH have a high prevalence of abnormal collagen deposition. There was correlation between HH formation and the presence on chromosome 2 of the COL3A1 gene encoding for type III collagen. COL3A1 was overexpressed in families with GERD and HH (23). Fei et al. compared biopsies taken at the crura in patients with GERD and HH, and in patients who underwent surgery for other reasons. A structural weakness in the muscular component of the crura, such as focal degeneration of myofibrils, swelling of sarcotubular structures and dilation of intermyofibrillar spaces, was found in patients with HH (24). Curci et al. found a 50% decrease of elastin in the phreno-esophageal and gastrohepatic ligament in patients with HH compared to controls with only GERD (25). Finally, von Diemen et al. compared phreno-esophageal biopsy samples from 29 patients with HH and GERD and 32 samples from cadavers without HH, and found that the total amount and the proportion of type I and type III collagen were about 60% lower in patients compared to controls (26).
Impact of HH and LES incompetence on the natural history of GERD
Besides the loss of tensile strength of the muscular crura and the loss of elasticity of the phreno-esophageal ligament, an additional factor that may account for progressive HH is the acquired esophageal shortening secondary to gastroesophageal reflux causing sustained contraction of the longitudinal esophageal muscle and fibrosis (27). Consequently, symptomatic patients with HH need to be properly investigated and treated given the potential for GERD progression.
The clinical relationship between HH and GERD has been extensively documented in epidemiological and clinical studies. Patients with HH are more likely to present with reflux symptoms, and the prevalence of GERD can reach 94%; on the other hand, symptomatic GERD patients are more likely to have HH compared to those without symptoms (28-30). Moreover, the prevalence of HH is higher in patients with Barrett’s esophagus and increases with the length of the metaplastic segment (31). Finally, the presence of HH more than doubles the risk of developing adenocarcinoma of the esophagus and gastric cardia (32).
The EGJ is an anatomically complex and dynamic region where both the smooth esophageal muscular fibers of the LES (intrinsic sphincter), and the striated muscular fibers of the crural diaphragm (extrinsic sphincter) work synergistically to protect the esophagus from reflux of gastroduodenal contents. The activity of these two sphincters overlaps and the basal tone and overall LES length and the extrinsic diaphragm compression maintain the LES pressure well above the 5 mmHg positive pressure gradient across the EGJ. This is enough in normal conditions to prevent reflux of gastric contents into the esophagus. Coughing or conditions that cause elevated intra-abdominal pressure, such as abdominal straining or compression, demonstrate the critical role of the crural diaphragm in increasing the LES pressure and restoring the antireflux barrier (33).
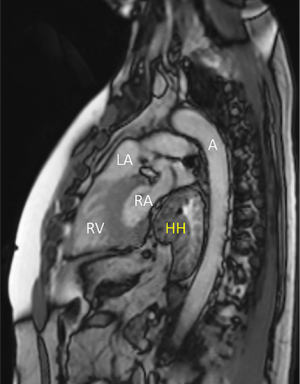
The failure of the antireflux barrier and consequent increase of esophageal acid exposure is secondary to incompetence of the crural diaphragm, with lack of “pinchcock effect”, incompetence of the LES, characterized by low basal pressure, short total length and/or intra-abdominal length, and reduced esophageal acid clearance caused by outlet obstruction and impairment of reflux-induced primary peristalsis. In patients with HH, the EGJ is displaced intrathoracically leading to a separation of the two sphincters (Figure 3).

Loss of the intra-abdominal LES segment and therefore its exposure to intra-abdominal pressure, and of the mucosal gastroesophageal flap valve visible by endoscopy further impair the antireflux barrier. HH has also been associated with transient LES relaxations, a phenomenon mediated via a vagal pathway occurring in response to gastric fundic distention (34). This mechanism is responsible for gastroesophageal reflux episodes which normally occur after meals and are associated with belching, independently of swallowing. An alternative physiological explanation is that gastric distention causes shortening of overall LES length (35). In patients with HH, a pouch forms between the upper margin of the LES and the diaphragmatic pinch and is filled with gastric contents after a reflux episode. The reflux material is then cleared by secondary esophageal peristalsis into the stomach, but a small amount of acid remains trapped in the sac and is subsequently regurgitated into the esophagus. Reiteration of this sequence of events can increase the overall esophageal acid exposure and leads to complications of GERD that are difficult to control with medical therapy (36).
Implications for surgical therapy
Current management of patients with GERD is largely based on proton-pump inhibitor therapy. According to the predominant paradigm, laparoscopic surgery is recommended only in patients with refractory GERD symptoms and in those with symptomatic large HH (Figure 4A,B).
Patients’ selection for antireflux surgery is critical for optimal outcomes. Campos et al. proved that the best predictors of a successful fundoplication are the presence of a typical primary symptom such as heartburn, an abnormal 24-hour pH score, and a clinical response to acid suppression therapy (37). Antireflux surgery has been performed for about 70 years now, with outcomes that are highly dependent on the surgeon’s expertise. With the advent of the laparoscopic approach, the traditional surgical techniques (mainly Nissen and Toupet) have been replicated with the added advantages of less pain, quick postoperative recovery, and short length of stay.
Louie et al. revisited the role of crural diaphragm and suggested that both hiatoplasty and fundoplication are physiologically crucial and equally contribute to restoration of EGJ competence. Importantly, this study also suggests that the fundoplication itself does not confer as much pressure as the crural repair, and its main role is probably to prevent LES shortening (38). The same theory applies to the magnetic sphincter augmentation (Linx® procedure), a novel surgical device placed laparoscopically around the EGJ without the need to alter gastric anatomy. Various studies have proven safety and efficacy of this procedure and shown less side effects compared to fundoplication (39,40). The encouraging outcomes of the Linx procedure in terms of symptoms relief, decreased medication use, and objective reflux control have led to expand its indications to patients with large HH (41). Improved subjective and objective outcomes with no increase in dysphagia rates have been reported in patients treated with Linx combined with formal crural repair compared to patients with minimal or no hiatal dissection (42,43). These results are consistent with the “two-sphincter hypothesis” and have been documented by high-resolution manometry (44).
Still, the problem of how to correctly address the axial (longitudinal) tension and the radial (lateral) tension at the EGJ remains unsolved. To reduce recurrence rates after surgical repair, it is clear that tension along these vectors must be minimized. Axial tension is generally recognized intraoperatively by measuring the intra-abdominal length of the esophagus; if this is shorter than 2 cm, a short esophagus should be suspected and an esophageal lengthening procedure (Collis gastroplasty) be performed. Unfortunately, there is often a tendency to overestimate the intra-abdominal esophageal length at laparoscopy due to the effect of pneumoperitoneum on the diaphragm (45). On the other hand, radial tension at the EGJ is not readily recognized and surgeons must rely on tactile and visual clues during hiatal repair. In the study of Bradley et al. (46), morphology of the hiatus area was assessed during laparoscopy. Four different hiatal shapes (slit, teardrop, D, and oval) were identified and appeared to influence the need for diaphragmatic relaxing incisions to release radial tension.
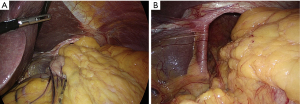
While laparoscopic cruroplasty and Toupet or Nissen fundoplication remain the current gold standard in antireflux surgery, the high rates of anatomical and clinical recurrence in patients with large HH still represent a matter of concern. This has led to an increasing interest for the use of prosthetic mesh to reinforce the esophageal hiatus (47) (Figure 3). However, the risk of erosion with nonabsorbable mesh has raised significant concerns, and many authors have suggested to use absorbable biological meshes to prevent recurrence and reoperation. Use of Surgisis® (Cook Biotech, IN, USA) has proven safe, but high recurrence rates have been reported (48). A recent randomized trial with 5-year follow-up showed no advantages for augmented crural repair using absorbable (Surgisis®, Cook Biotech, IN, USA) mesh versus nonabsorbable (TiMesh®, PFM Medical, Koln, Germany) versus suture repair alone. In fact, the incidence of small recurrent hernias was similar across all three patient groups and most patients remained asymptomatic (49). The new biosynthetic meshes, namely the Bio-A® (Gore, Flagstaff, AZ) and Phasix ST® (C.R. Bard, Inc./Davol, Inc., Warwick, RI, USA), seem to protect form early recurrence, to decrease the risk of reoperation, and to improve quality life compared to primary suture repair, but no long-term data is available (50-52).
Conclusions
The results of this review suggest that the pathogenesis of HH is multifactorial and correlates with progression of GERD. Physiologic aging combined with metabolic, genetic and mechanical factors play an important role in the natural history of the disease. Patients with reflux symptoms need to be carefully investigated for competency of the LES and adequate esophageal body contractility and clearance. A co-existing and even small hiatus hernia should not be underestimated and may play a significant role in the decision-making process and planning of antireflux surgery. If the esophagus is not foreshortened and the crural repair appears weak, reinforcement with onlay biosynthetic mesh may be indicated. Identification of HH and GERD in an early-stage can have a favorable impact on the natural history of the disease and may decrease post-surgical recurrence rates.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Lee L. Swanstrom and Steven G. Leeds) for the series “Hiatal Hernia” published in Annals of Laparoscopic and Endoscopic Surgery.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at available at http://dx.doi.org/10.21037/ales-20-26). The series “Hiatal Hernia” was commissioned by the editorial office without any funding or sponsorship. LB served as an unpaid editorial board member of Annals of Laparoscopic and Endoscopic Surgery from Oct 2019 to Sep 2021. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ronkainen J, Aro P, Storksrubb T, et al. High prevalence of gastroesophageal reflux symptoms and esophagitis with or without symptoms in the general adult Swedish population: a Kalixanda study report. Scand J Gastroenterol 2005;40:275-85. [Crossref] [PubMed]
- El-Serag HB, Sweet S, Winchester CC, et al. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut 2014;63:871-80. [Crossref] [PubMed]
- Ness-Jensen E, Lindam A, Lagergren J, et al. Changes in prevalence, incidence and spontaneous loss of gastro-oesophageal reflux symptoms: a prospective population-based cohort study, the HUNT study. Gut 2012;61:1390-7. [Crossref] [PubMed]
- Nocon M, Labenz J, Jaspersen D, et al. Health-related quality of life in patients with gastro-oesophageal reflux disease under routine care: results from the ProGERD study. Aliment Pharmacol Ther 2009;29:662-8. [Crossref] [PubMed]
- Malfertheiner P, Nocon M, Vieth M, et al. Evolution of gastroesophageal reflux over 5 years under routine medical care- the ProGERD study. Aliment Pharmacol Ther 2012;35:154-64. [Crossref] [PubMed]
- Allison PR. Reflux esophagitis, sliding hiatal hernia, and the anatomy of repair. Surg Gynecol Obstet 1951;92:419-31. [PubMed]
- Code CF, Fyke FE Jr, Schlegel JF. The gastroesophageal sphincter in healthy human beings. Gastroenterologia 1956;86:135-50. [Crossref] [PubMed]
- Liebermann-Meffert D, Allgower M, Schmid P, et al. Muscular equivalent of the lower esophageal sphincter. Gastroenterology 1979;76:31-8. [Crossref] [PubMed]
- Mittal RK, Rochester DF, McCallum RW. Electrical and mechanical activity in the human lower esophageal sphincter during diaphragmatic contraction. J Clin Invest 1988;81:1182-9. [Crossref] [PubMed]
- Mittal RK. The crural diaphragm, an external lower esophageal sphincter: A definitive study. Gastroenterology 1993;105:1565-7. [Crossref] [PubMed]
- Kumar D, Zifan A, Ghahremani G, et al. Morphology of the esophageal hiatus: is it different in 3 types of hiatus hernias? J Neurogastroenterol Motil 2020;26:51-60. [Crossref] [PubMed]
- Bonavina L. Paraesophageal hiatus hernia. In: Bland KI, Sarr MG, Büchler MW. editors. General Surgery. Principles and International Practice, 2nd Edition. London: Springer-Verlag, 2009:431-8.
- Kahrilas PJ, Kim HC, Pandolfino JE. Approaches to the diagnosis and grading of hiatal hernia. Best Pract Res Clin Gastroenterol 2008;22:601-16. [Crossref] [PubMed]
- Pandolfino JE, El-Serag HB, Zhang Q, et al. Obesity: a challenge to esophagogastric junction integrity. Gastroenterology 2006;130:639-49. [Crossref] [PubMed]
- Kusano M, Hashizume K, Ehara Y, et al. Size of hiatus hernia correlates with severity of kyphosis, not with obesity, in elderly Japanese women. J Clin Gastroenterol 2008;42:345-50. [Crossref] [PubMed]
- Polomsky M, Siddall KA, Salvador R, et al. Association of kyphosis and spinal skeletal abnormalities with intrathoracic stomach: a link toward understanding its pathogenesis. J Am Coll Surg 2009;208:562-9. [Crossref] [PubMed]
- Menon S, Trudgill N. Risk factors in the aetiology of hiatus hernia: a meta-analysis. Eur J Gastroenterol Hepatol 2011;23:133-8. [Crossref] [PubMed]
- Weber C, Davis CS, Shankaran V, et al. Hiatal hernias: a review of the pathophysiologic theories and implication for research. Surg Endosc 2011;25:3149-53. [Crossref] [PubMed]
- Milito P, Lombardi M, Asti E, et al. Influence of large hiatus hernia on cardiac volumes. A prospective observational cohort study by cardiovascular magnetic resonance. Int J Cardiol 2018;268:241-4. [Crossref] [PubMed]
- Light D, Links D, Griffin M. The threatened stomach: management of the acute gastric volvulus. Surg Endosc 2016;30:1847-52. [Crossref] [PubMed]
- El Sherif A, Yano F, Mittal S, et al. Collagen metabolism and recurrent hiatal hernia: cause and effect? Hernia 2006;10:511-20. [Crossref] [PubMed]
- Jansen PL, Merens PR, Klinge U, et al. The biology of hernia formation. Surgery 2004;136:1-4. [Crossref] [PubMed]
- Asling B, Jirholt J, Hammond P, et al. Collagen type III alpha I is a gastro-oesophageal reflux disease susceptibility gene and a male risk factor for hiatus hernia. Gut 2009;58:1063-9. [Crossref] [PubMed]
- Fei L, del Genio G, Brusciano L, et al. Crura ultrastructural alterations in patients with hiatal hernia: a pilot study. Surg Endosc 2007;21:907-11. [Crossref]
- Curci JA, Melman LM, Thompson RW, et al. Elastic Fiber Depletion in the Supporting Ligaments of the Gastroesophageal Junction: A Structural Basis for the Development of Hiatal Hernia. J Am Coll Surg 2008;207:191-6. [Crossref] [PubMed]
- von Diemen V, Trindade EN, Trindade MR. Hiatal hernia and gastroesophageal reflux: Study of collagen in the phrenoesophageal ligament. Surg Endosc 2016;30:5091-8. [Crossref] [PubMed]
- Mittal RK, Balaban DH. The esophagogastric junction. N Engl J Med 1997;336:924-32. [Crossref] [PubMed]
- Wright RA, Hurwitz AL. Relationship of hiatal hernia to endoscopically proved reflux esophagitis. Dig Dis Sci 1979;24:311-3. [Crossref] [PubMed]
- Petersen H, Johannessen T, Sandvik AK, et al. Relationship between endoscopic hiatus hernia and gastroesophageal reflux symptoms. Scand J Gastroenterol 1991;26:921-6. [Crossref] [PubMed]
- Zagari RM, Fuccio L, Wallander MA, et al. Gastro-oesophageal reflux symptoms, oesophagitis and Barrett’s oesophagus in the general population: the Loiano-Monghidoro study. Gut 2008;57:1354-9. [Crossref] [PubMed]
- Cameron AJ. Barrett’s esophagus: prevalence and size of hiatal hernia. Am J Gastroenterol 1999;94:2054-9. [Crossref] [PubMed]
- Chow WH, Finkle WD, McLaughlin JK, et al. The relation of gastroesophageal reflux disease and its treatment to adenocarcinomas of the esophagus and gastric cardia. JAMA 1995;274:474-7. [Crossref] [PubMed]
- Mittal RK, Fisher M, McCallum RW, et al. Human lower esophageal sphincter pressure response to increased intra-abdominal pressure. Am J Physiol 1990;258:G624-30. [PubMed]
- Kahrilas PJ, Shi G, Manka M, et al. Increased frequency of transient lower esophageal sphincter relaxation induced by gastric distention in reflux patients with hiatal hernia. Gastroenterology 2000;118:688-95. [Crossref] [PubMed]
- Bonavina L, Evander A, DeMeester TR, et al. Length of the distal esophageal sphincter and competency of the cardia. Am J Surg 1986;151:25-34. [Crossref] [PubMed]
- DeMeester TR, Lafontaine E, Joelsson BE, et al. Relationship of a hiatal hernia to the function of the body of the esophagus and the gastroesophageal junction. J Thorac Cardiovasc Surg 1981;82:547-58. [Crossref] [PubMed]
- Campos GM, Peters JH, DeMeester TR, et al. Multivariate analysis of factors predicting outcome after laparoscopic Nissen fundoplication. J Gastrointest Surg 1999;3:292-300. [Crossref] [PubMed]
- Louie BE, Kapur S, Blitz M, et al. Length and pressure of the reconstructed lower esophageal sphincter is determined by both crural closure and nissen fundoplication. J Gastrointest Surg 2013;17:236-43. [Crossref] [PubMed]
- Bonavina L, Saino G, Bona D, et al. One hundred consecutive patients treated with magnetic sphincter augmentation for gastroesophageal reflux disease: 6 years of clinical experience from a single center. J Am Coll Surg 2013;217:577-85. [Crossref] [PubMed]
- Ganz RA, Edmundowicz SA, Taiganides PA, et al. Long-term outcomes of patients receiving a magnetic sphincter augmentation device for gastroesophageal reflux. Clin Gastroenterol Hepatol 2016;14:671-7. [Crossref] [PubMed]
- Rona KA, Reynolds J, Schwameis K, et al. Efficacy of magnetic sphincter augmentation in patients with large hiatal hernias. Surg Endosc 2017;31:2096-102. [Crossref] [PubMed]
- Tatum JM, Alicuben E, Bildzukewicz N, et al. Minimal versus obligatory dissection of the diaphragmatic hiatus during magnetic sphincter augmentation surgery. Surg Endosc 2019;33:782-8. [Crossref] [PubMed]
- Irribarra MM, Blitz S, Wilshire CL, et al. Does treatment of the hiatus influence the outcomes of magnetic sphincter augmentation for chronic GERD? J Gastrointest Surg 2019;23:1104-12. [Crossref] [PubMed]
- Riva CG, Siboni S, Sozzi M, et al. High-resolution manometry findings after Linx procedure for gastro-esophageal reflux disease. Neurogastroenterol Motil 2020;32:e13750 [Crossref] [PubMed]
- Herbella FA, Del Grande JC, Colleoni R. Short esophagus or bad dissected esophagus? An experimental cadaveric study. J Gastrointest Surg 2003;7:721-5. [Crossref] [PubMed]
- Bradley DD, Louie BE, Farivar AS, et al. Assessment and reduction of diaphragmatic tension during hiatal hernia repair. Surg Endosc 2015;29:796-804. [Crossref] [PubMed]
- Frantzides CT, Madan AK, Carlson MA, et al. A Prospective, Randomized Trial of Laparoscopic Polytetrafluoroethylene (PTFE) Patch Repair vs Simple Cruroplasty for Large Hiatal Hernia. Arch Surg 2002;137:649-52. [Crossref] [PubMed]
- Oelschlager BK, Pellegrini CA, Hunter JG, et al. Biologic prosthesis to prevent recurrence after laparoscopic paraesophageal hernia repair: long-term follow-up from a multicenter, prospective, randomized trial. J Am Coll Surg 2011;213:461-8. [Crossref] [PubMed]
- Watson DI, Thompson SK, Devitt PG, et al. Five year follow-up of a randomized controlled trial of laparoscopic repair of very large hiatus hernia with sutures versus absorbable versus nonabsorbable mesh. Ann Surg 2020;272:241-7. [Crossref] [PubMed]
- Asti E, Lovece A, Bonavina L, et al. Laparoscopic management of large hiatus hernia: five-year cohort study and comparison of mesh-augmented versus standard crura repair. Surg Endosc 2016;30:5404-9. [Crossref] [PubMed]
- Abdelmoaty WF, Dunst CM, Filicori F, et al. Combination of surgical technique and bioresorbable mesh reinforcement of the crural repair leads to low early hernia recurrence rates with laparoscopic paraesophageal hernia repair. J Gastrointest Surg 2020;24:1477-81. [Crossref] [PubMed]
- Panici Tonucci T, Asti E, Sironi A, et al. Safety and Efficacy of Crura Augmentation with Phasix ST Mesh for Large Hiatal Hernia: 3-Year Single-Center Experience. J Laparoendosc Adv Surg Tech A 2020;30:369-72. [Crossref] [PubMed]
Cite this article as: Manzo CA, Asti E, Bonavina L. Hiatal hernia, lower esophageal sphincter and their combined effect on the natural history of gastroesophageal reflux disease: implications for surgical therapy. Ann Laparosc Endosc Surg 2021;6:44.