
Future perspectives: enhanced recovery in colorectal surgery
Introduction
When Eliud Kipchoge became the first man to run a marathon distance in under 2 hours, a major milestone of human achievement had been surpassed. Obtaining that goal was not the work of one man’s obsessive training; a complex interplay of talent, determination, expert advice, novel technologies and precise attention to all details, no matter how small, meant that the goal was possible: the concept of ‘marginal gains’ or ‘one percent advantages’.
A park in Vienna was chosen to minimise the effect of jetlag and to ensure perfect running weather. A roundabout was dug up and re-laid to ensure an easy turn around. 41 pacemaker runners were rotated in such a formation as to minimise wind resistance, devised using complex computer programming. He ran wearing shoes with an embedded carbon fibre plate (1).
Enhanced Recovery After Surgery (ERAS) can be seen to have a similar philosophy. All criteria in protocols are devised to minimize physiological insult to a patient, returning them to functional normality as soon as possible. Since the widespread adoption of minimally invasive surgery, not one element within the ERAS pathway can be said to have accounted for the improvement in care and outcomes for patients with colorectal disease. Rather it is the complex interplay of all the individual components aiming towards a common goal.
This article will address potential future advancements that could be made for patients undergoing colorectal surgery within an ERAS programme. Although many of the innovations may not become commonplace, and in isolation have minimal impact, it is probable that some may be incorporated and contribute to the ‘marginal gains’ that cumulatively have such a profound impact for our patients.
Pre-operative strategies
Prehabilitation
In the elective surgical oncology patient, there is often a window of opportunity between the time of diagnosis and surgery. This period may range from a few weeks in uncomplicated cases requiring primary surgery up to four months in the case of rectal cancer patients who require neoadjuvant therapy. It is during this period that there may be a role for a targeted preoperative intervention, in the form of ‘Prehabilitation’.
‘Prehabilitation’ describes an intervention aimed at enhancing an individual’s functional capacity in anticipation of a forthcoming physiological stressor (2).
Prehabilitation programmes have included supervised in-hospital education programmes, at home non-supervised programmes and psychological therapies. Many studies have been published in the area across a broad range of specialities. They have shown that prehabilitation in the form of pre-operative exercise regimens can have a positive impact on functional capacity and postoperative morbidity (3). Supervised exercise prehabilitation programmes have frequently demonstrated greater functional benefits and reduced drop-out rates when compared to self-administered interventions (2,4-6).
It has been difficult to strongly link prehabilitation to common criteria used when assessing surgical outcomes, for example length of stay and post-operative complications. Studies have however reported improvements across various physical and psychological parameters. A systematic review assessing exercise regimens by O’Doherty et al. reported on 10 studies, 4 of which were randomised controlled trials (RCT), regarding patients awaiting major abdominal surgery. Of these studies, seven used peak VO2 as their primary outcome and reported an improvement in this parameter following prehabilitation (7). Boereboom and colleagues reported eight studies (5 RCTs) comprising 518 patients. Five of these studies reported the ‘6 minute walking distance’ as primary outcome with improvements of up to 42 metres (8).
Targeted psychological interventions have yielded benefit. Anxiety and depression have shown to significantly affect post-operative outcome, impacting length of stay, functional capacity and infection rates. A systematic review of psychological prehabilitation in cancer patients (including breast, colorectal and prostate) showed improvement in quality of life and depression scores in the immediate and medium-term postoperative period, as well as distress related to body image. Psychological interventions included relaxation techniques, role play, coping strategies, problem- solving exercises and stress management (9).
Data regarding more conventional surgical outcomes is limited. Valkenet et al. showed that pulmonary complications were lower in those receiving inspiratory muscle training prior to cardiac and abdominal aortic aneurysm (AAA) surgery (10). Another review showed reduced post-operative pain, length of stay and improved physical function (11). Conflicting evidence does exist, however, that shows negligible improvement in physiological and functional parameters, and poor adherence to prehabilitation programmes (12).
It has been demonstrated that exercise-based prehabilitation programmes are safe, even in the context of ongoing chemotherapy. A 2018 systematic review of 33 articles concluded that prehabilitation regimens are not associated with an increased risk of adverse events (4).
Studies that directly address colorectal surgery are scarce. In curative rectal cancer patients, West and colleagues assigned 22 patients with locally advanced rectal cancer to receive neoadjuvant chemoradiotherapy (NACRT) and exercise, and 17 patients to NACRT alone. All subjects underwent cardiopulmonary exercise testing (as shown in Figure 1) at pre-determined intervals. The exercise group observed a significant improvement in anaerobic threshold compared with an unchanged control group between weeks 0 and 6. The control group suffered a sustained decline in fitness from weeks three to 14. In addition, there was a statistically significant difference in TNM tumour down-staging at restaging MRI favouring the exercise group (13). This paper was accompanied by an editorial entitled ‘Exercise: the new premed’ (14).

Prehabilitation does not exclusively target exercise. Bordes et al. assessed a tri-modal prehabilitation intervention in a randomised controlled trial in patients undergoing colorectal resection (15). This consisted of not only an exercise regimen but in addition nutrition counselling and the provision of stress reducing strategies. Although functional walking capacity was improved in the intervention group, there were no differences reported in physical activity, quality of life, 30-day complications, anxiety or depression.
While it has been shown that exercise and psychological coaching programmes during chemotherapy treatment are safe and feasible, and that certain elements of functional capacity can be improved, the evidence for prehabilitation is in its nascency, with mainly observational studies of low sample size. Studies are heterogeneous, with different interventions being trialled and little standardisation. This makes it difficult to recommend prehabilitation at present. Randomised trials are currently underway which should shed more light on the subject in the coming years.
Identifying and targeting sarcopenia
Sarcopenia is defined by the European Working Group for Sarcopenia (EWGSOP) as ‘a syndrome characterised by progressive and generalised loss of skeletal muscle mass and strength with a risk of adverse outcomes such as physical disability, poor quality of life and death’ (16). A vast amount of research has refined definitions, identification, prevention, and consequences of sarcopenia over the past decade (17). The primary diagnostic change from 2010 to 2018 was that muscle strength rather than muscle volume or even physical function came to the forefront as the best predictor of poor outcome.
Before any physical testing is performed, patients must be identified, either from self-referral [frailty, feeling weak, falls] or via the 5-question screening tool SARC-F. These questions evaluate Strength, Assistance in walking, ability to Rise from a chair, Climb stairs, and any Falls in the past year (18).
Measuring muscle strength is the core diagnostic element of sarcopenia and is recommended to be performed using a hand dynamometer. Handgrip strength is a powerful predictor of poor patient outcomes such as long length of hospital stay, infectious complications, chemotoxicity, and death (19). It is a cheap and readily available test (20).
Measuring muscle mass is more difficult and a gold standard technique less easy to recommend. The current ‘gold standard’ from EWGSOP is to use MRI or CT scanning to take cross sectional measurements of muscle volume using specialist software. The negatives of these two methods are the lack of portability, radiation dose in the case of CT, and cost.
Encouragingly, cross sectional analysis of CT in cancer patients, readily available as part of staging algorithms, has shown to be a strong predictor of disease-free survival and overall complication rates. ‘Body composition’ shows the most promise in predicting outcomes, with multiple studies showing sarcopenia patients have significantly poorer survival (21), longer lengths of hospital stay, higher 30 day morbidity and mortality (22) and increased chemotoxicity (23). A separate UK study of colorectal cancer patients showed an overall complication rate five times greater in the presence of sarcopenia (24).
Measurement of physical performance has several validated options, including gait speed, the short physical battery test, timed up and go test, and 400 m walk. For practical reasons of space and time, the EWGSOP recommend the 4-metre gait speed analysis (25,26). A 2018 study of cardiac surgery patients showed a two-fold increased risk of mortality for patients with slower walking speeds (27).
There are subcategories: primary and secondary sarcopenia, acute or chronic sarcopenia, and sarcopenic obesity (Figure 2). With regards to colorectal cancer the highest risk patients are the sarcopenic obese. This can be defined by BMI criteria or by excessive visceral fat on body composition analysis (28). A systematic review in 2015 showed reduced disease free and overall survival in the sarcopenic obese (22).
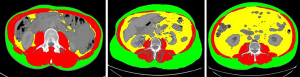
A logical target intervention therefore are exercise programs to reduce adiposity, irrespective of any muscle mass gained from the exercise. Even short interventions have demonstrated benefit. A 2017 Cochrane review of preoperative exercise showed benefits after only one week, reducing length of stay and pulmonary complications in lung cancer patients (29).
An international consensus has summarised the evidence for interventions following diagnosis. The evidence is however poor, comprising of two RCTs which did not use gold-standard measures (30). Thus far protein supplementation and resistance exercise regimens for adults with sarcopenia are the sole recommendations.
The prediction and modification of risk factors are key goals in ERAS. Sarcopenia is now established with an ICD-10 code. There are multiple ongoing studies that aim to attenuate the effects of sarcopenia which incorporate prehabilitation and nutritional supplementation. Until further evidence emerges, pre-operative identification of potentially sarcopenic patients is strongly recommended.
Mechanical bowel preparation (MBP) and antibiotics
The use of MBP continues to divide opinion.
It has been known that systemic antibiotics reduce surgical site infections (SSI) since the 1970s. They are therefore routinely given at induction.
It has been contested however that luminal organisms are unaffected by intravenous antibiotics, and that additional oral antibiotics are necessary. It has further been argued that reducing luminal contents with mechanical bowel preparation would reduce bacterial load further. Some colorectal specialists therefore assert that a combination of all 3 approaches would yield the best results.
Mechanical bowel preparation has potentially negative consequences, particularly in terms of electrolyte disturbance. This is compounded in the frail. MBP is also unquestionably unpleasant (18,19). Concerns regarding antibiotic stewardship are also valid in an age of increasing microbial resistance and nosocomial infection (31-33).
The first recommendation that can be made is that MBP alone (without synchronous oral antibiotics) is of no benefit. This is borne out by several meta-analyses assessing RCTs that compare MBP with no MBP, or a solitary rectal enema. When observational studies are assessed, a small benefit can be seen in term of surgical site infection for the use of MBP alone (34-37).
Some papers demonstrate that any benefit to MBP versus no MBP is negated when compared to the use of a rectal enema. A rectal enema carries few, if any of the risks of MBP. The use of a rectal enema in the context of rectal surgery is therefore potentially an interesting area of future study.
The second recommendation that can be made concerns the use of combined antibiotics—systemic therapy given at induction and an oral course perioperatively—with MBP. This has again been subject to rigorous meta-analyses that have compared the combined antibiotic approach with solely systemic antibiotics.
meta-analyses of RCTs only show that superficial SSI are reduced with the tri-modal approach, with no difference in other complications (28,38-40). One metanalysis of observational studies found significantly reduced rates of anastomotic leak, post-operative ileus, readmission and mortality (40). This question has been tackled by a large number of observational studies in the United States, with similar conclusions (41-43). Anastomotic leaks were also seen significantly less often in a recent Europe-wide audit by the ESCP (44).
The evidence therefore seems to converge on the idea is that if MBP is to be used, a combined antibiotic regimen should be employed.
What has not been answered however, is whether the causative factor in this reduction in complications are antibiotics, MBP, or their combination; namely, what is the effect of combined antibiotics in the absence of MBP?
At present no RCTs have addressed this issue. Two large retrospective database studies from the United States found opposite conclusions (45,46). Evidence regarding this question is limited. As highlighted in a recent Cochrane review, ‘it is not known whether oral antibiotics would still be effective when the colon is not empty’ (47).
Obtaining high level evidence is challenging. SSI and other commonly reported complication rates are relatively low and reducing (48). RCTs therefore require large numbers of patients to avoid being underpowered. Large scale observational or retrospective database studies are a realistic alternative, but these methodologies are known to have significant limitations.
Although work is currently in progress to answer outstanding issues surrounding MBP and antibiotics, it is an area of study that may require a novel approach to arrive at a consensus.
The microbiome and ERAS
A possible paradigm shift within colorectal surgery is the idea that anastomotic dehiscence is less affected by, for example, ischaemia, but rather by microbial pathogenesis, or the ‘microbiome’ of the colonic environment.
In a murine model, Shogan et al. found that topical application of antibiotics that acted on collagenase producing Enterococcus faecalis, pseudomonas species and the mucin degrading Ruminococci family inhibited anastomotic leak (49). The INTACT study that is currently in progress attempts to address the question of the microbiome as a secondary outcome (50). Work in this field may answer whether the important variable in anastomotic leakage is the mode of administration of antibiotics, rather than the utilisation of MBP.
Recent work has identified that the microbiome is involved in virtually all immune-mediated activation of intestinal inflammation (51). There is a growing body of evidence that the microbiome-host relationship may play an important role in healing and return to function (52-54). Murine studies have shown that subjects treated with antibiotics exhibit lower rates of Post-operative ileus (55).
In human studies, recent meta-analyses showed that pre-operative enteral antibiotics were associated with a reduction in POI. Some authors have concluded therefore that the microbiome plays an integral role in the pathogenesis of ileus. Ileus continues to be the overwhelming reason for longer than expected stays in hospital after elective colonic resection, and will be discussed further later in the article (40,56,57).
The exact mechanism explaining this is unclear. Possible explanations include effects upon gut wall permeability, alterations in the amount of Short Chain Fatty Acids produced by resident species and microbial metabolites stimulating serotonin release (58).
Novel techniques are being developed, including sequencing technologies and spectroscopic methods that allow rapid analysis of the microenvironment and complex host-microbe interactions that take place in the human gut for the first time. This may provide insight into potential new diagnostic and treatment pathways for common post-operative complications, which may at least in-part be influenced by a patient’s microbiome (57).
Operative strategies and anaesthesia
Minimally invasive surgery (MIS)
MIS is integral to ERAS protocols. Access trauma and the resulting stress response is greatly attenuated. Post-operative pain and therefore requirement for opiate analgesia is significantly reduced. MIS also reduces complications such as ileus, blood loss, pulmonary and wound infections, and has a significant impact on post-operative mobility. Laparoscopic surgery is now the accepted standard of care in colorectal surgery.
Different techniques for MIS have continued to develop that aim to facilitate removal of rectal tumours. This is particularly difficult in patients of large habitus, males, and those who have undergone radiotherapy. It is hoped that these techniques can reduce conversion rates and may have learning curves that are less steep for the trainee surgeon. Some techniques are purported to increase manual dexterity and precision, thereby possibly improving oncological outcomes and reducing complications.
Robotic surgery adoption has increased since the turn of the millennium (19). Early reviews in the United States—with many cases performed in low-volume centres—revealed higher complication rates, longer hospital stays, significantly increased costs, and poorer oncological outcomes (20). Recent reviews have been more favourable.
Regarding surgery above the pelvic brim, it has been suggested that robotic surgery results in potentially smaller incisions, reduced incisional hernias, and higher lymph node yields. A meta-analysis of 7 studies in 2017, one of which was randomized, demonstrated equivalent length of stay and marginally lower blood loss. Negatively, there were elevated costs and a significantly longer operating time despite not including theatre set up (21). Further trials continue to forge ahead but presently no superiority has been demonstrated over laparoscopic methods.
The case for robotics in rectal cancer surgery is stronger: the technical difficulty of operating in a narrow pelvis is challenging, and the oncological significance of total mesorectal excision whilst minimizing collateral damage of paramount importance.
A 2018 review assessed 14 retrospective and case-matched studies including more than 22,000 cases of robotic rectal surgery. Robotic surgery yielded lower conversion rates, subjectively improved TME specimen quality, fewer positive circumferential margins, and shorter lengths of stay (22). There was no benefit in disease-free or overall survival. ROLARR, a multi-centre randomized study comparing laparoscopic and robotic surgery reported in 2017: It concluded no advantage from robotic surgery (23).
The role of robotics in rectal cancer therefore remains controversial. No high-quality evidence exists to substantiate widespread adoption. It does not seem to offer significant benefits to the rectal cancer patient over standard laparoscopic surgery (24).
There are many proponents of the transanal approach, commonly known as ‘TATME’ (Transanal Total Mesorectal Excision). Several systematic reviews reveal no difference in specimen quality or anastomotic leak rates compared to laparoscopic and open surgery (25-28). A large prospective registry of cases has revealed anastomotic failure rates and specimen quality not dissimilar to databases of standard laparoscopy (29). A randomized trial for the trans-anal approach (COLOR III) has been initiated (30).
Currently, there is no evidence that the approach contributes to better recovery after surgery. Of significant concern is a recently published moratorium on TATME from Norway—where one hospital reported an unexpected and rapid pattern of recurrence in nearly 10% of patients—more than double what is expected. TATME has currently been halted in Norway until the end of the follow up period (59).
There is little evidence for superiority of one minimally invasive surgical approach over another. In the context of ERAS, the important element appears to be the avoidance of open surgery rather than the modality of minimally invasive technique. The adoption of novel surgical techniques can be problematic and should be encouraged only after rigorous scrutiny of performance and outcomes.
Potential future developments in anaesthesia
Until relatively recently, the delivery of anaesthesia was formulaic with anaesthetic agents delivered using simple algorithms based on weight or age. The concept of individualised care developed in the 1980s, and a ‘bespoke anaesthetic’ continues to be a major aim of modern anaesthesia (60).
Although not universally practised, and with heterogeneity in delivery, modern goal directed fluid therapy (GDFT) can be seen as an example of individualised care improving patient outcomes (60). Cardiac output monitoring has changed the emphasis from therapy guided by pressure (for example central venous pressure) to therapy guided by flow. This has allowed fluid administration to be more precise (61), avoiding the problems of both hypovolaemia and fluid overload. Both ultimately impair tissue oxygenation at the site of bowel anastomosis and elsewhere in the body. Huge variations have been described in fluid administration, ranging from 0.7–5.4 L of fluid for a patient weighing 75 kg undergoing a 4-hour procedure with minimal blood loss: the major determinant was the anaesthesia provider (62,63).
Hegemony however frequently changes particularly as other interventions may have an impact on one variable—for example Goal Directed Fluid Therapy. In their meta-analyses of 23 studies, Rollins et al. found that GDFT had significant benefits when patients were not managed on an ERAS pathway in terms of overall morbidity and length of hospital stay. When on an ERAS pathway, the difference between GDFT and non-GDFT were minimal (64). The authors suggested that this may have been due to better implementation of ERAS pathways over time, and that interpretation of GDFT has adapted: Earlier studies administered significantly more fluid. It serves as a reminder of how the idea of best practice constantly changes with other technical and technological improvements.
Benefits have also been seen using thromboelastometry and thromboelastography when transfusing blood acutely to deliver a normal coagulation profile (65).
Neuromuscular blockade
Neuromuscular blocking drugs were hailed as a major advance, yet their misuse carries significant risk, including accidental awareness under anaesthesia, inadequate reversal leading to hypoxia, hypercarbia and an increase in postoperative pulmonary complications (66). Formulaic approaches to drug administration potentially magnify these risks. Quantitative neuromuscular monitoring and the appropriate use of anticholinesterases or sugammadex to confirm adequate reversal from neuromuscular blockade is required (67).
The widespread adoption of laparoscopic surgery has stimulated interest in deep neuromuscular blockade, usually defined as a train-of four count of zero and a posttetanic count of 1-3. It is theorised that increasing depth of block may reduce required pressures for pneumoperitoneum, potentially preserving intraoperative cardiac output and organ perfusion and reducing post-operative morbidity and analgesic requirements. At the time of writing, no published papers have demonstrated a clear benefit in terms of reduction of peritoneal insufflation pressures nor improvement in outcomes (68).
Depth of anaesthesia
The advent of processed electroencephalographic (pEEG) monitoring such as the Bispectral Index monitor (BIS) has permitted the monitoring of depth of anaesthesia (69). This has allowed drugs to be better titrated, which has shown a reduction in post-operative delirium and cognitive dysfunction at 3 months in elderly patients due to a reduction in anaesthetic dosage (70). It is used with increasing frequency, and is recommended for monitoring depth of anaesthesia in patients undergoing totally intravenous anaesthesia (TIVA) with neuromuscular blockade (67).
This may reduce inadequate or excessive anaesthetic dosage, but it remains to be seen whether BIS monitoring is sufficient or whether interpretation of the formal EEG is necessary. This would however require the learning of a technically difficult skill (71). The triple low—low mean arterial pressure, low BIS readings and low minimum alveolar concentration of volatile anaesthetic—has been shown to be associated with increased mortality, however these findings may not be causal and are the subject of some controversy (72,73). It may be that low BIS values may reflect patient frailty and anaesthetic hypersensitivity rather than misuse of anaesthetic agents but tailored anaesthetic depth and appropriate monitoring seem a worthy goal to strive for.
Mechanical ventilation
Many studies identify that lung-protective ventilation [low tidal volume with Positive End Expiratory Pressure (PEEP)], as used on intensive care, may reduce post-operative complications (74,75). Zhou et al. found improved lung function post operatively in open abdominal surgery patients but had no effect on overall length of stay. Whether or not this approach can reduce post-operative pulmonary complications in laparoscopic abdominal surgery is the subject of ongoing work (76), but individualised ventilation strategies have been recommended based on low tidal volumes and PEEP by an international consensus of experts (77).
A more tailored strategy has been described: Plotting a dynamic compliance volume curve that allows for the assessment of intra-tidal compliance, allowing for 3 basic profiles: recruitment/derecruitment; constant respiratory system mechanics; and overdistension. This allows the titration of an optimum level of PEEP for each patient potentially reducing pulmonary injury and dysfunction. This may be helpful when operative strategy changes, for example when converting to an open procedure (78).
Opioid free anaesthesia
Opioids form the mainstay of intra-operative analgesia. Whilst classical side effects can be problematic such as nausea, vomiting, cough suppression, and delayed return to gut function (79), they may also negatively impact the gut microbiome in terms of opioid induced bowel disorders (80). Other issues with opioids include opioid tolerance and opioid induced hyperalgesia. Finally, the ‘opioid epidemic’ in the United States and similar patterns of dependence now emerging in the UK has highlighted the need for a different approach in terms of intraoperative anaesthesia. Surgical patients are the second most likely to be prescribed opioids after chronic pain patients, with a significant number becoming chronic opioid users (81).
‘Opioid free anaesthesia’ is an area of research that has gained much traction in recent years (82). A recent metanalysis of 23 RCTs showed that patients undergoing anaesthesia with intraoperative opioids as opposed to those without had similar levels of post-operative pain and increased post-operative nausea and vomiting (83). Opioid free anaesthesia may also benefit long term survival: potential effects of opioids on cancer recurrence have been postulated, as they may can have a dose-dependent effect on NK cell cytotoxicity and proinflammatory cytokine production (84).
Strategies for the avoidance of opioids include effective multi-modal analgesia, which include local anaesthetics, N-methyl-D-aspartate antagonists, paracetamol, NSAIDs and gabapentioids (85). It has been argued that multi-modal analgesia should be utilised in the post-operative period and be incorporated into enhanced recovery protocols (82).
Targeting nociception while under general anaesthesia is a novel approach to reducing opioid use. Previous methods of assessing nociception assessed autonomic responses such as sweating and could be considered crude. More recent methods include videopupillometry monitoring and the surgical plesythmographic index.
Most recent evidence points to the Analgesia Nociception Index (ANI) which utilises heart rate variability to assess pain. Analysis of high frequency components of the ECG allows assessment of the parasympathetic/sympathetic balance. High scores indicate a parasympathetic predominance and thus less nociception. This may allow anaesthetists to assess the quality of regional anaesthesia and to predict post-operative analgesic requirements (86).
Regional anaesthesia
ERAS society guidelines do not currently recommend epidural anaesthesia (EA) for laparoscopic colorectal surgery (87). Although it remains the gold standard for open procedures, EA has not shown the same analgesic benefits with minimally invasive approaches and may negatively impact on length of stay (88,89).
Spinal anaesthesia with low dose opioids for minimally invasive colorectal surgery is currently recommended by the ERAS society. It has demonstrated earlier return to mobility, lower post-operative opioid requirements and comparable analgesic effect (87,89,90).
Although yielding benefits such as improved post-operative pain, translation of these into long term outcomes is unclear, and are as yet unproven (91).
Anaesthetic type and long-term survival
Much research exists that potentially implicates certain types of anaesthesia in cancer recurrence. Volatile anaesthetic agents are used for most procedures carried out under general anaesthetic. TIVA, commonly administered using propofol and remifentanil, is the main alternative. Volatile anaesthesia has been demonstrated to be associated with negative effects on the function of natural killer cells and up-regulation of hypoxia-inducible factors, leading to the suggestion that volatile anaesthesia may promote cancer regrowth (92,93).
In a retrospective analysis of over 6,000 patients, with a near 50/50 split between volatile and intravenous anaesthesia, Wigmore et al. found a hazard ratio of 1.46 for mortality in patients submitted to a volatile anaesthetic. There was a more profound difference seen in gastrointestinal patients (94). Conflicting evidence exists in patients undergoing breast surgery, including one well designed randomised trial, concluding no difference (95,96). Given the uncertainty regarding the currently available findings and potentially huge impact, more prospective trials are urgently required.
Post-operative measures, recovery and follow up
Implementation of ERAS
Adherence to ERAS protocols varies widely. This is despite evidence that links increased compliance to ERAS protocols with lower complication rates as described by the ERAS compliance group and published in the Annals of Surgery in 2015. The group found that laparoscopic surgery, perioperative carbohydrate and fluid loading, and totally intravenous anaesthesia were associated with superior outcomes. Compliance rates ranged from 71% to 92% (97). Anecdotal evidence would suggest however, that adherence to ERAS principles would vary much greater than those figures.
Many components of protocols are part of long-established routine practice independent from ERAS, for example the use of MIS and prophylactic antibiotics. They are therefore easier to incorporate. More contentious facets of ERAS, for example the early removal of catheters, avoidance of opioids and in particular, the omission of bowel preparation have proved more difficult to implement (98).
Given that some features of ERAS are contentious and may not be appropriate for all patients, it is perhaps encouraging that it has been shown that all components of the protocol do not need to be adhered to achieve good results; protocols with more criteria do not necessarily result in better outcomes (99,100).
Adherence to ERAS protocols may improve long term survival. In their retrospective analysis, Gustafsson et al. compared nearly 1000 patients who had major surgery. In those who were treated on an ERAS protocol with compliance rates of over 70 percent, the risk of cancer specific death at 5 years was lowered by 42%. The authors suggest that better adherence to the protocol reduces surgical stress and therefore impacts long term survival (101). The incidence of post-operative complications has shown to be a critical factor in long term survival: it would be logical to assume that greater adherence to ERAS criteria and principles and the resulting reduction in surgical stress would benefit this in turn (102).
Although retrospective, it would appear the evidence regarding the benefits of adherence to ERAS protocols is strong. Prospective studies would add to this literature, and more importantly update it. As discussed elsewhere in this article, implementation and application of ERAS has changed over the last 2 decades, particularly in terms of surgical technique, and quantifying the impact of that change is important.
Modern technology, such as an application developed for smartphones, may make adherence to protocols easier and improve patient engagement. This forms the basis of an ongoing study (103).
Ileus
Post-operative ileus (POI) occurs in approximately 10-30% of patients (104-111). It is associated with significant morbidity, increased hospital length of stay and costs (107,112). POI may result from complications of surgery and can act as an early indicator of serious unidentified pathology, including anastomotic leak (108,113,114). The financial burden attributed to POI has been estimated to be between $750 million and >$1.3 billion per annum in the United States (112,115).
ERAS protocols aim to reduce the time to recovery of gastrointestinal (GI) function after colorectal surgery to an average of 1–2 days. As a result, POI is the focus of several items in ERAS protocols. Minimally invasive surgery, stringent fluid management, immediate resumption of oral fluids and diet, the avoidance of nasogastric tubes and sparing use of opiates have shown to reduce POI (116,117). Despite significant improvements, ileus remains the complication associated with the largest increase in length of hospital stay (118).
While it is widely accepted that POI exists as a clinical entity, some bowel dysmotility is considered a ‘normal’ physiological response following abdominal surgery and there is ambiguity defining exactly when it could be classified as ‘abnormal’.
There is now some consensus that POI occurs in two distinct phases; an early ‘neurogenic phase’ which is considered short-lived (<3 h), followed by a period of relative recovery, before a second longer lasting (>24 h) ‘inflammatory phase’ (119). The contributing factors to both initiation and resolution of the response and the implicated molecular pathways have yet to be fully explained.
One of the major hurdles in accurately defining POI is that the diagnosis relies upon clinical signs and symptoms. A lack of clarity in defining and recognising POI may have undermined previous research attempts and clinical trials. In a clinical context this results in delayed recognition and timely management (106).
There is a need for research and consensus to define what constitutes POI and how to best to identify it. Work is currently being conducted to develop non-invasive biosensor technologies and diagnostic biomarkers to determine their clinical utility in both the prediction and diagnosis of POI (120,121).
The use of mechanical bowel prep and pre-operative oral antibiotics to reduce the risk of POI is advocated by the American Society for Enhanced Recovery and Perioperative Quality Initiative. This guideline is based on retrospective analyses of two large US databases. These found a reduced rate of Ileus when MBP and OAB were used (42,122). These findings have not yet been reproduced in a prospective cohort and are not supported by the ERAS Society who advise against MBP as detailed in another section of this article (116).
Alvimopan (a peripheral µ-receptor antagonist) has been recommended in US and ERAS society guidelines, but the potential benefit is currently unclear. This drug is only available in the US and subject to strict FDA license arrangements, with a maximum of 15 dosages per patient. Concerns have also been cited regarding the cost of the medication, with each dose costing $65USD (116,119,123).
Other preventative strategies that have been tried include the use of coffee, chewing gum and oral magnesium. They have only weak evidence supporting their use, and their clinical utility is not yet established (116,123).
While improvements have been seen with regards to Ileus, return to GI function often remains unpredictable, and is possibly the biggest challenge still facing ERAS.
Early temporary stoma reversal
Temporary diverting ileostomies are commonplace and utilised to minimise consequences of an anastomotic leak. They are often created as part of an anterior or low anterior resection for rectal cancer in patients deemed at high risk of leak – elderly or comorbid patients for example. Morbidity after temporary stoma formation however can be considerable and can have a significant psychological and social impact.
Leakage, electrolyte disturbances and skin damage are common. Stomal obstruction can be particularly hazardous (124,125). Temporary ileostomies are typically reversed 6–12 months following the initial resection. The comorbid, patients undergoing chemotherapy, and those with more advanced cancer are more likely to have a greater delay to reversal, if they undergo the procedure at all (124).
To combat this morbidity, performing early reversal of temporary ileostomy—within 14 days of the initial procedure—is being investigated. The EASY trial, a prospective multi-centre RCT comparing early versus late closure following total mesorectal excision was published in 2017 and included 127 patients. Patients were excluded if leakage was suspected and for ‘other medical reasons’ for example steroid treatment or diabetes. Less than 10% of patients underwent radiotherapy. The mean number of complications was lower in the early reversal group, and the study concluded that early reversal was feasible and safe—albeit in selected patients (126). Another study in selected patients—including resections for benign disease—yielded similar conclusions, including lower operating time for early reversal (127). A 2015 systematic review assessed 4 studies, but only 2 were prospective with one RCT. The review reported lower complication rates in those undergoing early reversal. The authors advocated more studies in the area (128).
Within the context of ERAS, early reversal may potentially reduce stoma related complications. Given the selected nature of patients studied to date however, it could be argued that a temporary stoma may not have been necessary in the first instance. Perhaps the most important question is the accurate identification of patients requiring a defunctioning procedure, rather than the timeframe for reversal.
Patient reported outcome measures
Outcomes from cancer surgery have historically focussed on recurrence and hospital indicators such as length of stay and 30-day mortality. Improvements in care mean that quality of life and functional assessments are gaining prominence in gauging recovery.
Colorectal cancer survivors suffer many problems affecting their quality of life, ranging from psychological distress and fatigue, to urological, bowel and sexual dysfunction (129). There has thus been rebalancing of follow up programmes to assess quality of life (QOL) more formally, and incorporate patient reported outcomes (PROMS).
There are many potential benefits. Patients may be able to identify and obtain what support they require more quickly. Widespread reporting of PROMS would also enable pre-and post-operative counselling to better target functional and psychological recovery. Furthermore, evidence is emerging that PROMS improve survival in cancer patients (130). NICE has recommended that PROMS be a focus in future clinical trials regarding colorectal cancer (131).
Until recently, no consensus existed with regards to assessing QOL in colorectal cancer, particularly with regards to PROMS. The heterogeneity between tools is significant, and a wide variety are used: Mcnair et al. reported no fewer than 53 different PROMs measures from 104 different papers (132). Significant efforts have been made however towards an international consensus regarding a comprehensive tool to assess PROMS in recent years (133).
Within an ERAS context, research has been limited, with inconclusive results. One paper utilising PROMS suggested reduced fatigue in ERAS patients (134), but drawing meaningful conclusions at this time is impossible. PROMS could potentially add another dimension to assessing the impact of ERAS on a patient’s recovery.
Ultimately, the goal of care in elective colorectal surgery will move to ‘restoration of function’. This will require collaboration of peri-operative physicians, anaesthetists, surgeons, specialist nurses, and in no small measure, patients, in its assessment and delivery (135).
Smart phones, apps and accelerometers
Monitoring patient recovery post operatively has been limited to outpatient clinics and telephone-led follow up. The advent of wearable sensors and application-based technology usable with smartphones have the ability to transform follow-up programmes and rapidly increase usage of PROMs.
Accelerometer or podometer based mobility trackers are cheap and readily available. A study looking at elderly cardiac surgery patients found that they could be used reliably to assess mobility post operatively, and increased mobility in the immediate post-operative period correlated with a reduced length of stay (136). A more recent study in colorectal surgery patients showed that accelerometer based devices were accurate, and compared well with observed podometry (137).
Feedback provided by sensor technology has shown to be associated with increased short-term activity levels in the well (138). A 2018 systematic review of 36 papers assessing cardiometabolic conditions demonstrated similarly increased activity in the short term but only in the context of a supervised follow up programme. The paper highlighted limited data for long term improvements (139). A recently published study in the Annals of surgery demonstrated that in patients with peripheral vascular disease, wearable sensor technology significantly improved walking distance in patients with intermittent claudication when compared to supervised exercise programmes in isolation (140).
There is a potential for such advancements to make an impact in recovery from colorectal surgery: A smartphone application has been created by a colorectal surgery team for patients in Wales that explains the diagnosis, care pathway, and the benefits and components of ERAS (141). A sensor-based application has also been developed for patients with ostomies following colorectal procedures that reports on stoma output and the fullness of the bag. It has been intended for use both in the hospital ward and at home. It may prevent unpleasant leakage of stoma bag contents, and alert medical personnel and patients to impending dehydration before the development of irreversible kidney injury (142).
ERAS and the emergency patient
ERAS has shifted paradigms for what is now perceived as the standard of care in elective colorectal surgery. This level of improvement has not been seen in emergency patients, who most commonly present with a visceral perforation or obstruction. They represent a heterogenous group and range from having mild physiological derangements to being severely obtunded.
Although care has modernised, for example with the National Emergency Laparotomy Audit (143), many areas remain where advances can be made, particularly in terms of peri-operative pathophysiology. There have been calls to develop an ERAS style pathway with regards to emergency surgery, which could incorporate such criteria as goal directed treatment, higher level care triage, seniority of care providers, nutritional strategies and modalities for minimally invasive surgery (144).
Conclusions
In the future, new technology may conceivably enable more patients to have shorter hospital stays, avoid bowel preparation, and to be able to report problems and be monitored by smartphone applications without attending hospital. They may avoid opiates all together and have insignificant post-operative pain due to small incisions, quick but safe operations, and an anaesthetic tailored for them.
High risk, frail patients could be identified and made to feel stronger with the help of exercise programmes and dietary help. Fewer patients would need diverting stomas, and for potentially less time. A patient at risk of ileus may avoid it, having undergone a simple pathology test before and a few doses of tablet antibiotic.
The combination of these ‘marginal gains’ may have a profound effect on patients with colorectal disease in the future. No one measure discussed in this article amounts to a drastic shift from longstanding ERAS practice. Conversely, they do represent potential incremental improvements, and therefore embody a continuation of the ERAS philosophy.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2020.03.07). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Eliud Kipchoge: The man, the methods & controversies behind “moon-landing moment” [Internet]. BBC Sport 2019. Last accessed 7/2/20. Available online: https://www.bbc.co.uk/sport/athletics/50460861
- Carli F, Charlebois P, Stein B, et al. Randomized clinical trial of prehabilitation in colorectal surgery. Br J Surg 2010;97:1187-97. [Crossref] [PubMed]
- Carli F, Zavorsky GS. Optimizing functional exercise capacity in the elderly surgical population. Curr Opin Clin Nutr Metab Care 2005;8:23-32. [Crossref] [PubMed]
- Cave J, Paschalis A, Huang CY, et al. A systematic review of the safety and efficacy of aerobic exercise during cytotoxic chemotherapy treatment. Support Care Cancer 2018;26:3337-51. [Crossref] [PubMed]
- Youssef EF, Shanb AA. Supervised Versus Home Exercise Training Programs on Functional Balance in Older Subjects. Malays J Med Sci 2016;23:83-93. [Crossref] [PubMed]
- Loughney L, West M, Pintus S, et al. Comparison of oxygen uptake during arm or leg cardiopulmonary exercise testing in vascular surgery patients and control subjects. Br J Anaesth 2014;112:57-65. [Crossref] [PubMed]
- O’Doherty AF, West M, Jack S, et al. Preoperative aerobic exercise training in elective intra-cavity surgery: A systematic review. Br J Anaesth 2013;110:679-89. [Crossref] [PubMed]
- Boereboom C, Doleman B, Lund JN, et al. Systematic review of pre-operative exercise in colorectal cancer patients. Tech Coloproctol 2016;20:81-9. [Crossref] [PubMed]
- Tsimopoulou I, Pasquali S, Howard R, et al. Psychological Prehabilitation Before Cancer Surgery: A Systematic Review. Ann Surg Oncol 2015;22:4117-23. [Crossref] [PubMed]
- Valkenet K, Trappenburg JCA, Schippers CC, et al. Feasibility of Exercise Training in Cancer Patients Scheduled for Elective Gastrointestinal Surgery. Dig Surg 2016;33:439-47. [Crossref] [PubMed]
- Santa Mina D, Clarke H, Ritvo P, et al. Effect of total-body prehabilitation on postoperative outcomes: a systematic review and meta-analysis. Physiotherapy 2014;100:196-207. [Crossref] [PubMed]
- Lemanu DP, Singh PP, MacCormick AD, et al. Effect of preoperative exercise on cardiorespiratory function and recovery after surgery: A systematic review. World J Surg 2013;37:711-20. [Crossref] [PubMed]
- West MA, Loughney L, Lythgoe D, et al. Effect of prehabilitation on objectively measured physical fitness after neoadjuvant treatment in preoperative rectal cancer patients: A blinded interventional pilot study. Br J Anaesth 2015;114:244-51. [Crossref] [PubMed]
- Snowden CP, Minto G. Exercise: The new premed. Br J Anaesth 2015;114:186-9. [Crossref] [PubMed]
- Bordes J, Cardinal M, Kaiser E. Prehabilitation versus Rehabilitation. Anesthesiology 2015;122:1438. [Crossref] [PubMed]
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis. Age Ageing 2010;39:412-23. [Crossref] [PubMed]
- Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:16-31. [Crossref] [PubMed]
- Malmstrom TK, Miller DK, Simonsick EM, et al. SARC-F: a symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J Cachexia Sarcopenia Muscle 2016;7:28-36. [Crossref] [PubMed]
- Lieffers JR, Bathe OF, Fassbender K, et al. Sarcopenia is associated with postoperative infection and delayed recovery from colorectal cancer resection surgery. Br J Cancer 2012;107:931-6. [Crossref] [PubMed]
- Leong DP, Teo KK, Rangarajan S, et al. Prognostic value of grip strength: findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet 2015;386:266-73. [Crossref] [PubMed]
- Miyamoto Y, Baba Y, Sakamoto Y, et al. Sarcopenia is a Negative Prognostic Factor After Curative Resection of Colorectal Cancer. Ann Surg Oncol 2015;22:2663-8. [Crossref] [PubMed]
- Malietzis G, Currie AC, Athanasiou T, et al. Influence of body composition profile on outcomes following colorectal cancer surgery. Br J Surg 2016;103:572-80. [Crossref] [PubMed]
- Barret M, Antoun S, Dalban C, et al. Sarcopenia is linked to treatment toxicity in patients with metastatic colorectal cancer. Nutr Cancer 2014;66:583-9. [Crossref] [PubMed]
- Jones KI, Doleman B, Scott S, et al. Simple psoas cross-sectional area measurement is a quick and easy method to assess sarcopenia and predicts major surgical complications. Colorectal Dis 2015;17:O20-6. [Crossref] [PubMed]
- Peel NM, Kuys SS, Klein K. Gait Speed as a Measure in Geriatric Assessment in Clinical Settings : A Systematic Review. J Gerontol A Biol Sci Med Sci 2013;68:39-46. [Crossref] [PubMed]
- Studenski S, Faulkner K, Inzitari M, et al. Gait Speed and Survival in Older Adults. JAMA 2011;305:50-8. [Crossref] [PubMed]
- Afilalo J, Sharma A, Zhang S, et al. Gait Speed and 1-Year Mortality Following Cardiac Surgery. Journal of the American Heart Association 2018;7:e010139. [Crossref] [PubMed]
- Kalinkovich A, Livshits G. age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res Rev 2017;35:200-21. [Crossref] [PubMed]
- Cavalheri V, Granger C. Preoperative exercise training for patients with non-small cell lung cancer. Cochrane Database Syst Rev. 2017;6:CD012020. [Crossref] [PubMed]
- Dent E, Morley JE, Arai H, et al. International Clinical Practice Guidelines for Sarcopenia (ICFSR): Screening, Diagnosis and Management. J Nutr Health Aging 2018;22:1148-61. [Crossref] [PubMed]
- Wren SM, Ahmed N, Jamal A, et al. Preoperative Oral Antibiotics in Colorectal Surgery Increase the Rate of Clostridium Difficile Colitis. Arch Surg 2005;140:752-6. [Crossref] [PubMed]
- Krapohl GL, Phillips LR, Campbell DA Jr, et al. Bowel Preparation for Colectomy and Risk of Clostridium difficile Infection. Dis Colon Rectum. 2011;54:810-7. [Crossref] [PubMed]
- Kim EK, Sheetz KH, Bonn J, et al. A statewide experience. The Role of Full Bowel Preparation in Preventing Surgical Site Infection. Ann Surg. 2014;259:310-4. [Crossref] [PubMed]
- Rollins KE, Javanmard-emamghissi H, Lobo DN, et al. Impact of mechanical bowel preparation in elective colorectal surgery : a meta-analysis. World J Gastroenterol 2018;24:519-36. [Crossref] [PubMed]
- Güenaga KF, Matos D, Wille-Jørgensen P. Mechanical bowel preparation for elective colorectal surgery Cochrane Database Syst Rev 2011.CD001544. (Review). [Crossref] [PubMed]
- Dahabreh IJ, Steele DW, Shah N, et al. Oral mechanical bowel preparation for colorectal surgery: Systematic review and meta-analysis. Dis Colon Rectum 2015;58:698-707. [Crossref] [PubMed]
- Allegranzi B, Bischoff P, de Jonge S, et al. Surgical site infections 1 New WHO recommendations on preoperative measures for surgical site infection prevention: an evidence-based. Lancet Infect Dis 2016;16:e276-87. [Crossref] [PubMed]
- Koullouros M, Khan N, Aly EH. The role of oral antibiotics prophylaxis in prevention of surgical site infection in colorectal surgery. Int J Colorectal Dis 2017;32:1-18. [Crossref] [PubMed]
- Chen M, Song X, Chen LZ, et al. Comparing Mechanical Bowel Preparation with Both Oral and Systemic Antibiotics Versus Mechanical Bowel Preparation and Systemic Antibiotics Alone for the Prevention of Surgical Site Infection After Elective Colorectal Surgery: A Meta-Analysis of Randomized Controlled Clinical Trials. Dis Colon Rectum. 2016;59:70-8. [Crossref] [PubMed]
- McSorley ST, Steele CW, Mcmahon AJ. Meta-analysis of oral antibiotics, in combination with preoperative intravenous antibiotics and mechanical bowel preparation the day before surgery, compared with intravenous antibiotics and mechanical bowel preparation alone to reduce surgical-site inf. BJS Open 2018;2:185-94. [Crossref] [PubMed]
- Morris MS, Graham LA, Chu DI, et al. Oral Antibiotic Bowel Preparation Significantly Reduces Surgical Site Infection Rates and Readmission Rates in Elective Colorectal Surgery. Ann Surg 2015;261:1034-40. [Crossref] [PubMed]
- Kiran RP, Murray ACA, Chiuzan C, et al. Combined Preoperative Mechanical Bowel Preparation With Oral Antibiotics Significantly Reduces Surgical Site Infection, Anastomotic Leak, and Ileus After Colorectal Surgery. Ann Surg 2015;262:416-25; discussion 423-5. [Crossref] [PubMed]
- Midura EF, Jung AD, Hanseman DJ, et al. Combination oral and mechanical bowel preparations decreases complications in both right and left colectomy. Surgery 2018;163:528-34. [Crossref] [PubMed]
- 2017 European Society of Coloproctology (ESCP) collaborating group. Association of mechanical bowel preparation with oral antibiotics and anastomotic leak following left sided colorectal resection: an international, multi-centre, prospective audit. Colorectal Dis 2018;20 Suppl 6:15-32. [Crossref] [PubMed]
- Garfinkle R, Abou-Khalil J, Morin N, et al. Is there a role for oral antibiotic preparation alone before colorectal surgery? ACS-NSQIP analysis by coarsened exact matching. Dis Colon Rectum 2017;60:729-37. [Crossref] [PubMed]
- Kaslow SR, Gani F, Alshaikh HN, et al. Clinical outcomes following mechanical plus oral antibiotic bowel preparation versus oral antibiotics alone in patients undergoing colorectal surgery. BJS Open 2018;2:238-45. [Crossref] [PubMed]
- Nelson RL, Gladman E, Barbateskovic M. Antimicrobial prophylaxis for colorectal surgery. Cochrane Database Syst Rev 2014.CD001181. [PubMed]
- European Centre for Disease Control. Annual epidemiological report for 2017. Healthcare associated infections: Surgical site infections. Available online: https://www.ecdc.europa.eu/en/publications-data/healthcare-associated-infections-surgical-site-infections-annual-1
- Shogan BD, Carlisle EM, Alverdy JC, et al. Do we really know why colorectal anastomoses leak? J Gastrointest Surg 2013;17:1698-707. [Crossref] [PubMed]
- Armstrong G, Croft J, Corrigan N, et al. IntAct: intra-operative fluorescence angiography to prevent anastomotic leak in rectal cancer surgery: a randomized controlled trial. Colorectal Dis 2018;20:O226-34. [Crossref] [PubMed]
- Kinross JM, Darzi AW, Nicholson JK. Gut microbiome-host interactions in health and disease. Genome Med 2011;3:14. [Crossref] [PubMed]
- van Praagh JB, de Goffau MC, Bakker IS, et al. Intestinal microbiota and anastomotic leakage of stapled colorectal anastomoses: a pilot study. Surg Endosc 2016;30:2259-65. [Crossref] [PubMed]
- Gaines S, Shao C, Hyman N, et al. Gut microbiome influences on anastomotic leak and recurrence rates following colorectal cancer surgery. Br J Surg 2018;105:e131-41. [Crossref] [PubMed]
- Wiegerinck M, Hyoju SK, Mao J, et al. Novel de novo synthesized phosphate carrier compound ABA-PEG20k-Pi20 suppresses collagenase production in Enterococcus faecalis and prevents colonic anastomotic leak in an experimental model. Br J Surg 2018;105:1368-76. [Crossref] [PubMed]
- Pohl JM, Gutweiler S, Thiebes S, et al. Irf4 -dependent CD103 + CD11b + dendritic cells and the intestinal microbiome regulate monocyte and macrophage activation and intestinal peristalsis in postoperative ileus. Gut 2017;66:2110-20. [Crossref] [PubMed]
- Rollins KE, Javanmard-Emamghissi H, Acheson AG, et al. The Role of Oral Antibiotic Preparation in Elective Colorectal Surgery. Ann Surg. 2019;270:43-58. [Crossref] [PubMed]
- Alverdy JC. Microbiome Medicine: This Changes Everything. J Am Coll Surg 2018;226:719-29. [Crossref] [PubMed]
- Fayfman M, Flint K, Srinivasan S. Obesity, Motility, Diet, and Intestinal Microbiota — Connecting the Dots. Curr Gastroenterol Rep 2019;21:15. [Crossref] [PubMed]
- Larsen SG, Pfeffer F, Kørner H. Norwegian moratorium on transanal total mesorectal excision. Br J Surg 2019;106:1120-1. [Crossref] [PubMed]
- Fawcett WJ, Jones CN. Bespoke intra-operative anaesthesia - the end of the formulaic approach? Anaesthesia 2018;73:1062-6. [Crossref] [PubMed]
- Vincent JL, Pelosi P, Pearse R, et al. Perioperative cardiovascular monitoring of high-risk patients: A consensus of 12. Crit Care 2015;19:224. [Crossref] [PubMed]
- Minto G, Mythen MG. Perioperative fluid management: Science, art or random chaos? Br J Anaesth 2015;114:717-21. [Crossref] [PubMed]
- Lilot M, Ehrenfeld JM, Lee C, et al. Variability in practice and factors predictive of total crystalloid administration during abdominal surgery: Retrospective two-centre analysis. Br J Anaesth 2015;114:767-76. [Crossref] [PubMed]
- Rollins KE, Lobo DN. Intraoperative goal-directed fluid therapy in elective major abdominal surgery : A meta-analysis of randomized controlled trials. Ann Surg 2016;263:465-76. [Crossref] [PubMed]
- Weber CF, Go K, Meininger D, et al. Point-of-Care Testing. Anesthesiology 2012;117:531-47. [Crossref] [PubMed]
- Kirmeier E, Eriksson LI, Lewald H, et al. Post-anaesthesia pulmonary complications after use of muscle relaxants (POPULAR): a multicentre, prospective observational study. Lancet Respir Med 2019;7:129-40. [Crossref] [PubMed]
- Checketts MR, Alladi R, Ferguson K, et al. Recommendations for standards of monitoring during anaesthesia and recovery 2015: Association of Anaesthetists of Great Britain and Ireland. Anaesthesia 2016;71:85-93. [Crossref] [PubMed]
- Kopman AF, Naguib M. Laparoscopic surgery and muscle relaxants: Is deep block helpful? Anesth Analg 2015;120:51-8. [Crossref] [PubMed]
- Hajat Z, Ahmad N, Andrzejowski J. The role and limitations of EEG-based depth of anaesthesia monitoring in theatres and intensive care. Anaesthesia 2017;72:38-47. [Crossref] [PubMed]
- Chan MT, Cheng BC, Lee TM, et al. F1000Prime recommendations of: BIS-guided anesthesia decreases postoperative delirium and cognitive decline. J Neurosurg Anesthesiol 2013;25:33-42. [Crossref] [PubMed]
- Willingham MD, Avidan MS. Triple low, double low: It’s time to deal Achilles heel a single deadly blow. Br J Anaesth 2017;119:1-4. [Crossref] [PubMed]
- Willingham MD, Karren E, Shanks AM, et al. Concurrence of intraoperative hypotension, low minimum alveolar concentration, and low bispectral index is associated with postoperative death. Anesthesiology 2015;123:775-85. [Crossref] [PubMed]
- Kertai MD, White WD, Gan TJ. Cumulative duration of “triple low” state of low blood pressure, low bispectral index, and low minimum alveolar concentration of volatile anesthesia is not associated with increased mortality. Anesthesiology 2014;121:18-28. [Crossref] [PubMed]
- Wirth S, Kreysing M, Spaeth J, et al. Intraoperative compliance profiles and regional lung ventilation improve with increasing positive end-expiratory pressure. Acta Anaesthesiol Scand 2016;60:1241-50. [Crossref] [PubMed]
- Severgnini P, Selmo G, Lanza C, et al. Protective mechanical ventilation during general anesthesia for open abdominal surgery improves postoperative pulmonary function. Anesthesiology 2013;118:1307-21. [Crossref] [PubMed]
- Zhou ZF, Fang JB, Wang HF, et al. Effects of intraoperative PEEP on postoperative pulmonary complications in high-risk patients undergoing laparoscopic abdominal surgery: Study protocol for a randomised controlled trial. BMJ Open 2019;9:e028464. [Crossref] [PubMed]
- Young CC, Harris EM, Vacchiano C, et al. Lung-protective ventilation for the surgical patient: international expert panel-based consensus recommendations. Br J Anaesth 2019;123:898-913. [Crossref] [PubMed]
- Wirth S, Baur M, Spaeth J, et al. Intraoperative positive end-expiratory pressure evaluation using the intratidal compliance-volume profile. Br J Anaesth 2015;114:483-90. [Crossref] [PubMed]
- Vigneault L, Turgeon AF, Côté D, et al. Perioperative intravenous lidocaine infusion for postoperative pain control: A meta-analysis of randomized controlled trials. Can J Anesth 2011;58:22-37. [Crossref] [PubMed]
- Wang F, Meng J, Zhang L, et al. Morphine induces changes in the gut microbiome and metabolome in a morphine dependence model. Sci Rep 2018;8:3596. [Crossref] [PubMed]
- Makary MA, Overton HN, Wang P. Overprescribing is major contributor to opioid crisis. BMJ 2017;359:j4792. [Crossref] [PubMed]
- Elkassabany NM, Mariano ER. Opioid-free anaesthesia - what would Inigo Montoya say? Anaesthesia 2019;74:560-3. [Crossref] [PubMed]
- Frauenknecht J, Kirkham KR, Jacot-Guillarmod A, et al. Analgesic impact of intra-operative opioids vs. opioid-free anaesthesia: a systematic review and meta-analysis. Anaesthesia 2019;74:651-62. [Crossref] [PubMed]
- Lennon FE, Moss J, Singleton PA. The µ-opioid receptor in cancer progression: is there a direct effect? Anesthesiology 2012;116:940-5. [Crossref] [PubMed]
- Schwenk ES, Mariano ER. Designing the ideal perioperative pain management plan starts with multimodal analgesia. Korean J Anesthesiol 2018;71:345-52. [Crossref] [PubMed]
- Boselli E, Bouvet L, Bégou G, et al. Prediction of immediate postoperative pain using the analgesia/nociception index: A prospective observational study. Br J Anaesth 2014;112:715-21. [Crossref] [PubMed]
- Gustafsson UO, Scott MJ, Hubner M, et al. Guidelines for Perioperative Care in Elective Colorectal Surgery: Enhanced Recovery After Surgery (ERAS®) Society Recommendations: 2018. World J Surg 2019;43:659-95. [Crossref] [PubMed]
- Levy BF, Scott MJ, Fawcett W, et al. Randomized clinical trial of epidural, spinal or patient-controlled analgesia for patients undergoing laparoscopic colorectal surgery. Br J Surg 2011;98:1068-78. [Crossref] [PubMed]
- Hübner M, Blanc C, Roulin D, et al. Randomized clinical trial on epidural versus patient-controlled analgesia for laparoscopic colorectal surgery within an enhanced recovery pathway. Ann Surg 2015;261:648-53. [Crossref] [PubMed]
- Levy BF, Scott MJP, Fawcett WJ, et al. 23-Hour-stay laparoscopic colectomy. Dis Colon Rectum 2009;52:1239-43. [Crossref] [PubMed]
- Hopkins PM. Does regional anaesthesia improve outcome? Br J Anaesth 2015;115:ii26-33. [Crossref] [PubMed]
- Luo X, Zhao H, Hennah L, et al. Impact of is of lurane on malignant capability of ovarian cancer in vitro. Br J Anaesth 2015;114:831-9. [Crossref] [PubMed]
- Melamed R, Bar-Yosef S, Shakhar G, et al. Suppression of Natural Killer Cell Activity and Promotion of Tumor Metastasis by Ketamine, Thiopental, and Halothane, but Not by Propofol: Mediating Mechanisms and Prophylactic Measures. Anesth Analg 2003;97:1331-9. [Crossref] [PubMed]
- Wigmore TJ, Mohammed K, Jhanji S. Long-term survival for patients undergoing volatile versus IV anesthesia for cancer surgery: A retrospective analysis. Anesthesiology 2016;124:69-79. [Crossref] [PubMed]
- Yoo S, Lee HB, Han W, et al. Total Intravenous Anesthesia versus Inhalation Anesthesia for Breast Cancer Surgery: A Retrospective Cohort Study. Anesthesiology 2019;130:31-40. [Crossref] [PubMed]
- Sessler DI, Pei L, Huang Y, et al. Recurrence of breast cancer after regional or general anaesthesia: a randomised controlled trial. Lancet 2019;394:1807-15. [Crossref] [PubMed]
- ERAS Compliance Group. The impact of enhanced recovery protocol compliance on elective colorectal cancer resection: Results from an international registry. Ann Surg 2015;261:1153-9. [Crossref] [PubMed]
- Pędziwiatr M, Kisialeuski M, Wierdak M, et al. Early implementation of Enhanced Recovery After Surgery (ERAS®) protocol - Compliance improves outcomes: A prospective cohort study. Int J Surg 2015;21:75-81. [Crossref] [PubMed]
- Ahmed J, Khan S, Lim M, et al. Enhanced recovery after surgery protocols - compliance and variations in practice during routine colorectal surgery. Colorectal Dis 2012;14:1045-51. [Crossref] [PubMed]
- Nicholson A, Lowe MC, Parker J, et al. Systematic review and meta-analysis of enhanced recovery programmes in surgical patients. Br J Surg 2014;101:172-88. [Crossref] [PubMed]
- Gustafsson UO, Oppelstrup H, Thorell A, et al. Adherence to the ERAS protocol is Associated with 5-Year Survival After Colorectal Cancer Surgery: A Retrospective Cohort Study. World J Surg 2016;40:1741-7. [Crossref] [PubMed]
- Khuri SF, Henderson WG, DePalma RG, et al. Determinants of long-term survival after major surgery and the adverse effect of postoperative complications. Ann Surg 2005;242:326-41. [PubMed]
- Rauwerdink A, Jansen M, De Borgie CAJM, et al. Improving enhanced recovery after surgery (ERAS): ERAS APPtimize study protocol, a randomized controlled trial investigating the effect of a patient-centred mobile application on patient participation in colorectal surgery. BMC Surg 2019;19:125. [Crossref] [PubMed]
- Bauer AJ, Boeckxstaens GE. Mechanisms of postoperative ileus. Neurogastroenterol Motil 2004;16:54-60. [Crossref] [PubMed]
- Millan M, Biondo S, Fraccalvieri D, et al. Risk Factors for Prolonged Postoperative Ileus After Colorectal Cancer Surgery. World J Surg 2012;36:179-85. [Crossref] [PubMed]
- Vather R, Trivedi S, Bissett I. Defining Postoperative Ileus: Results of a Systematic Review and Global Survey. J Gastrointest Surg 2013;17:962-72. [Crossref] [PubMed]
- Vather R, Josephson R, Jaung R, et al. Development of a risk stratification system for the occurrence of prolonged postoperative ileus after colorectal surgery: A prospective risk factor analysis. Surgery 2015;157:764-73. [Crossref] [PubMed]
- Moghadamyeghaneh Z, Hwang GS, Hanna MH, et al. Risk factors for prolonged ileus following colon surgery. Surg Endosc 2016;30:603-9. [Crossref] [PubMed]
- Kim MJ, Min GE, Yoo KH, et al. Risk factors for postoperative ileus after urologic laparoscopic surgery. J Korean Surg Soc 2011;80:384-9. [Crossref] [PubMed]
- Svatek RS, Fisher MB, Williams MB, et al. Age and Body Mass Index Are Independent Risk Factors Paralytic Ileus After Radical Cystectomy. Urology 2010;76:1419-24. [Crossref] [PubMed]
- Chapuis PH, Bokey L, Keshava A, et al. Risk Factors for Prolonged Ileus After Resection of Colorectal Cancer. Ann Surg 2013;257:909-15. [Crossref] [PubMed]
- Murphy MM, Tevis SE, Kennedy GD. Independent risk factors for prolonged postoperative ileus development. J Surg Res 2016;201:279-85. [Crossref] [PubMed]
- Peters EG, Dekkers M, van Leeuwen-Hilbers FW, et al. Relation between postoperative ileus and anastomotic leakage after colorectal resection: a post hoc analysis of a prospective randomized controlled trial. Colorectal Dis 2017;19:667-74. [Crossref] [PubMed]
- Venara A, Alfonsi P, Cotte E, et al. Postoperative ileus concealing intra-abdominal complications in enhanced recovery programs-a retrospective analysis of the GRACE database. Int J Colorectal Dis 2019;34:71-83. [Crossref] [PubMed]
- Asgeirsson T, El-Badawi KI, Mahmood A, et al. Postoperative Ileus: It Costs More Than You Expect. J Am Coll Surg 2010;210:228-31. [Crossref] [PubMed]
- Gustafsson UO, Scott MJ, Schwenk W, et al. Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (ERAS(®)) Society recommendations. World J Surg 2013.259-84. [Crossref] [PubMed]
- Grass F, Slieker J, Jurt J, et al. Postoperative ileus in an enhanced recovery pathway — a retrospective cohort study. Int J Colorectal Dis 2017;32:675-81. [Crossref] [PubMed]
- Ahmed Ali U, Dunne T, Gurland B, et al. Actual versus estimated length of stay after colorectal surgery: which factors influence a deviation? Am J Surg 2014;208:663-9. [Crossref] [PubMed]
- Chapman SJ, Pericleous A, Downey C, et al. Postoperative ileus following major colorectal surgery. Br J Surg 2018;105:797-810. [Crossref] [PubMed]
- Kaneshiro M, Kaiser W, Pourmorady J, et al. Postoperative Gastrointestinal Telemetry with an Acoustic Biosensor Predicts Ileus vs. Uneventful GI Recovery. J Gastrointest Surg 2016;20:132-9. [Crossref] [PubMed]
- Hyšpler R, Tichá A, Kaška M, et al. Markers of perioperative bowel complications in colorectal surgery patients. Dis Markers 2015;2015:428535. [Crossref] [PubMed]
- Englesbe MJ, Brooks L, Kubus J, et al. A statewide assessment of surgical site infection following Colectomy : The Role of Oral Antibiotics. Ann Surg 2010;252:514-9; discussion 519-20. [PubMed]
- Hedrick TL, McEvoy MD, Mythen MMG, et al. American Society for Enhanced Recovery and Perioperative Quality Initiative Joint Consensus Statement on Postoperative Gastrointestinal Dysfunction Within an Enhanced Recovery Pathway for Elective Colorectal Surgery. Anesth Analg 2018;126:1896-907. [Crossref] [PubMed]
- Åkesson O, Syk I, Lindmark G, et al. Morbidity related to defunctioning loop ileostomy in low anterior resection. Int J Colorectal Dis 2012;27:1619-23. [Crossref] [PubMed]
- Persson E, Berndtsson I, Carlsson E, et al. Stoma-related complications and stoma size - a 2-year follow up. Colorectal Dis 2010;12:971-6. [Crossref] [PubMed]
- Danielsen AK, Park J, Jansen JE, et al. Early closure of a temporary ileostomy in patients with rectal cancer: A multicenter randomized controlled trial. Ann Surg 2017;265:284-90. [Crossref] [PubMed]
- Lasithiotakis K, Aghahoseini A, Alexander D. Is Early Reversal of Defunctioning Ileostomy a Shorter, Easier and Less Expensive Operation? World J Surg 2016;40:1737-40. [Crossref] [PubMed]
- Robertson JP, Puckett J, Vather R, et al. Early Closure of Temporary Loop Ileostomies: A Systematic Review. Ostomy Wound Manage 2015;61:50-7. [PubMed]
- UK Department of Health. Quality of Life of Colorectal Cancer Survivors in England 2015. Available online: https://www.england.nhs.uk/wp-content/uploads/2015/03/colorectal-cancer-proms-report-140314.pdf
- Basch E, Deal AM, Dueck AC, et al. Overall Survival Results of a Trial Assessing Patient-Reported Outcomes for Symptom Monitoring During Routine Cancer Treatment. JAMA 2017;318:197. [Crossref] [PubMed]
- NICE National Institute for Health Care and Excellence. Colorectal cancer: diagnosis and management | Guidance and guidelines | NICE. Clinical Guideline (CG131) 2014. Available online: https://www.nice.org.uk/guidance/cg131
- McNair AG, Whistance RN, Forsythe RO, et al. Synthesis and summary of patient-reported outcome measures to inform the development of a core outcome set in colorectal cancer surgery. Colorectal Dis 2015;17:O217-29. [Crossref] [PubMed]
- Zerillo JA, Schouwenburg MG, van Bommel ACM, et al. An International Collaborative Standardizing a Comprehensive Patient-Centered Outcomes Measurement Set for Colorectal Cancer. JAMA Oncol 2017;3:686. [Crossref] [PubMed]
- Zargar-Shoshtari K, Paddison JS, Booth RJ, et al. A Prospective Study on the Influence of a Fast-Track Program on Postoperative Fatigue and Functional Recovery After Major Colonic Surgery. J Surg Res 2009;154:330-5. [Crossref] [PubMed]
- Levy N, Grocott MPW, Lobo DN. Restoration of function: the holy grail of peri-operative care. Anaesthesia 2020;75:e14-7. [Crossref] [PubMed]
- Cook DJ, Thompson JE, Prinsen SK, et al. Functional recovery in the elderly after major surgery: Assessment of mobility recovery using wireless technology. Ann Thorac Surg 2013;96:1057-61. [Crossref] [PubMed]
- Skender S, Schrotz-King P, Böhm J, et al. Repeat physical activity measurement by accelerometry among colorectal cancer patients - Feasibility and minimal number of days of monitoring. BMC Res Notes 2015;8:222. [Crossref] [PubMed]
- Jauho AM, Pyky R, Ahola R, et al. Effect of wrist-worn activity monitor feedback on physical activity behavior: A randomized controlled trial in Finnish young men. Prev Med Rep 2015;2:628-34. [Crossref] [PubMed]
- Hodkinson A, Kontopantelis E, Adeniji C, et al. Accelerometer- and Pedometer-Based Physical Activity Interventions Among Adults With Cardiometabolic Conditions: A Systematic Review and Meta-analysis. JAMA Netw open 2019;2:e1912895. [Crossref] [PubMed]
- Normahani P, Kwasnicki R, Bicknell C, et al. Wearable sensor technology efficacy in peripheral vascular disease (wSTEP) a randomized controlled trial. Ann Surg 2018;268:1113-8. [Crossref] [PubMed]
- Shah P, Rees M, Brown D, et al. PWE-409 Colorectal cancer care apps for patient and staff education - bringing medical education into the 21st century. Gut 2015;64:A389.3-A390.
- Kontovounisios C, Smith J, Dawson P, et al. The Ostom-iTM Alert Sensor: a new device to measure stoma output. Tech Coloproctol 2018;22:697-701. [Crossref] [PubMed]
- Eugene N, Oliver CM, Bassett MG, et al. Development and internal validation of a novel risk adjustment model for adult patients undergoing emergency laparotomy surgery: the National Emergency Laparotomy Audit risk model. Br J Anaesth 2018;121:739-48. [Crossref] [PubMed]
- Foss NB, Kehlet H. Challenges in optimising recovery after emergency laparotomy. Anaesthesia 2020;75:e83-9. [Crossref] [PubMed]
Cite this article as: Singh R, Mackenzie P, White D, Allen S, Fawcett W, Rockall T. Future perspectives: enhanced recovery in colorectal surgery. Ann Laparosc Endosc Surg 2021;6:7.


