
Management of complications after paraesophageal hernia repair
Introduction
Laparoscopic paraesophageal hernia (PEH) repair can be done safely in expert hands with mortality rates of 1.3% in elective settings and 8% in emergencies (1,2). Due to the complexity of the operation, a significant learning curve is necessary for a surgeon to reduce complications rate to those of experts (3-5). The list of complications reported after PEH repair is long. Medical complications affecting the heart, lungs and kidneys are often associated with PEH repair because the operation is mostly performed for elderly patients with co-morbidities. Serious surgical complications result from iatrogenic injuries to the pleura, aorta and pericardium (6). Furthermore, traction on the gastric fundus, esophago-gastric junction (EGJ) and lower esophagus can cause immediate or delayed visceral perforation with life-threatening consequences (7). Hernia recurrence is common but not usually of clinical significance. Elective primary and revision PEH repair should therefore only be undertaken in units with experience and facilities to deal with respiratory failure, major bleeding, upper gastro-intestinal perforations and critical care. In this review the focus is on those complications directly related to laparoscopic PEH repair that may require revisional surgery.
Complications after PEH repair
CO2 retention and pneumothorax
Laparoscopic incision of the peritoneal sac in the mediastinum inevitably results in CO2 tracking up and accumulating in the mediastinal and subcutaneous spaces. CO2 retention manifests itself with a gradual rise in the end-tidal CO2 and surgical emphysema that can extend to the fingertips (8).
If CO2 retention is detected intra-operatively, the pneumoperitoneum pressure should be reduced to <11 mmHg or potentially de-insufflation for a period of time allowing for hyperventilation to lower the CO2 levels. A pneumoperitoneum pressure ≥14 mmHg is rarely required for surgery in the mediastinum. If the end-tidal CO2 remains high at the end of the procedure, with a risk of hypercarbia and respiratory depression, the patient should remain ventilated until CO2 levels drop into the normal range.
Although pneumoperitoneum can directly lead to pneumothoraces on either side, most pneumothoraces result from iatrogenic laceration of the pleura and direct insufflation of CO2 into the pleural cavities (8).
Intra-operative pneumothorax, or more accurately, capnothorax, is usually detected by the anesthesiologist as an increase in the ventilation pressures or a drop in the blood pressure. A left sided capnothorax will cause ballooning of the left diaphragm, which will be noticed by the surgeon. Reducing the pneumoperitoneum pressure to ≤11 mmHg will usually allow the positive pressure ventilation to re-expand the lung. If needed, the surgeon can further assist lung expansion using the laparoscopic suction device in the pleural space (9). At the end of the operation a capnothorax will almost always resolve without any intervention. However, on rare occasions an at-risk patient (poor lung function) or with persistent symptoms making extubation difficult chest tube drainage may be desired. On these occasions a thoracostomy drain can be avoided by placing a transhiatal soft tube drain via one of the laparoscopy port sites into the mediastinum or directly into the pleural space via the pleural defect which is a technique developed for laparoscopic esophagectomies (10). It is not usually necessary to connect the drain to an underwater device and it can usually be removed in a short time frame once the patient has stabilized.
A post-operative pneumothorax detected by a chest XR, ultrasound or CT scanning can be treated non-operatively if small and asymptomatic, because the capnothorax is usually absorbed rapidly. Symptomatic pneumothoraces may be due to an occult lung injury and should be treated with an intercostal chest drain insertion.
Post-operative bleeding
While uncontrollable intraoperative bleeding is quite rare, postoperative bleeding is one of the most common reasons for an early return to the OR. As PEH repair involves division of many large and small blood vessels, there is always a possibility of failure of a tissue seal or suture ligation and resulting bleeding. Such bleeding is most frequently from a short gastric vessel, or branch of the left gastric artery/vein. Anatomical distortion in a PEH can make identification of blood vessels more difficult which may lead to iatrogenic injury. Short gastric vessels may be stretched into the mediastinum and the surgeon must carefully seal and divide them near the fundic wall. In such cases the posterior gastric vessels connecting with the posterior aspect of the splenic hilum must be divided in the posterior aspect of the hernial sac. In PEH with organo-axial gastric volvulus the gastric fundus is in its original posterior position and the short gastric vessels short and close to the splenic hilum. If the surgeon wishes to do a fundoplication in such cases, once the distal stomach is reduced and the hiatus hernia repaired, a careful division of the proximal short gastric and posterior gastric vessels have to be performed to mobilize the fundus. The left gastric artery and its branches can also be distorted and stretched by the PEH hernial sac. In a large PEH there is destruction of posterior crural fibres which makes the upper border of the pancreas and left gastric artery appear to be withing the hiatus. Injury to the left gastric artery is avoided by dissecting out the entire hernial sac laterally and anteriorly in the mediastinum and delivering the sac, stomach and the esophago-gastric junction to the abdominal cavity. Resection of the hernial sac starts anteriorly to the left of the anterior vagus nerve and continues around the left side to near the midline. If the anatomy to the right of the vagus nerves and around the left gastric artery remain distorted and obscured by the posterior aspect of the hernial sac, this is best left in situ to avoid injury to the artery and nerves. If a patient has a sudden drop in blood pressure, tachycardia and increased abdominal pain in the first 48 hours, bleeding should be expected and urgent return to the OR planned.
Cardio-vascular injury
A potentially life-threatening complication of paraesophageal hernia repair is cardiac injury. Cardiac tamponade (11,12) and hydropneumopericardium (13) have been described. A primary cardiological event should be suspected in the first instance, and prompt diagnosis is key in patients developing intra- or postoperative hemodynamic instability (6). Most of the cases reported have happened after a large diaphragmatic defect was repaired by fixing a mesh with staples or tacks. All cases required a reoperation for pericardial drainage and repair. Fatal hemorrhage due to gastro-aortic fistula has been also reported, arising from a gastric ulceration at a suture site, an ischemic fundus, or mesh migration (14).
Perforation
Esophageal and gastric fundal perforations after PEH repair can occur due to traction on the lower esophagus, esophago-gastric junction and fundus, or a thermal injury from dissection with an energy device. When a perforation to the esophagus or stomach is recognized intra-operatively, good results are achieved by immediate repair with a dissolvable suture such as 3/0 Vicryl with either a two layer or single layer technique (15). A trans-hiatal tube drain is placed in the lower mediastinum adjacent to the esophageal repair at the end of the procedure. The patient should be restricted to having only sips of water for 72 hours before healing is confirmed by a colored drink test on the ward or a water-soluble contrast study in the XR department.
When the perforation presents after a few days, and the mediastinal drain has remained in situ from the primary operation, conservative management can continue if the patient is clinically stable. Cross-sectional imaging by CT scanning should be performed to delineate the extent of the liquid and air leak. Intravenous antibiotics and antifungal medication are administered. The patient is kept nil by mouth and a naso-gastric tube is placed to decompress and drain the stomach. The mediastinal drain is kept on free drainage, but suction can be applied in case of a large air leak. Intercostal chest drains are inserted for pleural effusions or pneumothoraces. Parenteral nutrition is usually administered but enteral nutrition can be provided by a naso-jejunal catheter or percutaneous jejunostomy (16).
If the perforation presents after a few days and there are no drains in situ, surgical intervention by laparoscopy, laparotomy or thoracotomy is usually indicated (17) (Figure 1). At laparoscopy all contaminating liquid should be aspirated and the mediastinum lavaged. Accurate placement of tube drains in the mediastinum is vital. Repair of the esophageal perforation can be attempted but is unlikely to be successful at this late stage, and repair over a t-tube drain could be considered as an alternative. Additional peritoneal and intercostal chest drains are placed intra-operatively or post-operatively by interventional radiology as appropriate. Endoscopic techniques such as endoscopic suturing and clipping, esophageal stenting with a removable covered self-expanding metal stent, or endoluminal sponge vacuum therapy have proven benefits as an alternative to surgery when dealing with esophageal perforation either as a primary procedure if there is not free peritoneal contamination or as an adjunct to surgery (18).
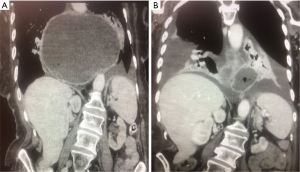
Perforation has also been described after intra-thoracic herniation and strangulation of a fundoplication wrap, leading to mediastinitis and empyema (19). A large hernia recurrence with acute gastric volvulus, as well as migration of the body of the stomach through the fundoplication ring, can also cause necrosis (19,20). Gastric necrosis with perforation requires urgent surgery with resection. A sleeve type gastrectomy can be done if only the fundus or greater curve is necrotic, but if both greater and lesser curves of the stomach are necrotic then a near total or total gastrectomy may be required (21). Immediate reconstruction by Roux loop can safely be done but delayed reconstruction has also been proposed.
Dysphagia
Post-surgical dysphagia is caused by delayed esophageal emptying due to mechanical compression on the esophago-gastric junction by a migrated fundoplication, a tight fundoplication, twisting of the EGJ by a distorted fundoplication, a tight diaphragmatic crural repair, or hiatal stenosis due to excessive scarring (22). In the long-term such EGJ compression can lead to esophageal dilatation, loss of peristalsis in the esophageal body and a clinical picture of iatrogenic pseudo-achalasia (23,24) (Figure 2).
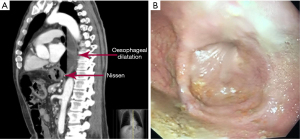
Some patients experience acute dysphagia, i.e., they are unable to swallow saliva or water in the early post-operative period. Early acute dysphagia can be caused by migration of the fundoplication into the hiatus or lower mediastinum due to post-operative retching and vomiting, but it can also be caused by over-tight crural sutures or fundoplication. In most cases early acute dysphagia is best treated by an early return to theatre to correct the obstruction at the EGJ. A migrated fundoplication is returned to the abdomen and the hiatus re-repaired. It is important to note the cause of the immediate herniation which is likely secondary to an insufficient mediastinal dissection which will need to be done at the return to surgery. On the other hand, if the crural repair is too tight, one or two crural sutures are removed and the 360° fundoplication is best converted to 180° by removing the anterior fundoplication sutures.
Most patients experience a temporary mild to moderate dysphagia for up to 6 weeks after repair. Occasionally, dysphagia persists beyond the expected 6 weeks and can become severely disabling. A moderate dysphagia persisting beyond the expected time frame should be investigated by a barium swallow XR to assess esophageal emptying and a gastroscopy to assess the hiatus repair and fundoplication. Persistent dysphagia with delay of passage at the EGJ is initially treated by balloon dilatation of the EGJ to 20 mm. Achalasia type pneumatic dilatation up to 30 mm can be used if initial dilatation is not successful (25). If pneumatic dilatation is unsuccessful then a re-operation should be considered. At re-operation for dysphagia the EGJ is mobilized, fundoplication opened anteriorly (360° to 180° conversion) and the hiatus repair assessed. In most cases the problem will be a hiatus hernia recurrence with partial migration of the fundoplication. In these cases, the hiatus should be repaired once again by non-absorbable sutures such as Ethibond with a patulous fundoplication. In cases where there is stenosis of the EGJ the excess scarring can be anterior adhesions between the left lobe of the liver, the fundoplication and hiatus. The fundoplication can be stenotic due to tight sutures and scar tissue formation between the fundoplication and EGJ as well as the repaired hiatus can be stenotic by tight sutures, excessive scar tissue or mesh. In such cases all the anterior adhesions should be divided, a hiatoplasty performed either anterior or posterior to the discretion of the surgeon and the fundoplication should be opened with all adhesions under the fundoplication divided restoring normal anatomy.
Hiatus hernia recurrence
Asymptomatic hiatus hernia recurrence does not warrant a repair. Hiatus hernia recurrence causing symptoms such as dysphagia, heartburn, regurgitation, vomiting, shortness of breath or chest pain should be considered for surgery. Pre-operative investigations include a gastroscopy, barium swallow XR and CT scan. Patients should be informed that the operation will take longer, is associated with more complications, and is less likely to resolve all the symptoms than their primary operation (26). Most operations to repair a recurrent hiatus hernia can be accomplished with a laparoscopic technique (2). The intra-operative findings are either slippage of the EGJ through an intact infra-diaphragmatic fundoplication, partial migration of the fundoplication into the mediastinum, complete migration of the fundoplication and proximal stomach, distortion/malconstruction of the fundoplication with migration, or volvulus of the distal stomach and/or greater curve into the previous hernia space (24,27,28). If the main pathology encountered at operation is gastric volvulus and it is deemed undesirable to perform a hiatus hernia repair due to patient risk factors or lack of surgical expertise, gastropexy can be performed by decompressing the stomach through a naso-gastric tube and then placing a row of non-absorbable sutures between the greater curve of the stomach and the anterior abdominal wall (29).
A few technical points in our experience can help achieving satisfactory results when repairing a recurrent PEH.
- Peritoneal adhesions—adhesions between the left lobe of the liver and stomach, and adhesions between the fundus and crura are best divided by diathermy scissors either mono-polar or bipolar.
- In cases where the entire hernia sac was not removed, the peritoneal hernial sac is dissected out of the mediastinum by developing a plane between the peritoneum and pleura, then peeling the sac off the mediastinal tissues (30). During dissection, traction should be applied to the peritoneal sac from lateral to medial, but no traction should be applied to the esophagus or stomach. Great care is taken not to try and pull or peel the peritoneal sac off the esophagus. Once the peritoneal sac has been released from both the right and left pleura, as well as anteriorly from behind the pericardium and posteriorly over the aorta, the esophago-gastric junction and hernial sac can be delivered to the abdomen without tension. During excision of the hernial sac great care should be taken not to injure the esophagus, fundus or vagus nerves. With additional esophageal mobilization, it is rarely (<4%) necessary to perform esophageal lengthening techniques.
- The previous fundoplication should be undone completely to re-assess the anatomy of the EGJ (31).
- The hiatus repair is performed with non-absorbable sutures such as Ethibond. The crura will have separated into a wide asymmetrical V-shape due to destruction of the anterior muscular component of the hiatus, and excessive stretching of the left crural fibers. The hiatus repair can start posteriorly through the crural muscles where the sutures will be under considerable tension (32). Lateral releasing incisions with or without mesh reinforcement can be used (5,33). As an alternative, the hiatus repair can start anteriorly through the central tendinous part of the diaphragm posterior to the pericardium (34,35). The start of an anterior repair is to the left of the midline in order to achieve equal lengths of the right and left crura. The first sutures through the tendinous part of the diaphragm are tied under tension to approximate the wide tips of the V-shaped hiatus, thus allowing the crura to assume a narrower oval shape. Three of four further anterior and one or two posterior sutures are placed to create the correct size crural aperture for the esophagus (Figure 3).
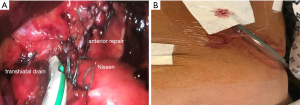 Figure 3 Anterior repair. (A) An anterior hiatal repair and 360° fundoplication. (B) A 24 French soft tube drain is inserted into the mediastinum via the transhiatal route at the end of the procedure. Two drains can be inserted if both right and left pleural spaces had pneumothoraxes that needed treatment.
Figure 3 Anterior repair. (A) An anterior hiatal repair and 360° fundoplication. (B) A 24 French soft tube drain is inserted into the mediastinum via the transhiatal route at the end of the procedure. Two drains can be inserted if both right and left pleural spaces had pneumothoraxes that needed treatment. - The fundoplication is redone in most cases as a 360° loose wrap, but a partial anterior or posterior fundoplication can also be done. Mobilization of the posterior extra-peritoneal aspect of the fundus may be required before a loose fundoplication can be achieved.
- The lower mediastinum and/or left and right pleural cavities are drained by placing one or two transhiatal tube drains in them (10) (Figure 3).
- A naso-gastric tube is not routinely used.
- Every effort is made to avoid retching and vomiting in the early post-operative period, i.e., anti-emetics are given routinely during anesthesia and afterwards, opiate use is kept to a minimum, and the patient is allowed only sips of water initially.
- Routine contrast swallow XR investigations are not performed (36), but a colored drink test with bedside observation of drain output can provide information about the integrity of the lower esophagus. A CT scan with oral contrast can provide additional information about possible leaks or hernia recurrence if the clinical situation warrants the investigation.
Excess intestinal gas
Hiatus repair and fundoplication affect gastric gas management with reduced belching (37) as well as reduced gastric accommodation (38) with most patients reporting some symptoms of increased intestinal gas (meteorism) post-operatively. Acute gastric dilatation with gas occurs in the immediate post-operative period with patients suffering severe epigastric pain and impaired respiration, but patients can present with these symptoms several months after the operation. When a concomitant gastric outlet obstruction, such as for SMA syndrome, is present, patients can develop closed loop obstruction with a dramatic rise of intraluminal pressure, which has been associated with acute pancreatitis (39), SMV thrombosis (40), stomach ischemia and perforation (41). The treatment is urgent decompression of the stomach by naso-gastric tube drainage. Excess gas accumulation in the intestines results in symptoms of bloating and cramps, visible abdominal distention (tympanites), and excess flatus (flatulence). Chronic excess gastric and intestinal gas is initially managed medically by diet modification, avoiding carbonated drinks and taking medication such as anti-spasmodics, charcoal, laxatives or simple analgesia. Patients with intractable symptoms and severe impairment of quality of life may be considered for redo surgery with suspicion of a technical error or early recurrence. Pre-operative investigations should include barium swallow to confirm good esophageal emptying, CT scanning to rule out a recurrent hiatus hernia and confirm gastric distention with gas, esophageal physiology to rule out GERD, isotope gastric emptying studies to rule out delayed gastric emptying and a gastroscopy to rule out gastritis. After excluding other pathology, the reasonable assumption is that the gastric gas bloat is caused by the configuration of the fundoplication. Patients should be warned that redo surgery is not guaranteed to resolve their symptoms and that opening the fundoplication may result in acid reflux. At laparoscopy, the fundoplication is opened anteriorly and converted from 360o to 180o, and the hiatus is opened anteriorly if there is any evidence of hiatal stenosis (42).
Delayed gastric emptying
Inadvertent truncal vagotomy or vagal nerve injury causes pyloric dysfunction with delayed gastric emptying, reflux and vomiting. Solid food retention is seen in the stomach at endoscopy or contrast XRs despite fasting. Confirmation of the diagnosis can be sought by radio-isotope gastric emptying studies. Treatment consists of pyloric dilatation up to 30 mm, pyloromyotomy via the POEM (POP) technique (43), or laparoscopic pyloroplasty. In rare cases, with severe reflux a Roux-en-Y gastric bypass or antrectomy with Roux-en-Y reconstruction can be done to relieve symptoms (44).
Suture, pledget and mesh erosion
Non-absorbable suture material, pledgets and mesh are often used for hiatus hernia repair and fundoplication to reduce recurrence rates (45). These materials can erode into the lumen or cause dense scarring with hiatal and esophageal stenosis. While eroded materials can be removed endoscopically, some patients require surgery, including resection such as esophagectomy and gastrectomy for mesh removal (46). Mesh erosion into the aorta can result in catastrophic bleeding that require urgent surgery (47).
Fistulas
A fistula connecting the distal esophagus and fundus has been described as causing persistent and worsening of pathological acid reflux post fundoplication (48). This was attributed to intraoperative tissue injury caused by deeply placed sutures, which in another instance have been held responsible for intra gastric wall abscess formation (49). Endoscopic and surgical repair can resolve the problem.
Splenic complications
Ligation of short gastric vessels can be associated with partial spleen infarction, usually causing no serious consequences (50). However, ‘wandering’ spleen and torsion on its mesentery, acute complete splenic infarction and a vanishing spleen leading to fatal pneumococcal sepsis have all been described after Nissen fundoplication, following minor trauma or in the presence of connective tissue disorders (51,52). Splenectomy may be required but is exceedingly rare in laparoscopic procedures.
Diarrhea and other medical complications
Up to 20% of patients experience some change in bowel habit after PEH repair, mostly diarrhea. Causes of new onset chronic diarrhea include vagotomy, small bowel bacterial overgrowth and bile salt malabsorption. Diarrhea is usually treated medically. Cardiac, respiratory and renal complications treated in the peri-operative period rarely persist in the longer term.
Conclusions
Medical and surgical complications of paraesophageal hernia repair can present intra-operatively, in the early post-operative phase or in a delayed manner. The complications can be life-threatening or severely impair quality of life. Primary surgery, peri-operative care and treatment of complications are best done in specialist centers by expert surgeons. Pneumothorax/capnothorax, hemorrhage and esophageal perforation that are immediately corrected during surgery have a good outcome. Delayed complications such as hiatus hernia recurrence, obstructing volvulus, gastric perforation, dysphagia, gas bloating, impaired gastric emptying and mesh erosion can successfully be dealt with by expert surgeons. Non-specialist surgeons confronted with emergency complications from paraesophageal hernias can employ temporary stabilizing techniques such as decompression and gastropexy before transfer.
Acknowledgments
The authors are grateful to Mr Mark Kelly and Dr Andrea De Zanna for their advice and contributions in the preparation of the manuscript.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Lee L. Swanstrom and Steven G. Leeds) for the series “Hiatal Hernia” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales-19-241). The series “Hiatal Hernia” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ballian N, Luketich JD, Levy RM, et al. A clinical prediction rule for perioperative mortality and major morbidity after laparoscopic giant paraesophageal hernia repair. J Thorac Cardiovasc Surg 2013;145:721-9. [Crossref] [PubMed]
- Dallemagne B, Quero G, Lapergola A, et al. Treatment of giant paraesophageal hernia: pro laparoscopic approach. Hernia 2018;22:909-19. [Crossref] [PubMed]
- Okrainec A, Ferri LE, Feldman LS, et al. Defining the learning curve in laparoscopic paraesophageal hernia repair: a CUSSUM analysis. Surg Endosc 2011;25:1083-7. [Crossref] [PubMed]
- Luketich JD, Nason KS, Christie NA, et al. Outcomes after a decade of laparoscopic giant paraesophageal hernia repair. J Thorac Cardiovasc Surg 2010;139:395-404. [Crossref] [PubMed]
- DeMeester SR. Laparoscopic paraesophageal hernia repair: critical steps and adjunct techniques to minimize recurrence. Surg Laparosc Endosc Percutan Tech 2013;23:429-35. [Crossref] [PubMed]
- Çalıkoğlu İ, Özgen G, Toydemir T, et al. Iatrogenic cardiac tamponade as a mortal complication of peri-hiatal surgery. Analysis of 30 published cases. Heliyon 2019;5:e01537 [Crossref] [PubMed]
- Sihvo EI, Salo JA, Räsänen JV, et al. Fatal complications of adult paraesophageal hernia: a population-based study. J Thorac Cardiovasc Surg 2009;137:419-24. [Crossref] [PubMed]
- Makay O, van den Broek WT, Yuan JZ, et al. Anesthesiological hazards during laparoscopic transhiatal esophageal resection: a case control study of the laparoscopic-assisted vs the conventional approach. Surg Endosc 2004;18:1263-7. [Crossref] [PubMed]
- Falk GL, D’Netto TJ, Phillips S, et al. Pneumothorax: laparoscopic intraoperative management during fundoplication facilitates management of cardiopulmonary instability and surgical exposure. J Laparoendosc Adv Surg Tech A 2018;28:1371-3. [Crossref] [PubMed]
- Gogalniceanu P, Crewdson K, Khan AZ, et al. Transhiatal chest drainage after esophagectomy. Ann R Coll Surg Engl 2007;89:535-6. [Crossref] [PubMed]
- Borrie AJ. Cardiac tamponade: a rare complication of Nissen fundoplication. ANZ J Surg 2018;88:E745 [Crossref] [PubMed]
- Cockbain AJ, Darmalingum A, Mehta SP. Acute deterioration after emergency paraesophageal hernia repair. Surgery 2016;159:1691-2. [Crossref] [PubMed]
- Lazopoulos G, Kalogerakos P, Kampitakis E, et al. Tension hydropneumopericardium following laparoscopic Nissen fundoplication. J Card Surg 2016;31:589. [Crossref] [PubMed]
- Mansour KA, Sharma J. Delayed intrathoracic rupture of herniated Nissen fundoplication: report of two cases. Ann Thorac Surg 2003;75:1957-9. [Crossref] [PubMed]
- Zhang LP, Chang R, Matthews BD, et al. Incidence, mechanisms, and outcomes of esophageal and gastric perforation during laparoscopic foregut surgery: a retrospective review of 1,223 foregut cases. Surg Endosc 2014;28:85-90. [Crossref] [PubMed]
- Imai TA, Soukiasian HJ. Management of complications in paraesophageal hernia repair. Thorac Surg Clin 2019;29:351-8. [Crossref] [PubMed]
- Urschel JD. Gastresophageal Leaks After Antireflux Operations. Ann Thorac Surg 1994;57:1229-32. [Crossref] [PubMed]
- Watkins JR, Farivar AS. Endoluminal therapies for esophageal perforations and leaks. Thorac Surg Clin. 2018;28:541-554. [Crossref] [PubMed]
- Salinas J, Georgiev T, González-Sánchez JA, et al. Gastric necrosis: a late complication of Nissen fundoplication. World J Gastrointest Surg 2014;6:183-6. [Crossref] [PubMed]
- Reyes-Zamorano J. Acute gastric volvulus: late complication of Nissen fundoplication. Report of two cases and review of the literature. Cir Cir 2014;82:541-50. [PubMed]
- Patel AD, Lin E, Lytle NW, et al. Combining laparoscopic giant paraesophageal hernia repair with sleeve gastrectomy in obese patients. Surg Endosc 2015;29:1115-22. [Crossref] [PubMed]
- Selima MA, Awad ZT, Filipi CJ. Hiatal Stenosis After Laparoscopic Nissen Fundoplication: A Report of 2 Cases. JSLS 2002;6:397-9. [PubMed]
- Bonavina L, Bona D, Saino G, et al. Pseudoachalasia occurring after laparoscopic Nissen fundoplication and crural mesh repair. Langenbecks Arch Surg 2007;392:653-6. [Crossref] [PubMed]
- Lai CN, Krishnan K, Kim MP, et al. Pseudoachalasia presenting 20 years after Nissen fundoplication: a case report. J Cardiothorac Surg 2016;11:96. [Crossref] [PubMed]
- Hui JM, Hunt DR, de Carle DJ, et al. Esophageal pneumatic dilation for post fundoplication dysphagia: safety, efficacy and predictors of outcome. Am J Gastroenterol 2002;97:2986-91. [Crossref] [PubMed]
- Brown AM, Nagle R, Pucci MJ, et al. Perioperative Outcomes and Quality of Life after Repair of Recurrent Hiatal Hernia Are Compromised Compared with Primary Repair. Am Surg 2019;85:556-60. [Crossref] [PubMed]
- Suppiah A, Sirimanna P, Vivian SJ, et al. Temporal patterns of hiatus hernia recurrence and hiatal failure: quality of life and recurrence after revision surgery. Dis Esophagus 2017;30:1-8. [Crossref] [PubMed]
- Carbo AI, Kim RH, Thomas Gates T, et al. Imaging Findings of Successful and Failed Fundoplication. Radiographics 2014;34:1873-84. [Crossref] [PubMed]
- Arevalo G, Wilkerson J, Saxe J. Acute Paraesophageal Hernia: Laparoscopic Repair With Adjunct T-Fastener Gastropexy for the High Operative Risk Patient. Surg Laparosc Endosc Percutan Tech 2018;28:123-7. [Crossref] [PubMed]
- Rochefort M, Wee JO. Management of the Difficult Hiatal Hernia. Thorac Surg Clin 2018;28:533-9. [Crossref] [PubMed]
- Antiporda M, Jackson C, Smith CD, et al. Strategies for surgical remediation of the multi-fundoplication failure patient. Surg Endosc 2019;33:1474-81. [Crossref] [PubMed]
- Bradley DD, Louie BE, Farivar AS, et al. Assessment and reduction of diaphragmatic tension during hiatal hernia repair. Surg Endosc 2015;29:796-804. [Crossref] [PubMed]
- Zhang C, Liu D, Li F, et al. Systematic review and meta-analysis of laparoscopic mesh versus suture repair of hiatus hernia: objective and subjective outcomes. Surg Endosc 2017;31:4913-4922. [Crossref] [PubMed]
- Nathanson L. Endoflip hiatal calibration during anterior partial fundoplication – Early outcomes. SAGES 2012 poster presentation.
- Wijnhoven BP, Watson DI, Devitt PG, et al. Laparoscopic Nissen fundoplication with anterior versus posterior hiatal repair: long-term results of a randomized trial. Am J Surg 2008;195:61-5. [Crossref] [PubMed]
- Shahzad K, Menon A. Routine versus selective contrast imaging to identify the need for early re-intervention following laparoscopic fundoplication: A retrospective cohort study. Int J Surg 2015;20:123-7. [Crossref] [PubMed]
- Oor JE, Broeders JA, Roks JD, et al. Reflux and Belching after Laparoscopic 270 degree Posterior Versus 180 degree Anterior Partial Fundoplication. J Gastrointest Surg 2018;22:1852-60. [Crossref] [PubMed]
- Pauwels A, Boecxstaens V, Broers C, et al. Severely impaired gastric accommodation is a hallmark of post-Nissen functional dyspepsia symptoms. Neurogastroenterol Motil 2017;29:10. [Crossref] [PubMed]
- Inoue M, Uchida K, Otake K, et al. Development of acute pancreatitis after Nissen fundoplication. Pediatr Int 2015;57:e48-9. [Crossref] [PubMed]
- Steele SR, Martin MJ, Garafalo T, et al. Superior mesenteric vein thrombosis following laparoscopic Nissen fundoplication. JSLS 2003;7:159-63. [PubMed]
- Petrosyan M, Estrada JJ, Giuliani S. Gastric perforation and pancreatitis manifesting after an inadvertent Nissen fundoplication in a patient with superior mesenteric artery syndrome. Case Rep Med 2009;2009:426162 [Crossref] [PubMed]
- Schwameis K, Zehetner J, Rona K, et al. Post-Nissen Dysphagia and Bloating Syndrome: Outcomes After Conversion to Toupet Fundoplication. J Gastrointest Surg 2017;21:441-5. [Crossref] [PubMed]
- Strong AT, Landreneau JP, Cline M, et al. Per-oral pyloromyotomy (POP) for medically refractory post-surgical gastroparesis. J Gastrointest Surg 2019;23:1095-103. [Crossref] [PubMed]
- Landreneau JP, Strong AT, Kroh MD, et al. Minimal invasive Roux-en-Y reconstruction as a salvage operation after failed nissen fundoplication. Surg Endosc 2020;34:2211-8. [Crossref] [PubMed]
- Skancke M, Brody F, Haskins IN, et al. Impact of operative times and mesh utilization on paraesophageal hernia repair: Analyses of 30-day outcomes from the American College of Surgeons National Surgical Quality Improvement Project Database. J Laparoendosc Adv Surg Tech A 2019;29:303-8. [Crossref] [PubMed]
- Tatum RP, Shalhub S, Oelschlager BK, et al. Complications of PTFE mesh at the diaphragmatic hiatus. J Gastrointest Surg 2008;12:953-7. [Crossref] [PubMed]
- Zügel N, Lang RA, Kox M, et al. Severe complication of laparoscopic mesh hiatoplasty for paraesophageal hernia. Surg Endosc 2009;23:2563-7. [Crossref] [PubMed]
- Chun CL, Dunnington G, Triadafilopoulos G. Where is the acid coming from? Esophago-gastric fistula following laparoscopic Nissen. Dig Dis Sci 2013;58:1486-90. [Crossref] [PubMed]
- Cole W, Zagorski S. Intramural gastric abscess following laparoscopic paraesophageal hernia repair. Endoscopy 2015;47 Suppl 1 UCTN:E227-8.
- Odabasi M, Abuoglu H, Arslan C, et al. Asymptomatic partial splenic infarction in laparoscopic floppy Nissen fundoplication and brief literature review. Int Surg 2014;99:291-4. [Crossref] [PubMed]
- Le K, Griner D, Hope W, et al. Splenic torsion requiring splenectomy six years following laparoscopic Nissen fundoplication. JSLS 2012;16:184-8. [Crossref] [PubMed]
- Dijkman KP, van Heurn LW, Leroy PL, et al. Vanishing spleen after Nissen fundoplication: a case report. Eur J Pediatr 2009;168:355-7. [Crossref] [PubMed]
Cite this article as: Botha AJ, Di Maggio F. Management of complications after paraesophageal hernia repair. Ann Laparosc Endosc Surg 2021;6:38.



