
Laparoscopic Roux-en-Y gastric bypass for the management of hiatal hernia and failed fundoplication in patients with obesity
Introduction
The United States has one of the highest rates of obesity in the world, with more than 30% of the adult population having a BMI greater than 30 kg/m2 (1). Gastroesophageal reflux disease is one of the most common comorbidities of obesity, with rates ranging from 22% to 70% (2). The pathophysiologic contributors to this association are many (3). One such contributor is the higher rate of hiatal hernias (HH), which in patients with obesity can be up to 37% (4). The majority of HH (over 95%) are sliding, or Type 1 HH. Type II-IV paraesophageal hernias (PEH) constitute the remainder (5). Laparoscopic HH repair with fundoplication has proven to be a definitive and safe treatment for PEH and symptomatic HH in the general population (6,7). However, the literature demonstrates that the long-term success rate of this procedure in obese patients with PEH is low (8,9). On the other hand, weight loss surgery, specifically a Roux-en-Y gastric bypass (RYGB) has proven to be effective in improving GERD. This video article advocates for the use of RYGB in obese patients presenting with HH or with history of failed fundoplication. This article reviews the laparoscopic HH repair and concurrent LRYGB technique for both scenarios.
Association of obesity with GERD and hiatal hernia
Patients develop GERD when the anatomic barriers to reflux are disrupted. The lower esophageal sphincter (LES), the angle of His, and gastric sling fibers make up some of the essential structures. The crural pillars play an important role in maintenance of the anti-reflux barriers of the esophagus and proximal stomach. Ayazi et al. showed that an increase in BMI was associated with increases in defective LES function as well as HH (10). In fact, in a retrospective case control study of 1,389 patients who underwent upper endoscopy, patients with BMI greater than 30 kg/m2 were 4 times more likely to have HH (11). Prospectively collected manometric data from 285 patients showed that obese patients were more likely to have increased intragastric pressures and GE junction disruption, which are risk factors for hiatal herniation (12).
Anti-reflux surgery addresses GERD and HH by restoring these anatomic barriers to reflux. In addition to the fundoplication, achieving adequate intra-abdominal esophagus and approximating the crura are key components of the HH repair (Video 1) (13). The laparoscopic Nissen fundoplication has been shown to be safe and effective therapy for the treatment of GERD (14,15). In fact, in a randomized control trial of 78 patients presenting to VA hospitals with GERD refractory to PPI, surgery was more effective than medical management (16). With recurrence rates less than 10% and long-term satisfaction scores as high as 96%, this procedure is universally accepted for the treatment of GERD and HH in non-obese patients. The Linx procedure, another anti-reflux intervention, has also shown promising results. At 5 years follow-up, these patients reported a decrease in daily PPI use from 100% to 15% in one study (14).
Unfortunately, the data to support anti-reflux surgery in obese patients is not as consistent. There is a lack of consensus due to studies having variable follow up, inadequate representation of morbidly obese patients, and low statistical power. However, the preponderance of data demonstrate that fundoplication is not as successful in obese patients. For example, in a study by Morgenthal et al., pre-operative BMI greater than 35 kg/m2 was a predictor of failure in obese patients undergoing laparoscopic Nissen fundoplication (9). Another study reported an overall symptomatic recurrence rate of 31.3% in obese patients compared to 4% in normal-weight patients (8). Interestingly, in a study describing anatomic findings in obese patients undergoing conversion from anti-reflux procedure to RYGB, there was an increased incidence of wrap disruption compared to herniated wrap, suggesting a different mechanism of failure compared to non-obese patients (17).
Repairing a HH can be done with a suture cruroplasty alone or with mesh reinforcement (Figure 1). There have been 5 RCTs to date, as summarized in a meta-analysis and systematic review by Memon et al., in which the recurrence rates between the two techniques are compared (18). The only trial with long term follow up of 5 years reported the most alarming recurrence rates of 59% and 54% respectively (19). Notably, the mean BMI in this trial was 31 kg/m2. Considering the fact that morbidly obese patients have a known increased rate of abdominal wall hernia recurrence, the HH recurrence rates reported in the aforementioned RCTs likely underestimate the true recurrence rate in the obese population (20).
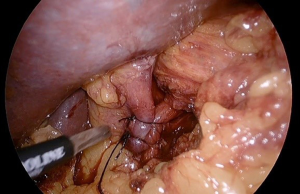
Concurrent bariatric surgery with hiatal hernia repair
Due to these concerns with traditional anti-reflux surgery in the obese population, bariatric surgeons often perform a concomitant weight loss procedure when addressing a HH in an obese patient. Bariatric surgery has two theoretical mechanisms of improving the success of a HH repair. First, bariatric surgery alone has been shown to improve GERD symptoms and decrease antacid use, with some studies suggesting better efficacy than medical therapy alone (15,21-23). Secondly, the weight loss that is induced ameliorates intraabdominal pressure, which is a significant risk for hernia repair failure. Surgeons have previously avoided repairing HH during RYGB due to concerns over increased morbidity and the belief that RYGB would resolve GERD without having to repair the HH (24). However, Kothari et al. established that HH during RYGB does not increase 30-day morbidity or mortality compared to patients who only undergo RYGB (25). Chaudhry et al. argued that concurrent HH repair with RYGB is not only safe, but also effective. At median follow up of 3 years, patients who underwent concomitant RYGB with HH repair had reductions in Gastroesophageal Reflux Disease Health-Related Quality of Life (GERD-HRQL) scores and decrease in antisecretory medications (26). Although there are no prospective controlled trials directly comparing cruroplasty vs. no cruroplasty for HH during RYGB, Borbély et al. reported objective findings in a series of 47 patients with persistent GERD symptoms following RYGB. They showed that 53% of these patients had a HH and 58% had a hypotensive LES (27). It should also be noted that failing to repair the hiatal hernia during RYGB has its own risks. Albeit rare, there are cases of patients presenting with obstructed roux limbs due to herniation. Moreover, repairing a HH containing an incarcerated gastric pouch is theoretically more dangerous than repairing the HH at the initial RYGB (28). Based on this data there, HH’s encountered during a RYGB should be definitively addressed.
In addition to the review by Kothari et al., database analyses of both the National Surgical Quality Improvement Program (NSQIP) and the Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program have reported on the safety of concurrent bariatric surgery with hiatal hernia repair. Both studies demonstrated similar 30-day morbidity and mortality rates between concurrent HH repair with bariatric surgery compared to bariatric surgery alone (29,30). In a subgroup analysis, the NSQIP study found that LSG in conjunction with HH repair had lower morbidity than RYGB with HH repair and the authors concluded that LSG should therefore be preferred when considering weight loss surgery during hiatal hernia repair. However, as articulated in the MBSAQIP retrospective review of over 80,000 patients, the increase in morbidity is likely due to the inherent higher morbidity of performing a RYGB compared to LSG (29,30).
More importantly, there is data to suggest LSG has the potential to worsen GERD, or even cause de novo GERD (31,32). LSG has become the most common bariatric procedure, accounting for about 70% of all weight loss procedures performed today (33). Yet at the 4th international consensus, GERD was the most commonly reported complication after LSG (33). In a systematic review of GERD rates after LSG, no agreement was achieved (34). More recently, an additional systematic review published in 2016 showed there was a trend in increased GERD prevalence after LSG, but again no definitive conclusions were reached (35). The difficulty in reaching conclusions among these publications are their variability in definitions of GERD, follow up times, and objective vs. subjective diagnostic measures of GERD. Despite this lack of agreement, some of the larger reviews remain troubling. For example, the rate of de novo GERD after LSG was found to be 8.6% and 9.3% in two respective reviews (31,36). Perhaps more concerning, there is an alarming increase in the incidence of Barrett’s in this population (37-39). Despite these data, albeit variable, only 23.3% of bariatric surgeons at the fifth international consensus conference on sleeve gastrectomy believed GERD is a contraindication to LSG (40). There are several factors that may contribute to the mechanism of increasing GERD after LSG (41). For example, the angle of His and gastric sling fibers are disrupted, and basal LES pressures are decreased (41). Also, in the setting of a hiatal hernia repair, the sleeve is not anchored into the abdomen the way a gastrojejunostomy anchors the gastric pouch in a RYGB. Because the diameter of the sleeve is essentially the same as the hiatal opening this can allow for easier re-herniation of the stomach.
With regard to RYGB, the literature supporting its utility in managing obese patients with GERD is more consistent. For example, Frezza et al. showed a decrease in reported heartburn symptoms from 87% to 22% as well as a decrease in antacid usage in a cohort of 152 obese patients (21). In a systematic review of bariatric surgery and GERD, RYGB was found to have a significant decrease in GERD compared to other bariatric procedures (42). Consistent with this finding, The Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) Foregut Task Force has determined that RYGB is the preferred surgical treatment for GERD in obese patients (43).
Conversion of failed fundoplication
The RYGB has not only demonstrated success in the primary management of GERD and HH in obese patients. The utility of this procedure can also be extended to the management of failed fundoplications (Figure 2). Of the patients who have undergone anti-reflux surgery, 3–10% require re-operative anti-reflux surgery (44). Furthermore, some of these patients go on to have multiple subsequent re-operative anti-reflux procedures. For example, in a study of 275 redo operations, Awais et al. showed that 11% needed another redo surgery at median follow up of 3.3 year (45). Redo anti-reflux surgery in general carries a higher intraoperative and postoperative complication rate compared to primary anti-reflux surgery (46). Satisfaction rates are also not as high after redo surgery (44). Among obese patients, treating a failed fundoplication with a RYGB has increased in popularity, and has proven to be successful. In one study, among 45 patients with mean BMI of 33 and history of failed fundoplication, 93.3% were symptom free at 11 months follow-up after RYGB (47). Similarly, Stefanidis et al. showed a 96% satisfaction rate after conversion RYGB, reflecting similar rates to primary anti-reflux surgery (48).
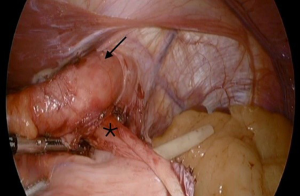
When performing RYGB for a failed fundoplication, the procedure begins with the same key components that would be performed during a redo fundoplication. For example, restoring normal anatomy by returning the GE junction 3 cm back into the abdomen, as well as taking down the fundoplication and returning the fundus to its normal position (Video 2) (44). Once normal anatomy is established and cruroplasty is accomplished, the surgeon proceeds by creating the gastric pouch. When operating on a patient with history of a prior fundoplication, the fundus may need to be resected from the remnant stomach as its blood supply is often compromised (Video 3).
Conclusions
GERD and HH present a challenge in surgical management of the patient with obesity. Although fundoplication has high success rates in the nonobese population, the rate of failure in obese patients warrants a reconsideration in using this procedure for all patients. RYGB has proved to be successful at treating GERD and should be considered the procedure of choice in the appropriate obese patient with a hiatal hernia. LSG is very popular among bariatric surgeons at present, but should be avoided in obese patients presenting with a hiatal hernia or history of GERD. RYGB is safe when performed concurrently with HH repair and effective when treating a failed fundoplication. As the rate of LSG has surpassed RYGB worldwide, we should not forget the utility of the RYGB when treating obese patients with GERD and HH.
Acknowledgments
Funding: Rory Carroll, MD is funded by NIH T32 CA148062 grant.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales-20-75). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hales CM, Carroll MD, Fryar CD, et al. Prevalence of Obesity Among Adults and Youth: United States, 2015-2016. NCHS Data Brief 2017;1-8. [PubMed]
- Prachand VN, Alverdy JC. Gastroesophageal reflux disease and severe obesity: Fundoplication or bariatric surgery? World J Gastroenterol 2010;16:3757-61. [Crossref] [PubMed]
- Frezza EE, Shebani KO, Robertson J, et al. Morbid obesity causes chronic increase of intraabdominal pressure. Dig Dis Sci 2007;52:1038-41. [Crossref] [PubMed]
- Che F, Nguyen B, Cohen A, et al. Prevalence of hiatal hernia in the morbidly obese. Surg Obes Relat Dis 2013;9:920-4. [Crossref] [PubMed]
- Landreneau RJ, Del Pino M, Santos R. Management of paraesophageal hernias. Surg Clin North Am 2005;85:411-32. [Crossref] [PubMed]
- Kubasiak J, Hood KC, Daly S, et al. Improved patient outcomes in paraesophageal hernia repair using a laparoscopic approach: a study of the national surgical quality improvement program data. Am Surg 2014;80:884-9. [Crossref] [PubMed]
- Kohn GP, Price RR, DeMeester SR, et al. Guidelines for the management of hiatal hernia. Surg Endosc 2013;27:4409-28. [Crossref] [PubMed]
- Perez AR, Moncure AC, Rattner DW. Obesity adversely affects the outcome of antireflux operations. Surg Endosc 2001;15:986-9. [Crossref] [PubMed]
- Morgenthal CB, Lin E, Shane MD, et al. Who will fail laparoscopic Nissen fundoplication? Preoperative prediction of long-term outcomes. Surg Endosc 2007;21:1978-84. [Crossref] [PubMed]
- Ayazi S, Hagen JA, Chan LS, et al. Obesity and gastroesophageal reflux: quantifying the association between body mass index, esophageal acid exposure, and lower esophageal sphincter status in a large series of patients with reflux symptoms. J Gastrointest Surg 2009;13:1440-7. [Crossref] [PubMed]
- Wilson LJ, Ma W, Hirschowitz BI. Association of obesity with hiatal hernia and esophagitis. Am J Gastroenterol 1999;94:2840-4. [Crossref] [PubMed]
- Pandolfino JE, El-Serag HB, Zhang Q, et al. Obesity: a challenge to esophagogastric junction integrity. Gastroenterology 2006;130:639-49. [Crossref] [PubMed]
- DeMeester SR. Laparoscopic paraesophageal hernia repair: critical steps and adjunct techniques to minimize recurrence. Surg Laparosc Endosc Percutan Tech 2013;23:429-35. [Crossref] [PubMed]
- Ganz RA, Edmundowicz SA, Taiganides PA, et al. Long-term Outcomes of Patients Receiving a Magnetic Sphincter Augmentation Device for Gastroesophageal Reflux. Clin Gastroenterol Hepatol 2016;14:671-7. [Crossref] [PubMed]
- Stefanidis D, Hope WW, Kohn GP, et al. Guidelines for surgical treatment of gastroesophageal reflux disease. Surg Endosc 2010;24:2647-69. [Crossref] [PubMed]
- Spechler SJ, Hunter JG, Jones KM, et al. Randomized Trial of Medical versus Surgical Treatment for Refractory Heartburn. N Engl J Med 2019;381:1513-23. [Crossref] [PubMed]
- Kellogg TA, Andrade R, Maddaus M, et al. Anatomic findings and outcomes after antireflux procedures in morbidly obese patients undergoing laparoscopic conversion to Roux-en-Y gastric bypass. Surg Obes Relat Dis 2007;3:52-7; discussion 58-9. [Crossref] [PubMed]
- Memon MA, Siddaiah-Subramanya M, Yunus RM, et al. Suture Cruroplasty Versus Mesh Hiatal Herniorrhaphy for Large Hiatal Hernias (HHs): An Updated Meta-Analysis and Systematic Review of Randomized Controlled Trials. Surg Laparosc Endosc Percutan Tech 2019;29:221-32. [Crossref] [PubMed]
- Oelschlager BK, Pellegrini CA, Hunter JG, et al. Biologic prosthesis to prevent recurrence after laparoscopic paraesophageal hernia repair: long-term follow-up from a multicenter, prospective, randomized trial. J Am Coll Surg 2011;213:461-8. [Crossref] [PubMed]
- Sauerland S, Korenkov M, Kleinen T, et al. Obesity is a risk factor for recurrence after incisional hernia repair. Hernia 2004;8:42-6. [Crossref] [PubMed]
- Frezza EE, Ikramuddin S, Gourash W, et al. Symptomatic improvement in gastroesophageal reflux disease (GERD) following laparoscopic Roux-en-Y gastric bypass. Surg Endosc 2002;16:1027-31. [Crossref] [PubMed]
- Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol 2013;108:308-28; quiz 29. [Crossref] [PubMed]
- Schietroma M, Piccione F, Clementi M, et al. Short- and Long-Term, 11-22 Years, Results after Laparoscopic Nissen Fundoplication in Obese versus Nonobese Patients. J Obes 2017;2017:7589408 [Crossref] [PubMed]
- Flanagin BA, Mitchell MT, Thistlethwaite WA, et al. Diagnosis and treatment of atypical presentations of hiatal hernia following bariatric surgery. Obes Surg 2010;20:386-92. [Crossref] [PubMed]
- Kothari V, Shaligram A, Reynoso J, et al. Impact on perioperative outcomes of concomitant hiatal hernia repair with laparoscopic gastric bypass. Obes Surg 2012;22:1607-10. [Crossref] [PubMed]
- Chaudhry UI, Marr BM, Osayi SN, et al. Laparoscopic Roux-en-Y gastric bypass for treatment of symptomatic paraesophageal hernia in the morbidly obese: medium-term results. Surg Obes Relat Dis 2014;10:1063-7. [Crossref] [PubMed]
- Borbély Y, Kröll D, Nett PC, et al. Radiologic, endoscopic, and functional patterns in patients with symptomatic gastroesophageal reflux disease after Roux-en-Y gastric bypass. Surg Obes Relat Dis 2018;14:764-8. [Crossref] [PubMed]
- Caceres M, Eid GM, McCloskey CA. Recurrent paraesophageal hernia presenting as obstruction of Roux limb after Roux-en-Y gastric bypass. Surg Obes Relat Dis 2010;6:197-9. [Crossref] [PubMed]
- Shada AL, Stem M, Funk LM, et al. Concurrent bariatric surgery and paraesophageal hernia repair: comparison of sleeve gastrectomy and Roux-en-Y gastric bypass. Surg Obes Relat Dis 2018;14:8-13. [Crossref] [PubMed]
- Hefler J, Dang J, Mocanu V, et al. Concurrent bariatric surgery and paraesophageal hernia repair: an analysis of the Metabolic and Bariatric Surgery Association Quality Improvement Program (MBSAQIP) database. Surg Obes Relat Dis 2019;15:1746-54. [Crossref] [PubMed]
- Gu L, Chen B, Du N, et al. Relationship Between Bariatric Surgery and Gastroesophageal Reflux Disease: a Systematic Review and Meta-analysis. Obes Surg 2019;29:4105-13. [Crossref] [PubMed]
- Himpens J, Dobbeleir J, Peeters G. Long-term results of laparoscopic sleeve gastrectomy for obesity. Ann Surg 2010;252:319-24. [Crossref] [PubMed]
- Angrisani L, Santonicola A, Iovino P, et al. IFSO Worldwide Survey 2016: Primary, Endoluminal, and Revisional Procedures. Obes Surg 2018;28:3783-94. [Crossref] [PubMed]
- Chiu S, Birch DW, Shi X, et al. Effect of sleeve gastrectomy on gastroesophageal reflux disease: a systematic review. Surg Obes Relat Dis 2011;7:510-5. [Crossref] [PubMed]
- Oor JE, Roks DJ, Unlu C, et al. Laparoscopic sleeve gastrectomy and gastroesophageal reflux disease: a systematic review and meta-analysis. Am J Surg 2016;211:250-67. [Crossref] [PubMed]
- DuPree CE, Blair K, Steele SR, et al. Laparoscopic sleeve gastrectomy in patients with preexisting gastroesophageal reflux disease: a national analysis. JAMA Surg 2014;149:328-34. [Crossref] [PubMed]
- Genco A, Soricelli E, Casella G, et al. Gastroesophageal reflux disease and Barrett's esophagus after laparoscopic sleeve gastrectomy: a possible, underestimated long-term complication. Surg Obes Relat Dis 2017;13:568-74. [Crossref] [PubMed]
- Sebastianelli L, Benois M, Vanbiervliet G, et al. Systematic Endoscopy 5 Years After Sleeve Gastrectomy Results in a High Rate of Barrett's Esophagus: Results of a Multicenter Study. Obes Surg 2019;29:1462-9. [Crossref] [PubMed]
- Felsenreich DM, Kefurt R, Schermann M, et al. Reflux, Sleeve Dilation, and Barrett's Esophagus after Laparoscopic Sleeve Gastrectomy: Long-Term Follow-Up. Obes Surg 2017;27:3092-101. [Crossref] [PubMed]
- Gagner M, Hutchinson C, Rosenthal R. Fifth International Consensus Conference: current status of sleeve gastrectomy. Surg Obes Relat Dis 2016;12:750-6. [Crossref] [PubMed]
- Braghetto I, Lanzarini E, Korn O, et al. Manometric changes of the lower esophageal sphincter after sleeve gastrectomy in obese patients. Obes Surg 2010;20:357-62. [Crossref] [PubMed]
- De Groot NL, Burgerhart JS, Van De Meeberg PC, et al. Systematic review: the effects of conservative and surgical treatment for obesity on gastro-oesophageal reflux disease. Aliment Pharmacol Ther 2009;30:1091-102. [Crossref] [PubMed]
- Nau P, Jackson HT, Aryaie A, et al. Surgical management of gastroesophageal reflux disease in the obese patient. Surg Endosc 2020;34:450-7. [Crossref] [PubMed]
- Grover BT, Kothari SN. Reoperative antireflux surgery. Surg Clin North Am 2015;95:629-40. [Crossref] [PubMed]
- Awais O, Luketich JD, Schuchert MJ, et al. Reoperative antireflux surgery for failed fundoplication: an analysis of outcomes in 275 patients. Ann Thorac Surg 2011;92:1083-9; discussion 1089-90. [Crossref] [PubMed]
- Furnée EJ, Draaisma WA, Broeders IA, et al. Surgical reintervention after failed antireflux surgery: a systematic review of the literature. J Gastrointest Surg 2009;13:1539-49. [Crossref] [PubMed]
- Kim M, Navarro F, Eruchalu CN, et al. Minimally invasive Roux-en-Y gastric bypass for fundoplication failure offers excellent gastroesophageal reflux control. Am Surg 2014;80:696-703. [Crossref] [PubMed]
- Stefanidis D, Navarro F, Augenstein VA, et al. Laparoscopic fundoplication takedown with conversion to Roux-en-Y gastric bypass leads to excellent reflux control and quality of life after fundoplication failure. Surg Endosc 2012;26:3521-7. [Crossref] [PubMed]
Cite this article as: Carroll R, Fontan F, Lehmann R, Smith J, Nau P. Laparoscopic Roux-en-Y gastric bypass for the management of hiatal hernia and failed fundoplication in patients with obesity. Ann Laparosc Endosc Surg 2021;6:23.


