
Complete mesocolic excision for right colon cancer—state of art: a systematic review of the literature
Introduction
Colon cancer (CC) is the third most commonly diagnosed cancer across countries but the second in terms of mortality; its incidence is estimated of 1,096,601 number of new cases per year and 881,000 deaths are estimated to occur in 2018 (1). Right-sided cancers (RSCs) are most commonly defined as cancers arising proximal to the splenic flexure while left-sided cancers (LSCs) are located distal to the splenic flexure (2-4). This cut point is often used because most of the transverse colon arises embryologically from the midgut, and only the distal third of it arises from the hindgut. Vascular supply has also been proposed as a defining characteristic of embryologic origin, the superior and inferior mesenteric arteries supplying the midgut and hindgut, respectively. Epidemiological studies have demonstrated gender and age relationship with a higher incidence of RSC in women and elderly people (5,6). There are also differences in pathologic appearance (7-10), and in the molecular biological pattern (11,12). Therefore, it has been suggested to consider colorectal cancer as three distinct tumor entities: RSC, LSC and rectal cancer. There is differential prognosis by stage between patients with right- and left-sided CCs. In literature stage II RSC is reported with a slightly better prognosis as compared to LSC due to a higher prevalence of good-prognosis MSI-high tumors, while stage III RSC has a slightly worse prognosis (3,4,8). Moreover, analyses of prospective clinical trials of patients with stage III colorectal cancer who received adjuvant chemotherapy also demonstrated lower disease-free survival (DFS) in those with right-sided location (HR, 0.70; 95% CI, 0.61–0.81) (13). Patients with metastatic RSC have worse prognosis as compared to those with LSC as well (14).
Before 90’s also rectal cancer had a poor prognosis because of high local recurrence rate. In 1988, Heald (15) introduced the concept of total mesorectal excision (TME) for rectal cancer which is based upon sharp dissection following embryological anatomical planes (16,17). TME provides a surgical specimen with an intact coverage, not only of the rectal tumor, but also of the main lymphatic drainage including the majority of regional lymph nodes, lymphatic vessels and surrounding fat tissue lying within mesorectum. Thereafter the development, standardization and widespread adoption of TME, surgery for rectal cancer has been reported with significant reduction of local recurrence rates and improvement of survival (15,18,19).
In 2009, Hohenberger (20) aimed at improving the outcome of patients with RSC by developing the same concept of TME also for RSC.
Therefore Hohenberger introduced the idea of complete mesocolic excision (CME) that is a new conception of right hemicolectomy for RSC based on three main issues: dissection of the embryological plane to remove a complete envelope containing the mesentery together with all the lymph nodes draining the tumor, a central vascular tie to remove the main lymph nodes in the central direction and resection of a sufficient length of bowel to remove the pericolic lymph nodes (20). The objectives of CME were to reduce local recurrence and to improve survival rates. The rationale behind this new concept of surgery for RSC was based on several points: the lymph node metastases of CC follow the supplying arteries; several studies have shown a survival benefit to higher lymph node yields after colonic resection (21); increasing negative lymph node count also correlates with survival in advanced colonic cancer (22,23) and finally, the ratio of lymph node metastases to the total number of harvested lymph nodes, known as the lymph node ratio (LNR), has been reported in several studies to be a better prognostic indicator than the number of involved lymph nodes, or pN status, alone (24).
In this last decade, many doubts and questions have been arised about safety and efficacy of CME together with its worldwide spread. Many authors have questioned whether a survival benefit from a greater lymph node yield truly exists considering that other factors are known to affect lymph node retrieval including patient age, immune status, tumor location, tumor characteristics and institutional factors (25,26).
Several studies have suggested that there isn’t any or only a minimal survival benefit in lymph node yield greater than 12 compared to that less than 12, particularly in the presence of good quality surgery and lymph node examination and that it is difficult to define a threshold number for lymph node retrieval (27,28).
Furthermore, several non-CME studies have failed to show a relationship between high vascular tie, and therefore greater lymph node yield and higher number of lymph node metastases, and/or improved survival (29,30). Others argue that metastases in lymph nodes outside conventional ranges of dissection represent distant metastases, and the extended resection will not influence the survival as they are related to a poor oncological outcome (31).
The aim of this systematic review was to investigate the safety, quality and outcomes of CME in patients with RSC.
We present the following article in accordance with the PRISMA reporting checklist (available at http://dx.doi.org/10.21037/ales-20-41).
Material and methods
Literature search and systematic review were done adhering to the Cochrane Collaboration guidance (32) to reduce the risk of bias and error. The systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (33). Data collection, randomized controlled trials (RCTs) and non-randomized controlled studies (non-RCSs) concerning CME were searched for this review. No language restrictions were adopted during articles search. This study was registered with PROSPERO (187448).
Search strategy
A systematic search of PubMed, Embase and the Cochrane Library was conducted on 12 December 2019.
The Cochrane database was searched using a combination of the following terms with the Boolean AND/OR operators: “Colonic Neoplasms”, “Colectomy”, “Colon”, “complete-mesocolic-excis”, “CME”, “central-vascular-ligat”, “D3”, “lymphadenect”, “lymph-nod”, “Lymph Node Excision”, “right”, “ileocol”, “ileo-col”.
For the PubMed and Embase database searches, these same keywords (and variants) were used as text words and Medical Subject Headings (MeSH terms), and were combined by using Boolean operators as follows: (“Colonic Neoplasms” OR “Colectomy” OR “Colon” OR colon*[tiab] OR colectom*[tiab]) AND (complete-mesocolic-excis*[tiab] OR CME[tiab] OR central-vascular-ligat*[tiab] OR (D3[tiab] AND (lymphadenect*[tiab] OR lymph-nod*[tiab] OR “Lymph Node Excision”))) AND (right*[tiab] OR ileocol*[tiab] OR ileo-col*[tiab]).
Outcomes of interest
- Safety of CME, including intra-operative and postoperative surgical complications and postoperative mortality;
- Quality of CME, including total number of lymph nodes retrieved, total number of metastatic lymph nodes; mean ileo-colic and middle colic main trunk vessels lengths; total area and integrity rate (%) of resected mesocolon; details of mesocolic resection;
- Survival Outcomes, including overall survival (OS) and DFS at 3 and 5 years.
Inclusion and exclusion criteria
Inclusion criteria were as follows
Only English articles were selected at the end. The inclusion criteria were the following:
- Studies including patients with pathologically verified CC;
- Studies with satisfactorily definition of CME technique, including a description of dissection in the embryologic mesocolic fascial planes and central vascular ligation (CVL) or D3 lymphadenectomy;
- Studies including at least one outcome of interest among their results.
Exclusion criteria were as follows
- Reviews and systematic reviews;
- Studies with abstract only, videos, oral communications, case reports and letters;
- Studies with less than 100 patients included;
- Studies from the same institution or overlapping patients (in these cases, we selected the study that included more patients or that was published later).
Studies selection
Our systematic review was conducted using Mendeley software. Five independent reviewers screened titles and abstracts identified by literature search. A total of 659 potentially suitable articles were initially identified. The first step was to merge the duplicates, resulting in 516 remnant studies. In the second step these studies were checked for the pertinence by reading the titles and analyzing their abstracts. All studies excluded at this second step were documented along with the reasons for exclusion (Figure 1). Any discrepancies among reviewers were solved through consensus.
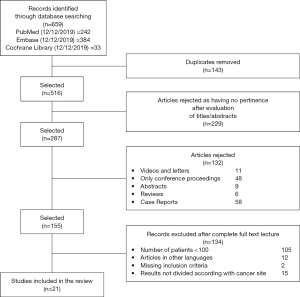
Full papers of selected abstracts were assessed to confirm whether also the full papers totally met inclusion criteria.
Data extraction was performed by five reviewers (FE, GO, LMR, LS, FS) and checked by a sixth reviewer (RR). Selected papers were identified by publication year and by the surname of the first author.
Results
Literature searches and inclusion assessment
Figure 1 summarizes the process of identification and selection of papers for inclusion in this systematic review, following the PRISMA guidelines (33).
Literature searches of electronic databases identified 659 articles. After de-duplication, 516 titles/abstracts were screened by reviewers and 229 articles were excluded as having no pertinence to this systematic review. Titles/abstracts of 287 potentially relevant papers were included for further evaluation. One hundred thirty-two studies have been excluded because they were reviews, abstracts, videos, oral communications, case reports and letters. Finally, 105 papers were excluded because the number of included patients was less than 100. Of these, 29 further papers were left out after examining in detail the full paper; the reasons for rejection are detailed in Figure 1.
Overall, we identified 21 studies about CME properly fulfilling inclusion and exclusion criteria (34-53).
Study characteristics
Of the 21 included studies, seven were from Korea, five from Europe, five from China, one was from Japan, one from Taiwan, one from North America and one from India. Most of the included papers were retrospective cohort studies and only two of them were prospective studies. The total number of included patients was 7,402 (Table 1).
Table 1
| Reference, year | Nation/region | Type of publication | Study period | N | Age (years), [range] | Gender, n male/n female (%) | Type of approach, n (%) | Operative time (min) | LOS (days) |
|---|---|---|---|---|---|---|---|---|---|
| Pramateftakis (50), 2010 | Greece | Retrospective | 1989–2008 | 115 | 65.6 [35–86] | 65 (56.52)/50 (43.48) | Open 115 (100.00) | NR | NR |
| Kang (40), 2014 | South Korea | Prospective | 2009–2012 | 128 | 66 [30–86] | 63 (49.22)/65 (50.78) | LS 128 (100.00) | 192 [118–363] | 5 [4–37] |
| Bae (37), 2014 | Korea | Prospective | 2006–2008 | 170 | 64.5 [29–94] | 92 (54.12)/78 (45.88) | LS 85 (50.00); open 85 (50.00) | 186.5 [41–664] | 11 [6–65] |
| Liang (42), 2015 | Taiwan | Retrospective | 2003–2008 | 244 | 64.48 [36–88] | NR | LS 244 (100.00) | 224 [168– 280] | 11 [8–14] |
| Cho (54), 2015 | Korea | Retrospective | 2000–2009 | 773 | 61.5 [39–84] | 421 (54.46)/352 (45.54) | MIS 205 (26.52); open 568 (73.48) | NR | 13.51 [2–27] |
| Subbiah (38), 2016 | India | Retrospective | 2009–2013 | 212 | 61 [34–82] | 163 (76.89)/49 (23.11) | LS 212 (100.00) | 142 | 5 |
| Huang (39), 2015 | China | Retrospective | 2006–2013 | 102 | 55.52 [39–71] | 61 (59.80)/41 (40.20) | LS 53 (51.96); open 49 (48.04) | 185.83 [75–308] | 12.44 [2–26] |
| Takahashi (36), 2017 | Japan | Retrospective | 2008–2014 | 202 | 57.75 [49–92] | 101 (50.00)/101 (50.00) | LS 202 (100.00) | 160.46 [157– 234] | NR |
| Siani (51), 2017 | Italy | Retrospective | 2008–2015 | 600 | 72 [57–87] | 333 (55.50)/267 (44.50) | LS 600 (100.00) | 149 [91–207] | 2.4 [1–6] |
| Kim (34), 2016 | South Korea | Retrospective | 2008–2013 | 215 | 67.92 [47–91] | 109 (50.70)/106 (49.30) | LS 116 (53.95); open 99 (46.05) | 175 [35–315] | 13.30 [1–40] |
| Sheng (48), 2017 | China | Retrospective | 2012–2014 | 150 | 61.20 [39–82] | 83 (55.33)/67 (44.67) | HAL-CME 78 (52.00); open 72 (48.00) | 143.52 [100–196] | 8,35 [5–13] |
| Kim (49), 2017 | Korea | Retrospective | 1995–2010 | 142 | 63.5 [41–86] | 66 (46.48)/76 (53.52) | LS 142 (100.00) | 309.0 [160–458] | 11.2 [2–20] |
| Shin (43), 2018 | South Korea | Retrospective | 2000–2013 | 2,249 | 61 median | 988 (43.93)/1,261 (56.07) | LS 1,010 (44.91); open 1,239 (55.09) | 150 median | 10.62 |
| Wang (52), 2017 | China | Retrospective | 2010–2015 | 172 | 67 [43–91] | 94 (54.75)/78 (45.35) | LS 172 (100.00) | 113.5 [44.7–182.3] | 8.7 [4.5–12.9] |
| Bertelsen (46), 2019 | Denmark | Retrospective | 2008–2013 | 256 | 73.5 median | 116 (45.31)/140 (54.69) | LS 107 (41.80); open 149 (58.20) | NR | NR |
| Li (35), 2018 | China | Retrospective | 2012–2018 | 108 | 67,26 [55–77] | 65 (60.18)/43(39.81) | LS 108 (100.00) | 135.74 [100–210] | 11.43 [8–29] |
| Spinoglio (45), 2019 | Italy | Retrospective | 2005–2013 | 202 | 71.2 [36–95] | 104 (51.49)/98 (48.51) | Robotic 101 (50.00); LS 101 (50.00) | 257.5 [95–540] | 7.9 [4–37] |
| Pelz (41), 2018 | Germany | Retrospective | 2009–2016 | 279 | 70.6 [17–93] | 149 (53.40/130 (46.59) | LS 24 (8.60); open 255 (91.40) | 152 [61–443] | 15.5 [2–83] |
| Lee (47), 2019 | Korea | Retrospective | 2005–2012 | 835 | 63 [56–71] | 461 (55.21)/374 (44.79) | NR | 207 [171–254] | 9 [7–12] |
| Sammour (44), 2020 | Texas, USA | Retrospective | 2009–2016 | 141 | 64 [38–90] | 74 (52.48)/67 (47.52) | LS 122 (86.52); robotic 19 (13.48) | 186 median | 3 median |
| Ouyang (53), 2019 | China | Retrospective | 2011–2013 | 107 | 57.6 [39–76] | 62 (57.94)/45 (42.06) | LS 107 (100.00) | 192.4 [130–255] | 7.2 [1–13] |
| Overall | 7402 | 64.76 [17–94]* | 3,670 (51.27)/3,488 (48.73) | Open 2,860 (44.95); LS 3,323 (52.23); HAL-CME 78 (1.23); robot 101 (1.59) | 182.74 [35–664]* | 9.86 [1–65]* |
*, All results are calculated as weighted arithmetic mean, all median values are excluded. LOS, length of stay; MIS, minimally invasive (robotic or laparoscopic); LS, laparoscopic; HAL-CME, hand assisted laparoscopic complete mesocolic excision; NR, not recorded.
Baseline patient characteristics
The weighted mean age of included patients was 65.42 (range, 17–94) years; 51.27% (3,670 patients) of them were men (one study did not include patients’ gender data) (42). The CME was performed by open technique in 45% of cases and with a minimally invasive approach in the remnant 55% of cases (robotics, laparoscopy and hand-assisted laparoscopy). The weighted mean operative time was 182.74 [35–664] minutes (min). The weighted mean length of stay was 9.86 [1–65] days. These baseline patient characteristics are reported in Table 1.
Pathologic outcomes
Pathologic data are summarized in Table 2. The weighted mean number of lymph nodes retrieved was 27.45 [1–88] and the mean number of metastatic nodes was 1.34 [0–48]. Only one study didn’t report the total number of lymph nodes yielded (50) while the number of metastatic nodes retrieved was detailed in only five articles (40,43,44,49,54). Subbiah (38) and Shin (43) were the only authors to report the rate of integrity of the mesocolic plane, which was high in both studies, 94% and 81% respectively. No one referred to Benz’s classification (55) to check the quality and completeness of dissection. Only Ouyang’s study (53) described the mean ileocolic vessel length, which was 12.6 (range, 10–15) cm. Nobody analyzed the length of middle colic trunk. Most of the analyzed patients (41.6%) had a TNM Stage II disease while 17% of them had a stage I, 39.7% a stage III and only the 1% of patients had a stage IV cancer.
Table 2
| Reference, year | N of lymph nodes, [range] | N of metastatic nodes, [range] | Ileocolic vessel length (cm), [range] | Middle colic trunk length (cm) | Pathological stage pTNM, n [%] | Mesocolic area (cm2) | Grade of integrity of mesocolic layer, n [%] | Benz score |
|---|---|---|---|---|---|---|---|---|
| Pramateftakis (50), 2010 | NR | NR | NR | NR | NR | NR | NR | NR |
| Kang (40), 2014 | 28 [3–88] | 0 [0–48] | NR | NR | NR | NR | NR | NR |
| Bae (37), 2014 | 27.5 [8–79] | NR | NR | NR | Stage I 15 [9], stage II 81 [48], stage III 74 [43] | NR | NR | NR |
| Liang (42), 2015 | 34.4 [18–51] | NR | NR | NR | Stage I 4 [2], stage II 38 [16], stage III 202 [83] | NR | NR | NR |
| Cho (54), 2015 | 33.7 [1–78] | 1.57 [0–9] | NR | NR | Stage I 100 [13], stage II 372 [48], stage III 301 [39] | NR | NR | NR |
| Subbiah (38), 2016 | 24 median | NR | NR | NR | Stage I 33 [16], stage II 96 [45], \stage III 83 [39] | NR | M plane 199 [94]; I plane 11 [6]; MP 2 [1] | NR |
| Huang (39), 2015 | 13.5 [2–26] | NR | NR | NR | Stage I 11 [11], stage II 54 [53], stage III 37 [36] | NR | NR | NR |
| Takahashi (36), 2017 | 23.47 [1–55] | NR | NR | NR | p T1 64 [32], pT2 27 [13], pT3 98 [49], pT4 13 [6]; pN0 132 [65], pN1 43 [21], pN2 20 [10], pN3 7 [4] | NR | NR | NR |
| Siani (51), 2017 | 27 [21–33] | NR | NR | NR | Stage I 153 [26], stage II 231 [39], stage III 216 [36] | NR | M plane 486 [81]; I plane 96 [16]; MP 18 [3] | NR |
| Kim (34), 2016 | 28.84 [5–55] | 1.46 [0–10] | NR | NR | Stage I 34 [17], stage II 88 [50], stage III 93 [43] | NR | NR | NR |
| Sheng (48), 2017 | 19.54 [14–25] | NR | NR | NR | Stage I 20 [14], stage II 65 [43], stage III 65 [43] | NR | NR | NR |
| Kim (49), 2017 | 21.9 [1–48] | NR | NR | NR | Stage I 21 [15], stage II 73 [51], stage III 48 [34] | NR | NR | NR |
| Shin (43), 2018 | 27.3 [2–52] | 1.32 [0–7] | NR | NR | Stage I 416 [18], stage II 973 [43], stage III 860 [38] | NR | NR | NR |
| Wang (52), 2017 | 23.3 [5–42] | NR | NR | NR | Stage I 10 [6], stage II 57 [33], stage III 105 [61] | NR | NR | NR |
| Bertelsen (46), 2019 | 38 [28–48] | NR | NR | NR | pT1 11 [4], pT2 27 [11], pT3 166 [65], pT4 52 [20]; pN0 165 [64], pN1 47 [18], pN2 44 [17] | NR | NR | NR |
| Li (35), 2018 | 18.45 [12–42] | NR | NR | NR | Stage I 9 [8], stage II 42 [39], stage III 57 [53] | NR | NR | NR |
| Spinoglio (45), 2019 | 29.3 [12–74] | NR | NR | NR | Stage I 47 [24], stage II 66 [33], stage III 70 [35], stage IV 17 [9] | NR | NR | NR |
| Pelz (41), 2018 | 31.8 [10–73] | NR | NR | NR | Stage 0 44 [16], stage I 47 [17], stage II 77 [28], stage III 70 [25], stage IV 41 [15] | NR | NR | NR |
| Lee (47), 2019 | 27 [19–37] | NR | NR | NR | Stage I 174 [21], stage II 365 [44], stage III 295 [35] | NR | NR | NR |
| Sammour (44), 2020 | 31 median | NR | NR | NR | Stage I 35 [25], stage II 49 [35], stage III 48 [34], stage IV 9 [6], | NR | NR | NR |
| Ouyang (53), 2019 | 23.2 [10–37] | NR | 12.6 [10–15] | NR | Stage I 15 [14], stage II 59 [55], stage III 33 [31] | NR | NR | NR |
| Overall | 27.45 [1–88]* | 1.34 [0–48]* | 12.6 [10–15]* | NR | Stage 0 44 [1] stage I 1,144 [17], stage II 2,786 [42], stage III 2,657 [40], stage IV 67 [1] | NR | M plane 685 [84]; I plane 107 [13]; MP 20 [3] | NR |
*, All results are calculated as weighted arithmetic mean, all median values are excluded. M plane, mesocolic plane; I plane, intramesocolic plane; MP, muscolaris propria; NR, not recorded.
Perioperative morbidity and mortality
Eighteen papers reported perioperative morbidity and mortality details (Table 3).
Table 3
| Reference, year | Intraoperative major vascular injuries, n (%) | Intraoperative visceral injuries, n (%) | Anastomotic leak, n (%) | Reoperation, n (%) | Perioperative blood loss volume (mL), [range] | Other surgical complication, n (%) | Nonsurgical complications, n (%) | Intraoperative mortality, n (%) | 30-days mortality, n (%) |
|---|---|---|---|---|---|---|---|---|---|
| Pramateftakis (50), 2010 | NR | NR | 1 (0.9) | NR | NR | Wound dehiscence 8 (6.9); enterocutaneus fistula 1 (0.9); abdominal bleeding 1 (0.9) | mesenteric vein Thrombosis 1 (0.9); deep venous thrombosis 1 (0.9); cerebral vascular accident 1 (0.9); atrial fibrillation 1 (0.9) | 0 | NR |
| Kang (40), 2014 | NR | NR | NR | NR | NR | Wound infection 1 (0.7) | Ileus 1 (0.7); urinary retention 4 (3.1) | 0 | NR |
| Bae (37), 2014 | NR | NR | 1 (0.58) | NR | 41.95 [0–304] | Wound infection 7 (4.1); abdominal bleeding 1 (0.6); abdominal abscess 1 (0.6); chylous leakage 15 (8.8) | Ileus 5 (2.9); pulmonary complication 1 (0.6); urinary tract infection 1 (0.6) | NR | 1 (0.58) |
| Liang (42), 2015 | NR | NR | 4 (1.6) | NR | 104.5 [58–150] | Wound infection 10 (4.0) | Urinary tract infection 8 (3.0); pneumonia 2 (0.8); ileus 4 (1.6); duodenal paralysis 7 (2.9); myocardial infarction 1 (0.4); cerebrovascular accident 1 (0.4); pulmonary embolism 1 (0.4); deep vein thrombosis 1 (0.4) | NR | NR |
| Cho (54), 2015 | NR | NR | 5 (0.6) | NR | NR | Wound infection 9 (1.2); abdominal bleeding 1 (0.1); bowel necrosis 1 (0.1) | Pulmonary disease 6 (0.8); ileus 9 (1.2); cerebrovascular infarction 1 (0.1); ARDS 1 (0.1) | 0 | 2 (0.2) |
| Subbiah (38), 2016 | NR | NR | 1 (0.5) | NR | NR | Wound infection 4 (2.0); abdominal abscess 1 (0.5) | Ileus 11 (5.0); urinary complication 2 (1.0); respiratory complication 7 (3.0); cardiac complication 1(0.5) | NR | NR |
| Huang (39), 2015 | NR | NR | NR | NR | 105.53 [0–350] | Wound infection 4 (4.0); anastomotic bleeding 1 (1.0) | Pneumonia 3 (3.0) | NR | NR |
| Takahashi (36), 2017 | NR | NR | 1 (0.5) | NR | 43 [20– 100] | Wound infection 10 (5.0); abdominal bleeding 1 (0.5); abdominal abscess 1 (0.5) | Ileus 4 (2.0); Thrombosis 1 (0.5); urinary complication 1 (0.5); cardiovascular complication 1 (0.5); pneumonia 1 (0.5) | NR | 0 |
| Siani (51), 2017 | NR | NR | 15 (2.5) | NR | 53 [0–123] | Wound infection 63 (10.6) | Pneumonia 59 (5.8); pleural effusion 38 (4.7); urinary tract infection 36 (6.0); ileus 9 (1.5); deep venous thrombosis 3 (0.5) | NR | NR |
| Kim (34), 2016 | NR | NR | 2 (0.9) | NR | 75 [0–371] | Wound infection 25 (11.6); abdominal bleeding 3 (1.4); abdominal abscess 4 (1.9); chylous leakage 8 (3.7) | Respiratory complication 11 (5.1); urinary complication 3 (1.4); ileus 13 (6.0) | NR | 4 (2) |
| Sheng (48), 2017 | NR | NR | 0 | NR | 0 | Wound infection 6 (4.0); chylous leakage 2 (1.3) | Ileus 4 (3.0); respiratory complication 1 (0.6); gastroplegia 3 (2.0) | NR | NR |
| Kim (49), 2017 | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Shin (43), 2018 | NR | NR | 18 (0.8) | NR | NR | Wound infection 61 (3.0) | Ileus 81 (4.0) | NR | 1 (0.04) |
| Wang (52), 2017 | 0 | 0 | 1 (0.6) | 2 (1.1) | 74.2 [18–130] | Wound infection 3 (1.74); chylous leakage 22 (12.8); anastomotic bleeding 1 (0.58) | Pneumonia 1 (0.6) | 0 | 0 |
| Bertelsen (46), 2019 | NR | NR | NR | NR | NR | NR | NR | NR | 13 (5) |
| Li (35), 2018 | NR | NR | 3 (2.8) | NR | 97 [50–300] | Wound infection 3 (2.8); chylous leakage 4 (3.7); abdominal abscess 3 (2.8) | Ileus 1 (0.9); gastroplegia 2 (1.8) | 0 | 0 |
| Spinoglio (45), 2019 | 1 (0.5) | NR | 2 (1.0) | 4 (2.0) | NR | Wound infection 15 (7.4); abdominal bleeding 8 (4.0) | Ileus 21 (10.4); pneumonia 4 (2.0); acute respiratory failure 3 (1.5); arrhythmia 3 (1.5); acute myocardial Infarction 1 (0.5); stroke 1 (0.5); urinary complication 4 (2.0) | NR | 1 (0.5) |
| Pelz (41), 2018 | NR | NR | NR | 52 (18.6) | NR | NR | NR | NR | 4 (1.4) |
| Lee (47), 2019 | NR | NR | NR | NR | NR | NR | NR | NR | 1 (0.1) |
| Sammour (44), 2020 | 0 | 0 | 2 (1.4) | 1 (0.7) | 50 median | NR | NR | 0 | 0 |
| Ouyang (53), 2019 | NR | NR | 1 (0.9) | 1 (0.9) | 108.4 [61–156] | Wound infection 3 (2.8); fat liquefaction wound 2 (1.9) | Ileus 5 (4.7); pulmonary infection 1 (0.9); urinary tract infection 1 (0.9) | 0 | 0 |
| Overall | 1 (0.2) | 0 | 57 (1.0) | 60 (7.0) | 65.14 [0–371]* | 314 (5.5) | 399 (6.9) | 0 | 27 (0.5) |
*, All results are calculated as weighted arithmetic mean, all median values are excluded. NR, not recorded.
Spinoglio et al. (45) described a case of major vessels injury, while no one described intraoperative visceral injuries. Overall surgical complications rate was 5.5% and anastomotic leakage occurred in 1% of patients. The weighted mean operative blood loss was 65.14 [0–371] mL.
Overall operative mortality rate for included studies was 0.5% and no one described intraoperative deaths.
Pelz (41) reported the higher reoperation rate (18.6%) among included studies, compared with a reintervention rate close to 1% of the others.
Survival outcomes
Ten studies detailed the adoption of adjuvant treatment. In total, 65% of patients in these studies underwent postoperative chemotherapy (34,37,44,46-51,54) (Table 4).
Table 4
| Reference | Adjuvant therapy, n (%) | 5-year local recurrence, n (%) | 5-year distant metastasis, n (%) | 3-year OS, % | 3-year DFS, % | 5-year OS, % | 5-year DFS, % |
|---|---|---|---|---|---|---|---|
| Pramateftakis (50), 2010 | 48 (41.7) | NR | NR | NR | NR | 72.4 | NR |
| Kang (40), 2014 | NR | NR | 7 (5.5) | NR | NR | NR | NR |
| Bae (37), 2014 | 133 (78.2) | 7 (4.1) | 20 (11.8) | NR | NR | 84 | 78 |
| Liang (42), 2015 | NR | NR | NR | NR | NR | NR | 66.8 |
| Cho (54), 2015 | 615 (79.6) | 34 (4.4) | 114 (14.7) | NR | NR | 84 | 82.8 |
| Subbiah (38), 2016 | NR | NR | NR | NR | NR | NR | NR |
| Huang (39), 2015 | NR | NR | NR | NR | NR | NR | NR |
| Takahashi (36), 2017 | NR | NR | NR | NR | NR | NR | NR |
| Siani (51), 2017 | 425 (70.8) | NR | NR | NR | NR | 83.0 | 78.3 |
| Kim (34), 2016 | 144 (71.3) | NR | NR | 87.4 | 78.6 | NR | NR |
| Sheng (48), 2017 | 127 (84.7) | NR | NR | NR | NR | NR | NR |
| Kim (49), 2017 | 99 (79.2) | 6 (4.2) | NR | NR | NR | 82.6 | 83.5 |
| Shin (43), 2018 | NR | 47 (2.0) | 270 (12.0) | 89.7 | NR | NR | 86 |
| Wang (52), 2017 | NR | 3 (1.7) | 14 (8.1) | 89.1 | 81.7 | NR | NR |
| Bertelsen (46), 2019 | 78 (30.4) | 25 (10.0) | NR | NR | NR | NR | NR |
| Li (35), 2018 | NR | 3 (3.0) | 16 (14.8) | NR | NR | 73.0 | 57.4 |
| Spinoglio (45), 2019 | NR | NR | NR | NR | NR | 75.0 | 84.2 |
| Pelz (41), 2018 | NR | NR | NR | NR | NR | NR | NR |
| Lee (47), 2019 | 491 (58.8) | NR | NR | NR | NR | 91.1 | 85.6 |
| Sammour (44), 2020 | 56 (39.7) | 0 | 26 (18.4) | NR | NR | NR | NR |
| Ouyang (53), 2019 | NR | 3 (2.8) | 6 (5.6) | 93.5 | 91.6 | NR | NR |
| Overall | 2,216 (65.0) | 128 (3.0) | 473 (12.3) | 89.6 | 82.4 | 84.3 | 82.8 |
OS, overall survival; DFS, disease-free survival; NR, not recorded.
Five-year local recurrence and distant metastasis rates were 3% and 12.3% respectively.
Most of the included papers reported 5-year DFS and OS survival rates and their weighted means were 82.8% and 84.3% respectively (35,37,43,45,47,49-51,54), whereas only four studies mentioned 3-year OS outcome (weighted mean 89.6%) (34,43,52,53) and only three studies detailed 3-year DFS (weighted mean 82.4%) (34,52,53).
Discussion
Some Authors would like to introduce CME as the standard of care for RSC based on the supposed evidence of its better oncologic outcomes provided with same operative morbidity and mortality as compared to the classic right hemicolectomy (20,24,56,57). CME is claimed as the adequate treatment for RSC because of the extensive lymph-node dissection which may include remote nodal basins and those along major first-order vessels such as the superior mesenteric artery.
Many studies showed that survival of CC is strictly related to the number of lymph nodes removed, despite the stage of the disease, patient demographics and tumor characteristics (21,58-60). Chen et al. reported that removing at least 15 lymph nodes increased median OS by 11 months in patients with stage I, 54 months in stage II and 21 months in stage III cancer. He stated that surgeons must remove at least 15 nodes in every dissection for CCs and concluded “I would advise surgeons to remember that the number of nodes makes a difference” (21). Prandi et al. showed a direct relationship between the number of lymph node yielded and survival which is even strongly significant in stage B (pN0) patients (58). Swanson et al. demonstrated that the prognosis of T3N0 CC is dependent on the number of lymph nodes examined (59). Finally, Le Voyer et al. stated that the number of lymph nodes analyzed for staging CCs is, itself, a prognostic variable of outcome, even in lymph node negative disease (60). This significant improvement of survival is related to many reasons; one of these is stage migration, especially in earlier stages (I and II). In the present review the overall weighted-mean number of lymph nodes yielded was high (28,57). This result demonstrates that CME enables surgeons to remove more nodes even than those suggested by Chen (21), providing a better staging and increasing the survival of the disease.
The concept of surgical dissection along embryologic fascial planes allowing the excision of an intact mesocolon containing its regional lymph nodes is another key point of CME. The surgery along embryological fascial planes enables to reduce the hazard of cancer spillage inside the abdominal cavity. West et al. (57) showed better survival outcomes in patients with preserved integrity of mesocolic plane of dissection especially for stage III disease. In this review only Subbiah (38) and Shin (43) analyzed the integrity of mesocolic plane in their studies, and no one referred to Benz classification to objectively define the quality of dissection. As one of the main items of CME is the dissection following embryological planes, this lack represents a major limit of the studies included in the present review. Moreover, the absence of an objective procedure to check the quality of surgery makes impossible to assess the real extension of the operations performed in each study and how much the procedures described in the different studies are homogeneous.
Recently Perrakis (61), claimed that CME can be implemented in surgical departments after previous adequate teaching without increasing postoperative complications and mortality. Despite a negative report by Pelz et al. (41), describing a high reoperation rate (18.6%), the overall postoperative outcomes of the present review showed acceptable findings: reoperation rate was 7%, surgical complications were 5.5%, intraoperative mortality was 0% and 30-day postoperative mortality was 0.5%. These results are consistent with those observed after standard nonCME right hemicolectomy (62,63). Anastomotic leak occurred in 1% of cases; showing the same incidence observed after standard right colectomy (64,65). Nevertheless, this is a really demanding procedure, requiring proved skill in advanced minimally invasive procedures.
Recently, a large retrospective study investigated postoperative and oncological outcomes of 3,518 patients submitted to right colectomy for RSC with different approaches (open, laparoscopic and robotic). The operative times were significantly different among the subgroups: 135.9±89.2 min for open surgery, 142.5±63.3 min for laparoscopy and 187.2±81.4 min for robotic procedures while the length of stay was significantly shorter after minimally-invasive approach (5.2±4.7 and 4.4±2.4 days after laparoscopic and robotic surgery respectively) compared to open surgery (7.9±7.7 days) (62). In the present review the resections were mostly performed with minimally invasive approach (55%); the weighted mean operative time and length of stay (LOS) were 182.74 min and 9.86 days respectively.
In a study of over 83,000 CC survivors based on the Surveillance Epidemiology End Results (SEER) database, the 5-year cause-specific survival was ≥80% for all stages together except from stage IV (48%) (66). In 2018, Qiu (67) detailed cancer-specific survival differences between RSCs and LSCs using SEER database, documenting a 5-year cause-specific survival of 68.1% after standard colectomy for RCS (92,8% in stage I, 85.5% in stage II, 64.9% in stage III and 11.2% in stage IV disease). Narayanan et al. recently published a large retrospective study of the National Cancer Database investigating the data from 379785 patients to assess the OS. The Authors found a 3- and 5-year OS of 61% and 51% (68). A recent analysis of 3,622 patients with CC derived from two French digestive cancer registries reported that 1.6% of patients with RSC developed a local recurrence 5 to 10 years after resection (69).
In the present study 3- and 5-year OS was 89.6% and 82.8%; Therefore, it was higher compared with survival results of standard right colectomies reported in recent literature.
This study has several limitations. First, all of the included studies were case series of a prospective or retrospective nature and there was no Level 1 evidence from a RCT. Second, these studies are not homogeneous as concerns the outcomes of interest of this review detailed in materials and methods section; most of them have important missing outcomes; none of them has investigated all of these items and furthermore, the majority of these studies considered only few outcomes of those requested. The last point is that the majority of included patients had early-stage diseases (58.6% stage I–II). This limitation represents a confounding factor for the final analysis of survival outcomes.
In conclusion, despite these limitations, data from extensive literature search document that CME with CVL for RCS is a promising procedure which can provide better oncological and survival outcomes without increasing postoperative morbidity and mortality as compared to standard right colectomy. However, to date, the quality of evidence is limited and does not consistently support the superiority of CME. More reliable data from large sample size RCTs are needed before CME can be recommended as the standard of care for right CC resections.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Marco Milone and Ugo Elmore) for the series “Right Colectomy 2.0” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at http://dx.doi.org/10.21037/ales-20-41
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales-20-41). The series “Right Colectomy 2.0” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Iacopetta B. Are there two sides to colorectal cancer? Int J Cancer 2002;101:403-8. [Crossref] [PubMed]
- Meguid RA, Slidell MB, Wolfgang CL. Is there a difference in survival between right- versus left-sided colon cancers? Ann Surg Oncol 2008;15:2388-94. [Crossref] [PubMed]
- Weiss JM, Pfau PR, O’Connor ES. Mortality by stage for right- versus left-sided colon cancer: analysis of surveillance, epidemiology, and end results—Medicare data. J Clin Oncol 2011;29:4401-9. [Crossref] [PubMed]
- Saltzstein SL, Behling CA. Age and time as factors in the left-to-right shift of the subsite of colorectal adenocarcinoma: a study of 213,383 cases from the California Cancer Registry. J Clin Gastroenterol 2007;41:173-7. [Crossref] [PubMed]
- Maruta M, Kotake K, Maeda K. Colorectal cancer in Japan. Rozhl Chir 2007;86:618-21. [PubMed]
- Papagiorgis P, Oikonomakis I, Karapanagiotou I, et al. The impact of tumor location on the histopathologic expression of colorectal cancer. J BUON 2006;11:317-21. [PubMed]
- Benedix F, Kube R, Meyer F. Comparison of 17,641 patients with right- and left-sided colon cancer: differences in epidemiology, perioperative course, histology, and survival. Dis Colon Rectum 2010;53:57-64. [Crossref] [PubMed]
- Nawa T, Kato J, Kawamoto H, et al. Differences between right- and left-sided colon cancer in patient characteristics, cancer morphology and histology. J Gastroenterol Hepatol 2008;23:418-23. [Crossref] [PubMed]
- Gervaz P, Bouzourene H, Cerottini JP, et al. Dukes B colorectal cancer: distinct genetic categories and clinical outcome based on proximal or distal tumor location. Dis Colon Rectum 2001;44:364-72; discussion 372-3. [Crossref] [PubMed]
- Bufill JA. Colorectal cancer: evidence for distinct genetic categories based on proximal or distal tumor location. Ann Intern Med 1990;113:779-88. [Crossref] [PubMed]
- Elnatan J, Goh HS, Smith DR. C-KI-RAS activation and the biological behaviour of proximal and distal colonic adenocarcinomas. Eur J Cancer 1996;32A:491-7. [Crossref] [PubMed]
- Sinicrope FA, Mahoney MR, Smyrk TC. Prognostic impact of deficient DNA mismatch repair in patients with stage III colon cancer from a randomized trial of FOLFOX-based adjuvant chemotherapy. J Clin Oncol 2013;31:3664-72. [Crossref] [PubMed]
- Schrag D, Weng S, Brooks G. The relationship between primary tumor sidedness and prognosis in colorectal cancer. J Clin Oncol 2016;34:abstr 3505.
- Heald RJ. The “Holy Plane” of rectal surgery. J R Soc Med 1988;81:503-8. [Crossref] [PubMed]
- Enker WE, Laffer UT, Block GE. Enhanced survival of patients with colon and rectal cancer is based upon wide anatomic resection. Ann Surg 1979;190:350-60. [Crossref] [PubMed]
- Enker WE, Kafka NJ, Martz J. Planes of sharp pelvic dissection for primary, locally advanced, or recurrent rectal cancer. Semin Surg Oncol 2000;18:199-206. [Crossref] [PubMed]
- Kapiteijn E, Putter H, Van De Velde CJH. Impact of the introduction and training of total mesorectal excision on recurrence and survival in rectal cancer in The Netherlands. Br J Surg 2002;89:1142-9. [Crossref] [PubMed]
- Wibe A, Møller B, Norstein J, et al. A national strategic change in treatment policy for rectal cancer - Implementation of total mesorectal excision as routine treatment in Norway. A national audit. Dis Colon Rectum 2002;45:857-66. [Crossref] [PubMed]
- Hohenberger W, Weber K, Matzel K, et al. Standardized surgery for colonic cancer: complete mesocolic excision and central ligation--technical notes and outcome. Colorectal Dis 2009;11:354-64. [Crossref] [PubMed]
- Chen SL, Bilchik AJ. More extensive nodal dissection improves survival for stages I to III of colon cancer: a population-based study. Ann Surg 2006;244:602-10. [PubMed]
- Tsai HL, Lu CY, Hsieh JS, et al. The prognostic significance of total lymph node harvest in patients with T2-4N0M0 colorectal cancer. J Gastrointest Surg 2007;11:660-5. [Crossref] [PubMed]
- Johnson PM, Porter GA, Ricciardi R, et al. Increasing negative lymph node count is independently associated with improved long-term survival in stage IIIB and IIIC colon cancer. J Clin Oncol 2006;24:3570-5. [Crossref] [PubMed]
- West NP, Hohenberger W, Weber K, et al. Complete mesocolic excision with central vascular ligation produces an oncologically superior specimen compared with standard surgery for carcinoma of the colon. J Clin Oncol 2010;28:272-8. [Crossref] [PubMed]
- Ratto C, Sofo L, Ippoliti M, et al. Accurate lymph-node detection in colorectal specimens resected for cancer is of prognostic significance. Dis Colon Rectum 1999;42:143-54. [Crossref] [PubMed]
- Gelos M, Gelhaus J, Mehnert P, et al. Factors influencing lymph node harvest in colorectal surgery. Int J Colorectal Dis 2008;23:53-9. [Crossref] [PubMed]
- Fielding LP, Fenoglio-Preiser CM, Freedman LS. The future of prognostic factors in outcome prediction for patients with cancer. Cancer 1992;70:2367-77. [Crossref] [PubMed]
- Nelson H, Petrelli N, Carlin A, et al. Guidelines 2000 for colon and rectal cancer surgery. J Natl Cancer Inst 2001;93:583-96. [Crossref] [PubMed]
- Willaert W, Ceelen W. Extent of surgery in cancer of the colon: is more better? World J Gastroenterol 2015;21:132-8. [Crossref] [PubMed]
- Willaert W, Mareel M, Van De Putte D, et al. Lymphatic spread, nodal count and the extent of lymphadenectomy in cancer of the colon. Cancer Treat Rev 2014;40:405-13. [Crossref] [PubMed]
- Chen H, Wang Y, Liu H, et al. Factors influencing apical node metastasis in colorectal cancer patients treated with laparoscopic radical resection with D3 lymphadenectomy: results from two centers in China. Surg Today 2015;45:569-75. [Crossref] [PubMed]
- Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. 2nd ed. Chichester: John Wiley & Sons, 2019.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097 [Crossref] [PubMed]
- Kim IY, Kim BR, Choi EH, et al. Short-term and oncologic outcomes of laparoscopic and open complete mesocolic excision and central ligation. Int J Surg 2016;27:151-7. [Crossref] [PubMed]
- Li J, Yudong L, Chen Y. Short- and long-term outcomes of laparoscopic complete mesocolic excision in elderly patients with right colon cancer. J BUON 2018;23:1625-32. [PubMed]
- Takahashi H, Takemasa I, Haraguchi N, et al. The single-center experience with the standardization of single-site laparoscopic colectomy for right-sided colon cancer. Surg Today 2017;47:966-72. [Crossref] [PubMed]
- Bae SU, Saklani AP, Lim DR, et al. Laparoscopic-assisted versus open complete mesocolic excision and central vascular ligation for right-sided colon cancer. Ann Surg Oncol 2014;21:2288-94. [Crossref] [PubMed]
- Subbiah R, Bansal S, Jain M, et al. Initial retrocolic endoscopic tunnel approach (IRETA) for complete mesocolic excision (CME) with central vascular ligation (CVL) for right colonic cancers: technique and pathological radicality. Int J Colorectal Dis 2016;31:227-33. [Crossref] [PubMed]
- Huang JL, Wei HB, Fang JF, et al. Comparison of laparoscopic versus open complete mesocolic excision for right colon cancer. Int J Surg 2015;23:12-7. [Crossref] [PubMed]
- Kang J, Kim IK, Kang SI, et al. Laparoscopic right hemicolectomy with complete mesocolic excision. Surg Endosc 2014;28:2747-51. [Crossref] [PubMed]
- Pelz JOW, Wagner J, Lichthardt S, et al. Laparoscopic right-sided colon resection for colon cancer-has the control group so far been chosen correctly? World J Surg Oncol 2018;16:117. [Crossref] [PubMed]
- Liang JT, Lai HS, Huang J, et al. Long-term oncologic results of laparoscopic D3 lymphadenectomy with complete mesocolic excision for right-sided colon cancer with clinically positive lymph nodes. Surg Endosc 2015;29:2394-401. [Crossref] [PubMed]
- Shin JK, Kim HC, Lee WY, et al. Laparoscopic modified mesocolic excision with central vascular ligation in right-sided colon cancer shows better short- and long-term outcomes compared with the open approach in propensity score analysis. Surg Endosc 2018;32:2721-31. [Crossref] [PubMed]
- Sammour T, Malakorn S, Thampy R, et al. Selective central vascular ligation (D3 lymphadenectomy) in patients undergoing minimally invasive complete mesocolic excision for colon cancer: optimizing the risk-benefit equation. Colorectal Dis 2020;22:53-61. [Crossref] [PubMed]
- Spinoglio G, Bianchi PP, Marano A, et al. Correction to: robotic versus laparoscopic right colectomy with complete mesocolic excision for the treatment of colon cancer: perioperative outcomes and 5-year survival in a consecutive series of 202 patients. Ann Surg Oncol 2019;26:884. [Crossref] [PubMed]
- Bertelsen CA, Neuenschwander AU, Jansen JE, et al. 5-year outcome after complete mesocolic excision for right-sided colon cancer: a population-based cohort study. Lancet Oncol 2019;20:1556-65. [Crossref] [PubMed]
- Lee JM, Han YD, Cho MS, et al. Impact of tumor sidedness on survival and recurrence patterns in colon cancer patients. Ann Surg Treat Res 2019;96:296-304. [Crossref] [PubMed]
- Sheng QS, Pan Z, Chai J, et al. Complete mesocolic excision in right hemicolectomy: comparison between hand-assisted laparoscopic and open approaches. Ann Surg Treat Res 2017;92:90-6. [Crossref] [PubMed]
- Kim MK, Lee IK, Kye BH, et al. Procedural difficulty differences according to tumor location do not compromise the clinical outcome of laparoscopic complete mesocolic excision for colon cancer: a retrospective analysis. Oncotarget 2017;8:64509-19. [Crossref] [PubMed]
- Pramateftakis MG. Optimizing colonic cancer surgery: high ligation and complete mesocolic excision during right hemicolectomy. Tech Coloproctol 2010;14:S49-51. [Crossref] [PubMed]
- Siani LM, Lucchi A, Berti P, et al. Laparoscopic complete mesocolic excision with central vascular ligation in 600 right total mesocolectomies: safety, prognostic factors and oncologic outcome. Am J Surg 2017;214:222-7. [Crossref] [PubMed]
- Wang Y, Zhang C, Zhang D, et al. Clinical outcome of laparoscopic complete mesocolic excision in the treatment of right colon cancer. World J Surg Oncol 2017;15:174. [Crossref] [PubMed]
- Ouyang M, Luo Z, Wu J, et al. Comparison of outcomes of complete mesocolic excision with conventional radical resection performed by laparoscopic approach for right colon cancer. Cancer Manag Res 2019;11:8647-56. [Crossref] [PubMed]
- Cho MS, Baek SJ, Hur H, et al. Modified complete mesocolic excision with central vascular ligation for the treatment of right-sided colon cancer: long-term outcomes and prognostic factors. Ann Surg 2015;261:708-15. [Crossref] [PubMed]
- Benz S, Tannapfel A, Tam Y, et al. Proposal of a new classification system for complete mesocolic excison in right-sided colon cancer. Tech Coloproctol 2019;23:251-7. [Crossref] [PubMed]
- Weber K, Merkel S, Perrakis A, et al. Is there a disadvantage to radical lymph node dissection in colon cancer? Int J Colorectal Dis 2013;28:217-26. [Crossref] [PubMed]
- West NP, Morris EJA, Rotimi O, et al. Pathology grading of colon cancer surgical resection and its association with survival: a retrospective observational study. Lancet Oncol 2008;9:857-65. [Crossref] [PubMed]
- Prandi M, Lionetto R, Bini A, et al. Prognostic evaluation of stage B colon cancer patients is improved by an adequate lymphadenectomy: results of a secondary analysis of a large scale adjuvant trial. Ann Surg 2002;235:458-63. [Crossref] [PubMed]
- Swanson RS, Compton CC, Stewart AK, et al. The prognosis of T3N0 colon cancer is dependent on the number of lymph nodes examined. Ann Surg Oncol 2003;10:65-71. [Crossref] [PubMed]
- Le Voyer TE, Sigurdson ER, Hanlon AL, et al. Colon cancer survival is associated with increasing number of lymph nodes analyzed: a secondary survey of intergroup trial INT-0089. J Clin Oncol 2003;21:2912-9. [Crossref] [PubMed]
- Perrakis A, Vassos N, Weber K, et al. Introduction of complete mesocolic excision with central vascular ligation as standardized surgical treatment for colon cancer in Greece. Results of a pilot study and bi-institutional cooperation. Arch Med Sci 2019;15:1269-77. [Crossref] [PubMed]
- Haskins IN, Ju T, Skancke M, et al. Right colon resection for colon cancer: does surgical approach matter? J Laparoendosc Adv Surg Tech A 2018;28:1202-6. [Crossref] [PubMed]
- Hinojosa MW, Konyalian VR, Murrell ZA, et al. Outcomes of right and left colectomy at academic centers. Am Surg 2007;73:945-8. [Crossref] [PubMed]
- Cirocchi R, Trastulli S, Farinella E, et al. Intracorporeal versus extracorporeal anastomosis during laparoscopic right hemicolectomy - systematic review and meta-analysis. Surg Oncol 2013;22:1-13. [Crossref] [PubMed]
- Milone M, Elmore U, Allaix ME, et al. Fashioning enterotomy closure after totally laparoscopic ileocolic anastomosis for right colon cancer: a multicenter experience. Surg Endosc 2020;34:557-63. [Crossref] [PubMed]
- Chang GJ, Hu CY, Eng C, et al. Practical application of a calculator for conditional survival in colon cancer. J Clin Oncol 2009;27:5938-43. [Crossref] [PubMed]
- Qiu MZ, Pan WT, Lin JZ, et al. Comparison of survival between right-sided and left-sided colon cancer in different situations. Cancer Med 2018;7:1141-50. [Crossref] [PubMed]
- Narayanan S, Gabriel E, Attwood K, et al. Association of clinicopathologic and molecular markers on stage-specific survival of right versus left colon cancer. Clin Colorectal Cancer 2018;17:e671-8. [Crossref] [PubMed]
- Bouvier AM, Launoy G, Bouvier V, et al. Incidence and patterns of late recurrences in colon cancer patients. Int J Cancer 2015;137:2133-8. [Crossref] [PubMed]
Cite this article as: Reddavid R, Osella G, Evola F, Puca L, Spidalieri L, Rorato LM, Sangiuolo F, Solej M, Degiuli M. Complete mesocolic excision for right colon cancer—state of art: a systematic review of the literature. Ann Laparosc Endosc Surg 2020;5:42.


