
Complex and acute paraesophageal hernias—type IV, strangulated, and irreducible
An overview of paraesophageal hernia
Complex and acute paraesophageal hernias (PEH) are a subset of the broader category of PEH and as such we will begin with an overview of evaluation and management for all PEH.
Definition
Hiatal hernias (HH) are classified into four types (Table 1). This classification is based on the position of the gastroesophageal junction (GEJ), the extent of herniated stomach, and the presence of other organs in the hernia sac (1). Type I HH, also referred to as sliding hernias, are by far the most common type, accounting for more than 95% of all HH (1). Types II–IV HH are all classified as PEH, and account for 5% of all HH (2,3). By definition, PEH means that at least a significant portion of the fundus is herniated superior to the GEJ, whether the GEJ is in normal anatomic position or is itself herniated. Type II PEH is rare and is defined as having gastric fundus herniating through the diaphragmatic hiatus alongside a normally positioned GEJ. Type III PEH is the most common PEH and is a combination of type I and II, whereby there is displacement of the GEJ together with the fundus above the diaphragm. Finally, while some have included giant hernias containing only stomach in the category of type IV PEH, by far the most common definition is that they include type II or III but additional herniation of other abdominal viscera such as colon, small intestine, spleen and pancreas (1,3,4). This chapter deals primarily with complex (high severity), acute, and type IV PEHs.
Table 1
| Hernia type | Definition |
|---|---|
| I | Sliding hernia |
| II | Gastric fundus has herniated superior to gastroesophageal junction (GEJ)—GEJ remains in normal position |
| III | Combination of type I and type II—GEJ herniates together with stomach |
| IV | Includes type II and type III, with the addition of either colon, small bowel, pancreas or spleen in the hernia sac |
Presentation
PEHs are diagnosed in one of three scenarios: (I) the patient is asymptomatic and the PEH is discovered after performing imaging for an unrelated reason; (II) the patient is symptomatic but not acute; or (III) the patient presents with features of acute obstruction or incarceration of stomach and/or involved organs, which may include ischemia, gangrene, and sepsis (5).
The most common symptoms for all paraesophageal hernia types are due to partial chronic obstruction of the herniated fundus and include early satiety, or pain with larger volumes of food, particularly solids. Other chronic symptoms may include nausea, dysphagia, anemia due to low-grade occult bleeding from Cameron ulcers, or dyspnea. Gastroesophageal reflux symptoms are not uncommon but they often fade over time as the hernia enlarges and the stomach compresses or angulates the esophagus. In an acutely large PEH respiratory symptoms may be prominent due to compression of the lung and the mediastinum by the filled intrathoracic stomach (3). However, acute incarceration can result from volvulus and cause gangrene leading to perforation (5). In patients who develop severe epigastric pain, one must suspect incarceration and/or obstruction (3).
Generally, patients with type IV hernias have the same clinical characteristics as those with type II or III, with symptoms driven primarily by factors related to the herniated fundus. Dysphagia and postprandial discomfort still remain the most common presentations that occur in more than 50% of cases of type IV PEH (1). The other herniated organs are often not associated with additional symptoms, although there may be chronic symptoms of subacute large or small bowel obstruction, or postural pain from impingement of herniated pancreas (6,7). Interestingly, some patients may have type IV HH as a consequence of, or in conjunction with, congenital shortened esophagus (8). However, these particular patients typically present as infants or young children with symptoms such as dysphagia, vomiting, and failure to thrive (8). In acute herniation of type IV hernias, complete obstruction of the colon and/or small bowel, either exclusively or in conjunction with gastric volvulus is not uncommon.
Diagnostic modalities
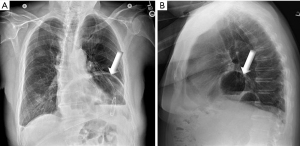
Diagnosis of both the acute and non-acute PEH is via chest X-ray, CT scan, contrast swallow, and upper endoscopy (EGD) (5). On plain X-ray a hiatal hernia is seen as a rounded soft-tissue opacity or an air-filled hyperlucency in the retrocardiac region, with or without an air-fluid level (Figure 1). Contrast swallow [upper gastrointestinal (UGI)] gives the best definition of the location of the GEJ, the portion of stomach herniated, and the characteristics of herniation such as location in right vs. left chest, organo- or meso-axial herniation of the stomach, angulation, emptying of the herniated fundus, and clues regarding the presence of short esophagus, such as esophageal tortuosity, mucosal damage or stricture. The CT scan defines the anatomy of the hiatus and contents of the mediastinum (3), and is most helpful in visualizing the characteristics of the diaphragmatic hiatus/other defects in the diaphragm and identifying other herniated organs comprising type IV hernias. EGD is an important diagnostic modality for ruling out mucosal abnormalities such as Barrett’s esophagus, ulcerations or ischemia, but is somewhat limited in defining the extent and location of herniated stomach and cannot identify other herniated organs associated with type IV hernias. Manometry can be performed prior to elective repair, though if a partial fundoplication is planned or if urgent surgery is needed it may be omitted.
Management
Timing and indications
The risk of requiring an emergent operation in a patient with a paraesophageal hernia has been reported as ranging from 0.7% to 7% (9). However, for the asymptomatic patient, the long-term risk of complications from PEH is not completely known but is thought to be 1–2% per year (10), thus the decision to continue living with this condition without corrective surgery can be appropriate depending on the patient’s age, co-morbidities and inclinations (11-13). Symptoms, particularly progressive, create a higher recommendation to consider repair (10). Of course, the acutely incarcerated or strangulated type IV hernia requires immediate intervention.
Traditionally, all patients with type IV PEHs were recommended for surgery given the risk of complications such as incarceration causing strangulation and the need to intervene emergently (14-17). Patients undergoing emergent surgery for hiatal hernia repair have more adverse prognostic factors and more major complications compared to those undergoing elective repair (18-20). Tam et al. emphasize that this is still the case in symptomatic large PEHs (18), but it may not be necessary for the asymptomatic patient.
Standard surgical technique
The main goal of surgery for the repair of PEH is to reduce the contents back into the abdominal cavity and repair the diaphragmatic defect in such a way as to eliminate the symptoms and complications of herniation and prevent recurrent herniation. A secondary goal is to rebuild the antireflux mechanism with a fundoplication. Though reflux may not be a significant symptom prior to repair, the reduction of herniated stomach will reduce gastroesophageal angulation which may lead to reflux unless a fundoplication is performed simultaneously. However, the addition of a fundoplication may not always be appropriate. Laparoscopic repair has emerged as the preferred approach and in experienced hands and is rare to require conversion to open surgery.
The standard approach is to focus on reducing the hernia sac which will simultaneously reduce the stomach; to remove the hernia sac; to obtain adequate length of intra-abdominal esophagus; to securely close the diaphragmatic hiatus around the esophagus—which may require a diaphragmatic relaxing incision and/or reinforcement of the closure—and to perform a total or partial fundoplication. Our standard technique for closure of the hiatus includes simple as well as pledget-reinforced horizontal mattress sutures with the addition of biologic mesh. If there is undue tension on the hiatal closure, we induce a left pneumothorax, and in the worst cases a relaxing incision in either the right or left hemidiaphragm, closed with permanent mesh is added. In addition to total or partial fundoplication we also routinely add Hill sutures to secure the GEJ down to the preaortic fascia and unload axial tension on the fundoplication. Hill sutures are the only intra-abdominal fixation which will secure the GE junction—rather than just the fundus—intra-abdominally. With the addition of Hill sutures, we have seen a substantial reduction in long-term anatomic hernia recurrence rates (21). We utilize partial fundoplication for patients who are elderly or who have poor esophageal motility on preoperative manometry, and rarely we will omit the fundoplication, particularly for quite fragile patients or in the acute setting in an unstable patient. In those cases, the fundus is fixed with multiple sutures to the dome of the left hemidiaphragm to prevent re-herniation.
Morbidity and mortality for emergent PEH
Mortality after emergent repair of PEHs has been studied both in institutional series as well as in large population-based studies, with a wide variation in reported mortality. Historical studies report mortality rates as high as 40% (22,23), while more contemporary studied report patient mortality ranging from 0 to 22% (19,24). Luketich et al. reported a 30-day mortality rate of 7.5% among patients who underwent emergency surgery in one of the largest series published to date (25). A large population-based study of the Nationwide Inpatient Sample from 2014 revealed a mortality rate of 3.2% among the subset of patients treated on an emergency basis (20). Thus, mortality has reduced significantly over the years, in patients undergoing emergent surgery for PEH (2).
Type IV PEH
Type IV PEH is defined as type II or III with the additional herniation of other organs within reach of the esophageal hiatus, including transverse colon (most commonly), small bowel, spleen or pancreas (Figure 2). The exact incidence and distinct clinical manifestations of type IV PEH are not completely defined (26).

Herniated organs
The specific organs that have herniated in addition to the stomach may have a significant impact on presentation, clinical course and management. Transverse colon or small bowel herniation may result in chronic symptoms of constipation or intermittent partial bowel obstruction, or may be the primary organ affected by acute obstruction (3,7,27). There have even been reports of herniation of sigmoid colon (28). Pancreas herniation is relatively rare with some case reports of head (6), body (29-31), tail (32) or complete (33) herniation of pancreas reported in the literature. Herniated pancreas does not typically result in specific symptoms, though there can be mechanical pressure effects resulting in postural discomfort. The possibility of spleen herniation should be considered at the time of surgical repair as it may add to the complexity and risk of the procedure (34). There is a least 1 report of spontaneous rupture of herniated spleen in a type IV hernia.
Management
For non-acute type IV patients elective repair may not be indicated and age, comorbidity, and the severity and progression of symptoms should be considerations, just as for type II and III hernias (1). There is little objective evidence that the herniation of other organs significantly increases the risk of catastrophe beyond that of the risk of type III herniation, though the subjective concern is higher. The preoperative work-up is generally the same as that for type II or III, and includes UGI, CT and EGD, as well as optional manometry knowing that the pressure readings can be skewed due to the abnormal anatomy. Evaluation in the acute setting is primarily focused on identification of anatomy and ruling out major complications of herniation such as incarceration, strangulation and organ ischemia. Some modalities like EGD may have the advantage of being both diagnostic and therapeutic and so should be considered early in the clinical course if possible.
The operative technique is also largely the same as for type II and III, with the additional burden of the herniated small bowel, colon, pancreas or spleen. The operation can be more challenging and lead to additional risk associated with repair. The key to reduction of the additional organs lies in properly dissecting out the hernia sac and reducing it. With this done, the viscera typically will follow. We have found that attempting to reduce the organs prior to reducing the anterior portion of the hernia sac can at best be difficult or frustrating and sometimes dangerous from excessive traction on viscera in a typically older more fragile patient. However, once the anterior 180 degrees or more of the sac is mobilized and reduced, particularly out of the left chest, the organs can then be largely reduced providing considerably more room for additional dissection. The one exception may be the pancreas, where adherence to the left crus from repeated trauma can make dissection treacherous. Injury is best avoided by meticulous dissection, continuing to reduce hernia sac rather than pancreas, and working posteriorly from both left and right sides of the esophagus and stomach.
Diaphragmatic closure may also be more challenging in these patients due to the sheer size of the defect. We have correlated the shape of the hiatus with the likelihood of excess tension on the repair (35). Our basic technique is as described above but is more likely to include inducing a pneumothorax via the left pleura, closure both posteriorly and anteriorly, and the use of a relaxing incision, typically in the right crus, closed with permanent mesh. We do not utilize permanent mesh in direct contact with the esophageal hiatus, but frequently do add biologic mesh for reinforcement of the hiatus itself.
Acute patients should be treated similarly to acute patients with gastric herniation, though there is the additional risk of non-viability of the colon or small bowel and the possible need for resection or a second-look operation (see below).
The “irreducible” hernia
Irreducibility refers to the inability of the surgeon to bring incarcerated organs back into the abdomen at the time of operation using standard technique. The exact incidence of this is unknown but it is fair to say that it is quite a rare situation and that it is relative to the efforts and techniques expended and to some extent to the experience of the surgeon. Generally, it is true, particularly in the acute setting, that distended fundus or other viscera can create a dumbbell configuration in which reducing the viscera can be difficult. If a nasogastric tube has not been placed previously it may be helpful to pass it at this time; endoscopy may be even more effective also permitting assessment of the mucosa for ischemia.
A relatively small incision of the diaphragmatic hiatus, typically left anterolateral, can be dramatically beneficial in helping reduce contents, without a significant technical cost. Extensive dense adhesions between viscera and the posterior left crus may form from repeated trauma of the herniation, holding viscera and the hernia sac from reducing. Careful but bold division of scar tissue along the left crus may be needed to free the adhesions.
Finally, an unusual but problematic herniation is that of the fundus or body of the stomach herniating posterior to the GEJ into the right chest. Dissecting into the right chest is often more difficult because of trocar angles and obstruction from the liver. Advance awareness in terms of planning trocar placements as well as including the right chest wall in the sterile field to allow for thoracoscopic assistance should be employed. As a last resort, conversion to open surgery may be necessary, though not necessarily easier due to some loss of both exposure and the benefit of pneumoperitoneum. Thoracotomy can be considered, though in our experience it has almost never been needed when a pure laparoscopic procedure was planned.
The “strangulated” hernia
Presentation and diagnosis
The most serious complication of PEH is when the obstruction progresses to incarceration and strangulation of the stomach. While the terms incarceration and strangulation have sometimes been used interchangeably the term strangulation should only be used if the blood supply to the stomach is so compromised that its viability is questioned and resection must be seriously considered (22). Strangulation is most likely to occur in the organo-axial type of gastric volvulus when the degree of rotation continues beyond 180 degrees (22) (Figure 3). As the gastric distension worsens, several obstruction points may ensue, including the esophagus, the mid portion of the stomach and the duodenum, all at the level of the diaphragmatic hiatus (5). Prompt relief of this obstruction is necessary, otherwise strangulation of the stomach occurs. Although strangulation is relatively rare in hiatal hernia, it is a more common occurrence in traumatic diaphragmatic hernias (22).
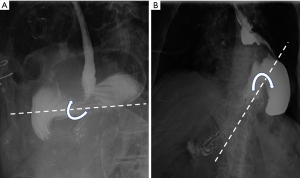
Strangulation is suspected when a patient with a known large hiatal hernia presents with acute chest or upper abdominal pressure and pain, most typically after a meal. In this constellation of symptoms known as Borchardt’s triad, patients have severe distress, presenting with chest pain and retching with inability to vomit, and inability to pass a nasogastric tube (5). Chest X-ray and clinical symptoms may be adequate for diagnosis. A complete blood count (CBC) and blood chemistries should be done early to assess for ischemia.
Initial management
Urgent decompression is mandatory and can often be accomplished with a nasogastric tube (23,36). If decompression with a nasogastric tube is unsuccessful, prompt EGD almost always succeeds, and at any rate should be performed in the early course of the disease to assess viability of the gastric mucosa (36). If decompression is accomplished and warning signs of ischemia are not present the condition has been converted to a semi-elective situation, allowing time for recovery from the acute event, planning for repair, and possible manometry. The timing for semi-elective repair, however, remains unclear. Currently, no study has directly compared surgery during the index admission versus surgery after discharge from the hospital. However, it seems delaying definitive surgery may lead to recurrent admissions. Wirsching et al. noted that patients repaired within 30 days had a 10% emergency department admission rate while waiting for surgery, compared to 31% for patients scheduled for surgery after 30 days from their first presentation (36).
If the herniation cannot be decompressed or there is evidence of ischemia, prompt surgical intervention is warranted (37,38). Hill showed that mortality can be as high as 50% in those patients needing immediate surgery due to unsuccessful nasogastric decompression (23). However, a more contemporary study showed no difference in mortality between patients repaired within 24 hours compared to those repaired after a day, although this was a national database study that did not report reasons for early or delayed surgery (39).
Surgical management
While strangulation presents a more challenging scenario, the operative strategy is not unlike that for the non-strangulated hernia; this can either be via a transthoracic or transabdominal approach (40), though the laparoscopic approach is generally preferred, particularly for type IV hernias (26,41). Generally, the pneumoperitoneum induced by CO2 dilates the hiatus and makes reduction possible. Viability of the stomach and/or other organs must be carefully determined, and may require intraoperative endoscopy to assess gastric mucosa as well as laparoscopic assessment of the stomach wall (42). If gangrene appears to be full thickness and particularly if the amount and location of non-viable stomach is not extensive nor in a critical area, it is more expeditious to proceed with stapled resection of the non-viable portion at the time of the first operation. The need for major gastric resection with reconstruction is unlikely but possible; a detailed description of the technique of the procedure is beyond the scope of this work. Alternatively, for borderline situations repeat endoscopy 24 hours later may be preferable. The same holds true for non-viability of colon or small bowel, though resection is obviously considerably more complicated, and endoscopy is not helpful for follow up assessment. A second-look laparoscopy 24 hours later may be advisable. If resection is necessary it is inadvisable to perform a relaxing incision with permanent mesh closure (43).
Morbidity and mortality
While no specific data exist on the outcomes of strangulated type IV PEHs, Tam et al. found that non-elective repair was independently associated with a 1.7 times increased odds of major adverse events and trended toward an increase of 2.7 times for odds of mortality compared to elective repair, after accounting for age and comorbid index score (18). Augustin et al. demonstrated that in unadjusted analysis, patients undergoing emergent surgery for PEH demonstrated 11 times greater odds of mortality compared with individuals who underwent elective PEH repair, although, on multivariable analyses emergent surgical indication did not independently predict mortality (2). However, Shea et al. showed that mortality was not statistically different when a propensity matched cohort comparing emergent and elective patients was investigated (44). They concluded that in experienced hands, complication rates of emergency operations appear to approach those of elective patients when matched for baseline characteristics, which they state may be particularly true for high volume centers (44). They therefore recommended that patients presenting with emergent indications for repair should be evaluated if possible at high-volume centers, where complication rates of emergency operations appear to approach those of elective repair. (44). Despite this divergence in evidence emergent repair is at the very least extraordinarily disruptive, and at worst is likely associated with inability to prepare adequately for a major operation in an often frail elderly patient, with subsequent higher risk of morbidity/mortality.
Other reports suggest that morbidity and mortality is largely dependent on timing of surgery (36,39). Bhayani et al. showed a morbidity rate of 23% vs. 31% and mortality rate of 5.4% and 4% between early and interval repair groups from their analysis of acutely incarcerated and obstructed patients in a national database (39). Wirsching et al. showed that overall mortality can be as low as 0.2% if patients undergo a staged approach, decompression followed by semi-elective repair (36). Both studies showed similar pulmonary, wound and urinary complications between early and interval repair patients. They therefore, recommend resuscitation, decompression and semi-elective repair to optimize the patient with an incarcerated, potentially strangulated, PEH.
Additional factors associated with worse outcomes include older age, higher BMI and comorbidities. Luketich et al. showed that age over 70, BMI over ≥35 kg/m2, and Charlson comorbidity index score ≥3 were associated with increased mortality (25). Similarly, Augustin et al. discovered that factors such as higher frailty score and preoperative sepsis were associated with increased odds of mortality (2). On the other hand, laparoscopic repair and BMI 25–30 and BMI ≥30 were associated with reduced odds of mortality (2), while underweight patients (as defined by BMI <18.5) have an increased risk of mortality (9.6 times increased odds of mortality compared to patients with BMI ≥30, P value <0.001) (2).
Conclusions
Although complex and acute PEHs are only a small portion of all PEHs, they present a particular challenge to the surgeon in terms of their management. Unlike less complex PEHs where the surgeon has the luxury of outpatient evaluation and elective repair, acute PEHs require more prompt evaluation and intervention. Evaluation needs to identify life-threatening complications, such as strangulation. Expedient decompression either with nasogastric tube or endoscopy is necessary and will sometimes allow for semi-elective repair, allowing for resuscitation in a patient who may be older or frail at baseline. Ultimately, surgical repair is essential, although timing for surgery remains controversial. Type IV hernias can present some unique clinical and technical challenges but for the most part present and are managed similarly to type II and III hernias.
Acknowledgments
We would like to thank Dr. Brian E. Louie for feedback on this project. We would also like to acknowledge Dale E. Shultz for assistance in identifying patients with appropriate images for the manuscript.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Lee L. Swanstrom and Steven G. Leeds) for the series “Hiatal Hernia” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales-20-7). The series “Hiatal Hernia” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Banimostafavi ES, Tayebi M. Large hiatal hernia with pancreatic body herniation: Case-report. Ann Med Surg (Lond) 2018;28:20-2. [Crossref] [PubMed]
- Augustin T, Schneider E, Alaedeen D, et al. Emergent Surgery Does Not Independently Predict 30-Day Mortality After Paraesophageal Hernia Repair: Results from the ACS NSQIP Database. J Gastrointest Surg 2015;19:2097-104. [Crossref] [PubMed]
- Biswas S, Gogna S, Patel P. Jejunal Diverticular Perforation Causing Small Bowel Obstruction in a Type 4 Hiatal Hernia: A Rare Case Report of a Nonagenarian Patient and Review of Relevant Literature. Case Rep Surg 2017;2017:8412927 [Crossref] [PubMed]
- Maiti A, Saha D, Basu A. Type 4 Hiatal Hernia. Am J Med Sci 2016;351:e5 [Crossref] [PubMed]
- Abbara S, Kalan MM, Lewicki AM. Intrathoracic stomach revisited. AJR Am J Roentgenol 2003;181:403-14. [Crossref] [PubMed]
- Rozas MG, González MM. A rare complication of hiatal hernia. Gastroenterology 2010;139:e1-e2. [Crossref] [PubMed]
- Hsu CT, Hsiao PJ, Chiu CC, et al. Terminal ileum gangrene secondary to a type IV paraesophageal hernia. World J Gastroenterol 2016;22:2642-6. [Crossref] [PubMed]
- Weller S, Powers C, Sly B, et al. Severe type IV hiatal hernia secondary to congenital shortened esophagus. JAAPA 2017;30:1-3. [Crossref] [PubMed]
- Sihvo EI, Salo JA, Räsänen JV, et al. Fatal complications of adult paraesophageal hernia: a population-based study. J Thorac Cardiovasc Surg 2009;137:419-24. [Crossref] [PubMed]
- Stylopoulos N, Gazelle GS, Rattner DW. Paraesophageal hernias: operation or observation? Ann Surg 2002;236:492-500. [Crossref] [PubMed]
- George D, Apostolos PV, Athanasios P, et al. Struggling with a Gastric Volvulus Secondary to a Type IV Hiatal Hernia. Case Rep Med 2010;2010:257497 [Crossref] [PubMed]
- Horgan S, Eubanks TR, Jacobsen G, et al. Repair of paraesophageal hernias. Am J Surg 1999;177:354-8. [Crossref] [PubMed]
- Dahlberg PS, Deschamps C, Miller DL, et al. Laparoscopic repair of large paraesophageal hiatal hernia. Ann Thorac Surg 2001;72:1125-9. [Crossref] [PubMed]
- Wiechmann RJ, Ferguson MK, Naunheim KS, et al. Laparoscopic management of giant paraesophageal herniation. Ann Thorac Surg 2001;71:1080-6; discussion 1086-7. [Crossref] [PubMed]
- Hawasli A, Zonca S. Laparoscopic repair of paraesophageal hiatal hernia. Am Surg 1998;64:703-10. [PubMed]
- Gantert WA, Patti MG, Arcerito M, et al. Laparoscopic repair of paraesophageal hiatal hernias. J Am Coll Surg 1998;186:428-32; discussion 432-3. [Crossref] [PubMed]
- Krähenbühl L, Schafer M, Farhadi J, et al. Laparoscopic treatment of large paraesophageal hernia with totally intrathoracic stomach. J Am Coll Surg 1998;187:231-7. [Crossref] [PubMed]
- Tam V, Luketich JD, Winger DG, et al. Non-Elective Paraesophageal Hernia Repair Portends Worse Outcomes in Comparable Patients: a Propensity-Adjusted Analysis. J Gastrointest Surg 2017;21:137-45. [Crossref] [PubMed]
- Polomsky M, Jones CE, Sepesi B, et al. Should elective repair of intrathoracic stomach be encouraged? J Gastrointest Surg 2010;14:203-10. [Crossref] [PubMed]
- Jassim H, Seligman JT, Frelich M, et al. A population-based analysis of emergent versus elective paraesophageal hernia repair using the Nationwide Inpatient Sample. Surg Endosc 2014;28:3473-8. [Crossref] [PubMed]
- Levy G, Aye RW, Farivar AS, et al. A Combined Nissen Plus Hill Hybrid Repair for Paraesophageal Hernia Improves Clinical Outcomes and Reduces Long-Term Recurrences Compared with Laparoscopic Nissen Alone. J Gastrointest Surg 2017;21:121-5. [Crossref] [PubMed]
- Beardsley JM, Thompson WR. Acutely obstructed hiatal hernia. Ann Surg 1964;159:49-62. [Crossref] [PubMed]
- Hill LD. Incarcerated paraesophageal hernia. A surgical emergency. Am J Surg 1973;126:286-91. [Crossref] [PubMed]
- Poulose BK, Gosen C, Marks JM, et al. Inpatient mortality analysis of paraesophageal hernia repair in octogenarians. J Gastrointest Surg 2008;12:1888-92. [Crossref] [PubMed]
- Luketich JD, Nason KS, Christie NA, et al. Outcomes after a decade of laparoscopic giant paraesophageal hernia repair. J Thorac Cardiovasc Surg 2010;139:395-404, 404.e1. [Crossref] [PubMed]
- Lugaresi M, Mattioli B, Daddi N, et al. Surgery for Type III-IV hiatal hernia: anatomical recurrence and global results after elective treatment of short oesophagus with open and minimally invasive surgery. Eur J Cardiothorac Surg 2016;49:1137-43. [Crossref] [PubMed]
- Tagaya N, Tachibana M, Kijima H, et al. Laparoscopic treatment of paraesophageal hiatal hernia with incarceration of the pancreas and jejunum. Surg Laparosc Endosc Percutan Tech 2007;17:313-6. [Crossref] [PubMed]
- Grushka JR, Grenon SM, Ferri LE. A type IV paraesophageal hernia containing a volvulized sigmoid colon. Dis Esophagus 2008;21:94-6. [Crossref] [PubMed]
- Carvalheiro J, Mendes S, Sofia C. Hiatal hernia involving pancreas body: An unusual finding. GE J Port Gastrenterol 2014;21:85-7. [Crossref]
- Indiran V. Isolated Focal Herniation of Pancreatic Body Through Esophageal Hiatus in a Patient With Scoliosis. Clin Gastroenterol Hepatol 2016;14:e39-40. [Crossref] [PubMed]
- Valente T, Rossi G, Lassandro F, et al. Asymptomatic isolated partial hiatal herniation of the pancreas: MDCT evaluation and anatomical explanation: case report and review of literature. Clin Anat 2013;26:1008-13. [Crossref] [PubMed]
- Katz M, Atar E, Herskovitz P. Asymptomatic diaphragmatic hiatal herniation of the pancreas. J Comput Assist Tomogr 2002;26:524-5. [Crossref] [PubMed]
- Saxena P, Konstantinov IE, Koniuszko MD, et al. Hiatal herniation of the pancreas: diagnosis and surgical management. J Thorac Cardiovasc Surg 2006;131:1204-5. [Crossref] [PubMed]
- Szwerc MF, Landreneau RJ. Splenic rupture as a consequence of giant paraesophageal hernia. Ann Thorac Surg 2000;70:1727-8. [Crossref] [PubMed]
- Bradley DD, Louie BE, Farivar AS, et al. Assessment and reduction of diaphragmatic tension during hiatal hernia repair. Surg Endosc 2015;29:796-804. [Crossref] [PubMed]
- Wirsching A, El Lakis MA, Mohiuddin K, et al. Acute Vs. Elective Paraesophageal Hernia Repair: Endoscopic Gastric Decompression Allows Semi-Elective Surgery in a Majority of Acute Patients. J Gastrointest Surg 2018;22:194-202. [Crossref] [PubMed]
- Díez Ares JÁ, Peris Tomas N, Estelles Vidagany N, et al. Gastric necrosis secondary to strangulated giant paraesophic hiatal hernia. Rev Esp Enferm Dig 2016;108:498-500. [PubMed]
- Hoff R, Qazi B. Strangulated Paraesophageal Hiatal Hernia. J Am Osteopath Assoc 2018;118:207. [PubMed]
- Bhayani NH, Kurian AA, Sharata AM, et al. Wait only to resuscitate: early surgery for acutely presenting paraesophageal hernias yields better outcomes. Surg Endosc 2013;27:267-71. [Crossref] [PubMed]
- Gryglewski A, Kuta M, Pasternak A, et al. Hiatal hernia with upside-down stomach. Management of acute incarceration: case presentation and review of literature. Folia Med Cracov 2016;56:61-6. [PubMed]
- Nattakom T, Schuerer D, Batra S, et al. Emergency laparoscopic repair of a paraesophageal hernia. Surg Endosc 1999;13:75-6. [Crossref] [PubMed]
- Liao K, Ramirez J, Carryl S, et al. A new approach in the management of incarcerated hernia. Emergency laparoscopic hernia repair. Surg Endosc 1997;11:944-5. [Crossref] [PubMed]
- Crespin OM, Yates RB, Martin AV, et al. The use of crural relaxing incisions with biologic mesh reinforcement during laparoscopic repair of complex hiatal hernias. Surg Endosc 2016;30:2179-85. [Crossref] [PubMed]
- Shea B, Boyan W, Decker J, et al. Emergent Repair of Paraesophageal Hernias and the Argument for Elective Repair. JSLS 2019;23:e2019.00015.
Cite this article as: Baison GN, Aye RW. Complex and acute paraesophageal hernias—type IV, strangulated, and irreducible. Ann Laparosc Endosc Surg 2021;6:42.


