
The short esophagus—lengthening techniques
Introduction
Management of the ‘short esophagus’ found during surgery for gastroesophageal reflux disease and hiatal hernia repair has been the subject of surgical controversy since the first descriptions in the early 1900s. Shortening of the esophagus in adults, currently accepted as an acquired condition, was theorized in 1938 (1). Long-standing reflux damage resulting in fibrotic shortening of the esophagus as well as chronic gastric herniation resulting in mediastinal fixation can lead to situations in which standard dissection will not restore the gastroesophageal junction to an appropriate intraabdominal position, and an additional ‘lengthening’ procedure is required. This paper will attempt to meld that historical background into a discussion of the current definition, etiology, diagnosis and various surgical options for the short esophagus.
Treatment of the short esophagus has revolved around goals of treating the associated hiatal hernia and of resolving gastroesophageal reflux. Any additional length of intra-abdominal esophagus below the hiatus is a matter of reflux barrier physiology and preferred esophageal length required by some antireflux procedures. The changing causes of esophageal shortening and methods to treat reflux does make for an interesting story.
Definition of a short esophagus and relation to Nissen fundoplication
Although conceptually a short esophagus may be relatively straightforward, the reported incidence ranges from 2% to 80% in patients with giant hiatal hernias (2,3).
Current laparoscopic literature regarding a short esophagus frequently presumes a fundoplication will accompany the hiatal hernia repair. An esophagus is ‘short’ if less than 2–3 cm of visible esophagus does not rest easily within the abdominal cavity after routine dissection. Fundoplication can still be completed without a lengthening procedure, but with a greater risk of failure (4). The origin of the concept is interesting. First, the precise mark of the gastroesophageal junction is rarely defined; many diagrams show measurements taken on the right side of the esophagus, presumably where esophageal adventitia transitions to gastric serosa, e.g., Horvath, 2000 (4). The phrenoesophageal membrane and the angle of His more reproducibly mark the transition from esophagus to stomach and are normally adjacent to the abdominal side of the diaphragmatic crura, not 2–3 cm below. It would seem that achieving 2–3 cm of intra-abdominal esophagus may be unnatural, as it would be stretching a normal esophagus. Where then does the need for 2–3 cm of length arise?
One goal of antireflux surgery has been to restore abdominal length to the lower esophageal sphincter. By manometry and surgical findings, the lower esophageal sphincter does extend 2–3 cm below the angle of His. These findings indicate that a Heller myotomy must extend onto the anterior gastric serosa to divide the esophageal sling fibers all the way down to the junction of the oblique gastric sling fibers, which mark the lower margin of the LES. Accomplishing restoration of manometrically-determined abdominal length does not require an additional 2–3 cm of visible abdominal esophageal length above the phrenoesophageal membrane.
However, the bulk of a Nissen or similar fundoplication does require the stomach be placed around 2–3 cm of tubular esophagus. This is likely a major reason that fundoplication and hernia repairs accompanied by fundoplication fail when that 2–3 cm of additional length is not obtained. Procedures that do not involve a bulk around the distal esophagus do not require that additional length (4).
Historical background
The current definition of a ‘short esophagus’ is due to the requirements of a Nissen fundoplication. This is important as it relates to early surgical description and treatment of the ‘short esophagus.’
Philip Allison in 1948 described the ‘short esophagus’ as a radiologic finding in a review of 63 patients with peptic ulcers of the esophagus (5). The radiologic findings defining a hiatal hernia were known; Allison’s paper however recognized 8 patterns of a short esophagus, 5 of which were associated with various degrees of stricture. Allison also observed in his postmortem and surgical dissections that “the lowest part of the esophagus is enclosed in a thick-walled tunnel of the diaphragmatic crura…. there is in life no such thing as the abdominal oesophagus. This part is embedded in the diaphragmatic muscle except for a small triangular bare area in front.” Allison’s paper sets the stage: “The rational treatment is to cure the deformity which allows acid to reach the oesophagus. The success of this depends on the oesophagus being elastic enough to reach below the diaphragm.” At that time, due to the severity of stricture rendering the esophagus inelastic, partial esophagectomy was the most common surgical solution, even though it did not cure the deformity.
At the same time, observations by rigid endoscopy and resected specimens indicated that sometimes a segment of what appeared to be esophagus by its tubular nature above the hiatus, had instead a columnar-lined epithelium and peritoneal covering consistent with stomach. Norman Barrett in 1950 considered columnar lining always indicative of gastric tissue (6). Allison in 1953, in describing what for many years has been called the ‘cardia’, made a keen observation: “The oesophagus (below a stricture), which is lined by gastric mucosa, retains its tubular contour, although it may be a little dilated. The position of the cardia can be identified where the lumen widens again to form the sac of the herniated stomach….” The cardia is an intermediate tubular structure that represents esophagus lined by gastric epithelium without “a demonstrable change in mucosal pattern” and results from reflux (7). It is not often that surgeons are known to change their opinion; however, Barrett in 1957 aligned with Allison that the lower part of the esophagus can be “lined by columnar epithelium extending upward for a short or long distance above the esophagogastric junction,” and had his name eponymously used henceforth as “Barrett’s esophagus” (8).
At this time, the barrier to reflux was called the ‘cardiac valve’ and more attention was paid to the flap-valve effect of the angulated gastroesophageal junction and the pinchcock effect of the diaphragmatic hiatus, and little was known about what is now defined by manometry as the lower esophageal sphincter. In a sliding hiatal hernia, competence of the ‘cardiac valve’ was attained by restoring parts to their normal anatomic position by repair of the phrenoesophageal membrane (9). In 1954 Collis described a similar restoration of the cardiac valve by closing the hiatal opening anterior to the esophagus (as opposed to posterior, as Allison had done), and in 1968 reported 80% 4-year control of reflux with this procedure (10,11).
A different understanding of, and approach to the treatment of reflux esophagitis was taken by Rudolf Nissen (12). He believed that reflux was due to a functional insufficiency of the gastroesophageal sphincter and its sequalae. “Consequently, the principal purpose of the operation is not the elimination of the hernia, but the restoration of the sphincteral function (13).” In Nissen’s original description, which he used in all cases except those of the paraesophageal type, the peritoneal covering of the gastroesophageal junction was incised so that the intraabdominal esophagus could be “pulled down approximately 10 cm[sic]!” Reduction of the herniated stomach relied on a Boerama anterior gastropexy, no closure of the hiatal opening was performed.
In 1957 Collis published a technique, which still bears his name, to treat hiatus hernia in the setting of a markedly short esophagus (14). The technique involved creating a tube of gastric tissue to act as a conduit between the lower end of the esophagus and the main body of the stomach. Collis justified the use of acid-secreting gastric tissue as prior studies demonstrated that the proximal stomach (the cardia) had 50% of the parietal cell density as the rest of the stomach. The procedure was performed through a thoracic approach, placing two clamps longitudinally along the herniated stomach parallel and very close to the lesser curve, then dividing between the clamps and oversewing the divided tissue (Figure 1). The remnant, non-connecting portion is placed below the diaphragm and then “the limbs of the right crus are now sutured together and in front of the connecting tube. This produces an acute angle between the connecting tube and the main body of the stomach.” No fundoplication was performed; prevention of reflux relied upon recreation of the acute angle at the entry to the main body of the stomach by closing the hiatus anterior to the esophagus as had been described in his 1954 paper.
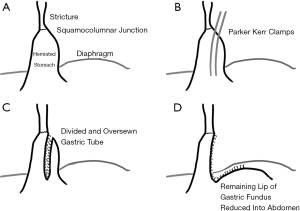
Collis also astutely observed that some patients undergoing paraesophageal hernia repair developed reflux when none was present preoperatively. Confirmed by other studies, current ‘dogma’ is to perform an antireflux procedure—typically a Nissen fundoplication—in all paraesophageal hernia repairs regardless of preoperative reflux findings.
Thal et al. in 1965 described an antireflux procedure which left the stomach above the diaphragm.
In 1971 Pearson et al. reported their initial experience of combining a Collis gastroplasty with a Belsey-type partial fundoplication performed through a thoracotomy. Although the initial indication for gastroplasty was obvious shortening due to peptic stricture, Pearson recognized that more subtle shortening occurred “in cases of gross ulcerative esophagitis, even without a peptic stricture”. The gastroplasty was created over a 48-Fr Maloney dilator and was generally 5 cm long. The partial fundoplication used in this situation was longer than a typical Belsey Mark IV operation. When the operation was completed, the upper end of the gastric tube would be at or just above the thoracic side of the diaphragmatic hiatus. The objective of the operation was “to achieve an antireflux repair that is free of tension and therefore highly unlikely to suffer an anatomic recurrence of the hiatus hernia” (15), a mantra that has stayed the course of time.
Orringer and Sloan in 1978 described combining the Collis procedure with a 360-degree Nissen fundoplication, as reports of the Collis-Belsey indicated an unexpectedly high (30–46%) failure rate in controlling reflux (16). The reason for failure, according to the paper was that “construction of the gastroplasty tube so reduces the amount of gastric fundus available for fundoplication that a standard 240-degree Belsey wrap around the new distal esophagus cannot be achieved.” This is a curious statement, as the paper does not go on to explain how a 360-degree wrap can be constructed when there is insufficient funds for a 240-degree wrap. Orringer’s thoracic approach used a 56-60F dilator in the lumen of the esophagus and performed the gastroplasty with two applications of a GIA surgical stapler creating a “functional distal esophagus” of 5–10 cm. The subsequent 360-degree fundoplication was created around a 46Fr dilator, of 6 cm in length, and enfolded 3–4 cm of gastric fundus as well as 3–4 cm of the gastroplasty tube.
Up until the report by Steichen in 1986, the Collis gastroplasty was commonly performed through a left thoracotomy (17). Using an abdominal approach, Steichen described using an end-to-end anastomotic stapler (EEA) to create a ‘buttonhole’ uniting anterior and posterior gastric walls 5 cm distal to, and in vertical alignment of the angle of His. Subsequently a gastrointestinal anastomotic (GIA) stapler could be passed cranially through this buttonhole, parallel to the lesser curve, to divide the fundus up to the angle of His—creating in a cranially-directed fashion what had previously been done caudally-directed through the chest. The flap of fundus created was then used to create a 360-degree fundoplication.
Other procedures for the short esophagus included creating an intrathoracic Nissen fundoplication (18), if necessary, accompanied by a longitudinal opening of the stricture and suturing the gastric wall as a patch over the esophageal opening (Thal-Nissen procedure), as well as a stapled uncut gastroplasty. Neither of these gained wide acceptance (19). The Collis procedure did reduce the need for esophageal resection in cases of short esophagus associated with strictures not refractory to dilation (19).
The era of laparoscopic surgery and potent acid-suppression medications
By the mid-1990s, two developments—laparoscopic fundoplication and proton pump inhibitors (PPIs)—altered the landscape of the short esophagus. With the use of PPIs, refractory transmural strictures leading to irreversible esophageal shortening became less frequent. Laparoscopic fundoplication in the short-term increased the number of patients receiving an abdominal, minimally invasive approach to antireflux surgery.
Early descriptions of laparoscopic fundoplication recognized that failure to obtain that additional 2–3 cm of length was associated with a higher failure rate. Careful preoperative endoscopic and radiologic evaluation to identify a fixed hernia which would likely need a lengthening procedure was promoted as laparoscopic methods of performing a Collis gastroplasty were lacking. A fixed hernia of >5 cm from diaphragm to the gastroesophageal junction seemed to be the most reliable landmark (20).
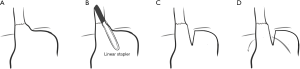
In 1996 Swanstrom and colleagues reported the outcomes of 34 patients with a >5 cm axial hernia on preoperative endoscopy. In 10 patients the EGJ could easily be brought 2 cm or more below the diaphragm. Extensive trans-abdominal mediastinal dissection (performed under direct laparoscopic visualization, which had not been performed during open surgery) was successfully used in 17 of the remaining 24. Of the remaining 7 patients, 4 had crural repair and gastropexy. The remaining 3 had a novel minimally invasive Collis procedure performed with an endoscopic linear GIA stapler inserted through the right chest under thoracoscopic visualization, with the mediastinal dissection, crural repair, and fundoplication performed laparoscopic technique (Figure 2). Based on improved outcomes of the 3 Collis patients compared to the 17 with extended mobilization, the paper concluded that laparoscopic Collis gastroplasty was the treatment of choice for the shortened esophagus (21).
Johnson in 1998 reported that 26% of 220 patients undergoing laparoscopic antireflux surgery, had esophageal foreshortening by preoperative evaluation using the >5 cm fixed height criterion. After hiatal dissection, 9 of these patients (16% of those suspected to have foreshortening) did not have 2 cm of esophagus resting easily below the diaphragm and required Collis gastroplasty. A minimally invasive variation of the EEA/GIA stapling technique described by Steichen in 1986 was used to perform the gastroplasty in these patients (22).
In 2002 Jobe reported follow-up on fifty-two patients with laparoscopic adaptation of a Hill posterior gastropexy repair in giant hiatal hernia with short esophagus without gastroplasty (23). Small, asymptomatic axial recurrences were noted in 4 patients; larger hernias due to disruption of the hiatal repair—not the gastropexy—were seen in 7. The study concluded that the Hill procedure avoided the need for gastroplasty because it does not require the 2–3 cm of additional esophageal length of a fundoplication.
In 2004 Terry reported results of laparoscopic resection of a wedge of gastric fundus to create a Collis gastroplasty, obviating the need for an EEA stapled buttonhole (which was difficult to place with laparoscopy) or the double-lumen endotracheal tube required for the right chest approach (Figure 3) (24).
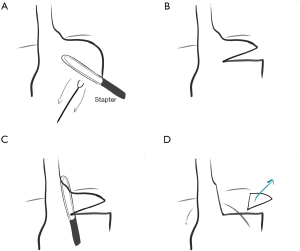
In 2014 Wilson and colleagues described a left chest approach to a Collis using an articulated endoscopic stapler. A left sided pneumothorax is induced and through a single port in the 4th intercostal space the articulated stapler is advanced gently (largely by feel) through the mediastinal pleural defect and the hiatus and with jaws open along the gastric fundus starting at the angle of His to complete the gastroplasty. A 46Fr Bougie is used to size the gastroplasty (25).
Disadvantages to the Collis procedure
The gastric conduit created by the Collis gastroplasty places the acid-secreting mucosa of the gastric fundus against an already diseased esophagus (26). In one study, 7/15 patients were found to have acid-secreting parietal cells in the neo-esophagus above the wrap and esophagitis or the need for acid-suppressive medication has been reported (27).
Whether the gastric tube of a Collis retains peristalsis remains unclear. Theoretically, this segment should be aperistaltic (27).
Collis gastroplasty can make ablation difficult in distal esophagus secondary to acid production by the tubularized oxyntic gastric tissue (28).
Alternatives to Collis procedure.
Recognizing variations in degree of esophageal shortening, O’Rourke and colleagues described extended mediastinal dissection of the esophagus 7–10 cm circumferentially above the hiatus as an option to reduce reflex recourse to a Collis gastroplasty (29). This extended, “Type II” mediastinal dissection was performed in 92% of patients with a mixed paraesophageal hernia, and in 26% of patients undergoing routine fundoplication. The report does not mention the frequency with which this procedure was inadequate and an intraoperative decision to perform a Collis gastroplasty occurred but suggests it would be in the 2–4% of cases.
Oelschlager noted that a frequent finding in a short esophagus was tension from the vagus nerves. Division of one (in 26 patients) or both (in 4 patients) vagal nerves would in these situations provide 2–3 cm of additional esophageal length, negating the need for a Collis procedure. Post-vagotomy side effects in the unilateral vagotomy group were no different than in matched controls; 4/4 patients undergoing bilateral vagotomy did report persistent dumping (30).
The possibility of vagal mobilization rather than vagal division was described by Herbella in 2009. Although no data are presented, conceptually this could enable preservation of vagal function while still obtaining additional intraabdominal esophageal length (31).
In 2017, Bellevue et al. (32) compared a laparoscopic modified Hill gastropexy with Nissen fundoplication to a laparoscopic Collis-Nissen procedure in 106 patients with a short esophagus by preoperative testing. Though not meeting statistical significance, postoperative pH and endoscopic findings favored the Hill-Nissen, whereas recurrent hernia >2 cm favored the Collis-Nissen.
Emerging methods
Magnetic sphincter augmentation initially used as an alternative to fundoplication in patients with <3 cm hiatal hernias, has demonstrated utility in patients with large hiatal hernias and paraesophageal hernias (33,34). As it has a cranial-caudal dimension on the esophagus of ~6 mm and in most instances is placed just above the GEJ, it does not necessitate obtaining the additional and ‘artificial’ 2–3 cm of visible abdominal esophageal length that a Nissen fundoplication requires. MSA with LINX may have a role controlling reflux without the need for gastroplasty (which would not be advisable with an implant). Results in controlling reflux and preventing recurrent herniation with MSA in patients with giant hernias (without resorting to gastroplasty) have been encouraging (34).
Conclusions
The purpose of this review has not been to detail the success or failure of these various techniques to lengthen the esophagus. Variations in definition, surgical judgement and technique, and outcomes assessments would make any such assessment fraught with potential error. Some principles do, however, emerge that may guide a surgeon in managing this complex issue.
The need for additional visible length of intra-abdominal esophagus to perform an esophagogastric fundoplication is rarely mentioned in reports of Collis-Nissen procedures. The native external esophago-gastric junction is at the insertion of the phrenoesophageal membrane to the inferior edge of the diaphragmatic crura, and manometrically the lower esophageal sphincter extends beyond this as is recognized during Heller myotomy. The additional 2–3 cm needed to perform a fundoplication is in many respects unnatural and may to some extent contribute to fundoplication failures.
Early descriptions by Collis reflect a gastroplasty performed along a “broad neck of gastric tissue” and the EGJ is clearly well above the hiatus. This broad neck of gastric tissue in some cases probably represented a dilated distal esophagus, in which the angle of His had dehisced (Figure 4). In these instances, the so-called gastroplasty would not contain oxyntic, acid-secreting mucosa. More modern descriptions show a gastroplasty starting at the angle of His, creating an acid-secreting neo-esophagus.
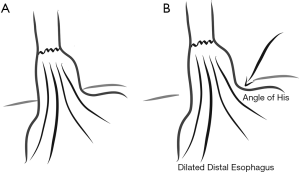
Initial descriptions of the short esophagus dealt with patients in whom uncontrollable acidic gastric reflux led to transmural fibrotic shortening. With modern acid-suppression medication, such transmural shortening is uncommon. More often the esophageal shortening results from accordion-like periesophageal fixation as the hernia becomes more fixed in the mediastinum combined with paraesophageal herniation of the stomach. Extensive mediastinal mobilization will often allow the esophagus to ‘regain’ its native length. Additionally, untethering the Vagus nerves can provide additional length.
A technical aspect to esophageal mobilization which is difficult to quantify and thus to study is that of dissecting peri-esophageal adventitial fibrosis. Untethering the vagus nerves down to the lesser curve and up 5–10 cm on the esophagus will release a fair portion of the adventitial fibrosis that accompanies long-standing herniation (Figure 5). Frequently an artery running along the antero-medial surface of the esophagus is an additional locus of fibrosis. As experience is gained, careful denuding of the esophagus will provide even further gain in esophageal length as it is this adventitial scarring that limits esophageal length. With the technique of esophageal adventitial dissection, the author’s personal rate of Collis gastroplasty in paraesophageal hernias with short esophagus decreased from 18% (35) to 0% (personal data, unpublished). Lastly, MSA may fill the void for the need for increased intra-abdominal length of the esophagus to accommodate a bulky fundoplication.

As is often said regarding other hernia repairs, the best repair is the one the surgeon knows the best. Recognizing the problem must be followed by meticulous, practiced technique; but it is not clear that one technique is better than another.
Synopsis
Original descriptions of the ‘short esophagus’ by Allison and Collis were in patients with severe acid-reflux related esophageal strictures resulting in transmural fibrosis and shortening, when medical therapy for reflux was limited. Surgical intervention following Collis’ method was performed with an open thoracic approach, positioned the neo-esophago-gastric junction just below the diaphragm, and initially did not include a fundoplication.
With the widespread use of potent acid-suppressive medication, most esophageal shortening is due to long-standing herniation with resultant mediastinal fixation and associated peri-esophageal adventitial fibrosis. Extensive trans-abdominal laparoscopic mediastinal mobilization often frees up this accordioned esophagus and restores native esophageal length with the esophago-gastric junction below or inferior to the diaphragm. Posterior fundoplication requires an additional 2–3 cm of visible abdominal esophagus to ensure the plication is properly placed around distal esophagus. In such instances additional length has been obtained by performing a gastroplasty following Collis’ principles, though now performed with minimally invasive laparoscopic technology. Alternatives to gastroplasty include extensive dissection of peri-esophageal fibrosis with vagus nerve mobilization or division, performance of a Hill posterior gastropexy with or without fundoplication or use of MSA.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Lee L. Swanstrom and Steven G. Leeds) for the series “Hiatal Hernia” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales-20-55). The series “Hiatal Hernia” was commissioned by the editorial office without any funding or sponsorship. RCWB reports personal fees from Ethicon EndoSurgery, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Herbella FA, Patti MG, Del Grande JC. When did the esophagus start shrinking? The history of the short esophagus. Dis Esophagus 2009;22:550-8. [Crossref] [PubMed]
- Hashemi M, Peters JH, DeMeester TR, et al. Laparoscopic repair of large type III hiatal hernia: objective followup reveals high recurrence rate. J Am Coll Surg 2000;190:553-60; discussion 560-51. [Crossref] [PubMed]
- Maziak DE, Todd TR, Pearson FG. Massive hiatus hernia: evaluation and surgical management. J Thorac Cardiovasc Surg 1998;115:53-60; discussion 61-52. [Crossref] [PubMed]
- Horvath KD, Swanstrom LL, Jobe BA. The short esophagus: pathophysiology, incidence, presentation, and treatment in the era of laparoscopic antireflux surgery. Ann Surg 2000;232:630-40. [Crossref] [PubMed]
- Allison PR. Peptic ulcer of the oesophagus. Thorax 1948;3:20-42. [Crossref] [PubMed]
- Barrett NR. Chronic peptic ulcer of the oesophagus and 'oesophagitis'. Br J Surg 1950;38:175-82. [Crossref] [PubMed]
- Allison PR, Johnstone AS. The oesophagus lined with gastric mucous membrane. Thorax 1953;8:87-101. [Crossref] [PubMed]
- Barrett NR. The lower esophagus lined by columnar epithelium. Surgery 1957;41:881-94. [PubMed]
- Allison PR. Reflux esophagitis, sliding hiatal hernia, and the anatomy of repair. Surg Gynecol Obstet 1951;92:419-31. [PubMed]
- Collis JL, Kelly TD, Wiley AM. Anatomy of the crura of the diaphragm and the surgery of hiatus hernia. Thorax 1954;9:175-89. [Crossref] [PubMed]
- Collis JL. Surgical control of reflux in hiatus hernia. Am J Surg 1968;115:465-71. [Crossref] [PubMed]
- Nissen R. A simple operation for control of reflux esophagitis. Schweiz Med Wochenschr 1956;86:590-2. [PubMed]
- Nissen R. The treatment of hiatal hernia and esophageal reflux by fundoplication. In: Nyhus LM, Harkins HN. Editors. 1st edition. Philadelphia, PA: Lippincott, 1964:488-96.
- Collis JL. An operation for hiatus hernia with short oesophagus. Thorax 1957;12:181-8. [Crossref] [PubMed]
- Pearson FG. Complications and pitfalls: Belsey and Collis-Belsey antireflux repairs. Chest Surg Clin N Am 1997;7:513-32. [PubMed]
- Orringer MB, Sloan H. Combined Collis-Nissen reconstruction of the esophagogastric junction. Ann Thorac Surg 1978;25:16-21. [Crossref] [PubMed]
- Steichen FM. Abdominal approach to the Collis gastroplasty and Nissen fundoplication. Surg Gynecol Obstet 1986;162:272-4. [PubMed]
- Moghissi K. Intrathoracic fundoplication for reflux stricture associated with short oesophagus. Thorax 1983;38:36-40. [Crossref] [PubMed]
- Adler RH. Collis gastroplasty: origin and evolution. Ann Thorac Surg 1990;50:839-42. [Crossref] [PubMed]
- Urbach DR, Khajanchee YS, Glasgow RE, et al. Preoperative determinants of an esophageal lengthening procedure in laparoscopic antireflux surgery. Surg Endosc 2001;15:1408-12. [Crossref] [PubMed]
- Swanstrom LL, Marcus DR, Galloway GQ. Laparoscopic Collis gastroplasty is the treatment of choice for the shortened esophagus. Am J Surg 1996;171:477-81. [Crossref] [PubMed]
- Johnson AB, Oddsdottir M, Hunter JG. Laparoscopic Collis gastroplasty and Nissen fundoplication. A new technique for the management of esophageal foreshortening. Surg Endosc 1998;12:1055-60. [Crossref] [PubMed]
- Jobe BA, Aye RW, Deveney CW, et al. Laparoscopic management of giant type III hiatal hernia and short esophagus. Objective follow-up at three years. J Gastrointest Surg 2002;6:181-8; discussion 188. [Crossref] [PubMed]
- Terry ML, Vernon A, Hunter JG. Stapled-wedge Collis gastroplasty for the shortened esophagus. Am J Surg 2004;188:195-9. [Crossref] [PubMed]
- Wilson JL, Bradley DD, Louie BE, et al. Laparoscopy with left chest collis gastroplasty: a simplified technique for shortened esophagus. Ann Thorac Surg 2014;98:1860-2. [Crossref] [PubMed]
- Martin CJ, Cox MR, Cade RJ. Collis-Nissen gastroplasty fundoplication for complicated gastro-oesophageal reflux disease. Aust N Z J Surg 1992;62:126-9. [Crossref] [PubMed]
- Jobe BA, Horvath KD, Swanstrom LL. Postoperative function following laparoscopic collis gastroplasty for shortened esophagus. Arch Surg 1998;133:867-74. [Crossref] [PubMed]
- Lagergren J, Ye W, Lagergren P, et al. The risk of esophageal adenocarcinoma after antireflux surgery. Gastroenterology 2010;138:1297-301. [Crossref] [PubMed]
- O'Rourke RW, Khajanchee YS, Urbach DR, et al. Extended transmediastinal dissection: an alternative to gastroplasty for short esophagus. Arch Surg 2003;138:735-40. [Crossref] [PubMed]
- Oelschlager BK, Yamamoto K, Woltman T, et al. Vagotomy during hiatal hernia repair: a benign esophageal lengthening procedure. J Gastrointest Surg 2008;12:1155-62. [Crossref] [PubMed]
- Herbella FA. Vagotomy during hiatal hernia repair: anatomic observations. J Gastrointest Surg 2009;13:393-4; author reply 395. [Crossref] [PubMed]
- Bellevue OC, Louie BE, Jutric Z, et al. A Hill Gastropexy Combined with Nissen Fundoplication Appears Equivalent to a Collis-Nissen in the Management of Short Esophagus. J Gastrointest Surg 2018;22:389-95. [Crossref] [PubMed]
- Rona KA, Reynolds J, Schwameis K, et al. Efficacy of magnetic sphincter augmentation in patients with large hiatal hernias. Surg Endosc 2017;31:2096-102. [Crossref] [PubMed]
- Buckley FP 3rd, Bell RCW, Freeman K, et al. Favorable results from a prospective evaluation of 200 patients with large hiatal hernias undergoing LINX magnetic sphincter augmentation. Surg Endosc 2018;32:1762-8. [Crossref] [PubMed]
- Bell RC, Fearon J, Freeman KD. Allograft dermal matrix hiatoplasty during laparoscopic primary fundoplication, paraesophageal hernia repair, and reoperation for failed hiatal hernia repair. Surg Endosc 2013;27:1997-2004. [Crossref] [PubMed]
Cite this article as: Bell RCW, Freeman K. The short esophagus—lengthening techniques. Ann Laparosc Endosc Surg 2021;6:34.


