Robot-assisted spleen preserving distal pancreatectomy: case report
Introduction
According to the Miami international evidence-based guidelines on minimally invasive pancreas resection, minimally invasive distal pancreatectomy (MIPD) for benign or low-grade malignant tumors is to be considered over open distal pancreatectomy, since it is associated with a shorter hospital stay, reduced blood loss, and equivalent complication rates (1). Additionally, whenever oncologically indicated, the spleen should be preserved because of hematological and immunological advantages (2). During MIPD the spleen can be spared along with the splenic vessels (3) or despite the sacrifice of the splenic vessels (so called procedure) (4). In the Warshaw procedure, spleen supply is maintained through the left gastro-epiploic arcade and the short gastric vessels. This collateral circulation, however, may not be sufficient to ensure spleen viability in all patients, while sometimes the lack of optimal venous outflow results in sinistral portal hypertension with the development of gastric varices (5). Robotic assistance facilitates spleen preservation during MIPD, possibly because of the enhanced dexterity offered by the robotic system (6).
We herein describe a case of robot-assisted MIPD with preservation of the spleen and the splenic vessels. The attached video demonstrates the technical details of this procedure as defined in a center with experience with over 380 robotic pancreatic resections.
We present the following case in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/ales.2020.03.14).
Case presentation
A 28-year-old female patient was incidentally diagnosed with a cystic lesion located in the body-tail of the pancreas during routine abdominal ultrasonography. She was otherwise completely healthy (American Society of Anesthesiologists score class 1).
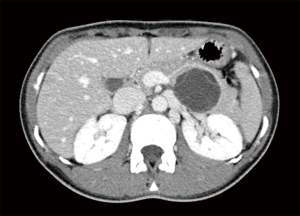
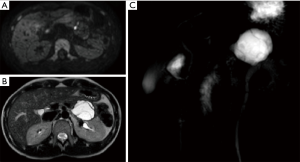
The patient was further investigated by assay of tumor markers (Ca19.9, Ca15.3 and Ca125), contrast-enhanced computed tomography (CT) scan and magnetic resonance imaging (MRI). Tumor markers were negative. CT confirmed the presence of a pancreatic cystic lesion, measuring 40 mm with internal septae (Figure 1). MRI demonstrated also the presence of two mural nodules showing restricted diffusion (Figure 2). The case was discussed at a multidisciplinary tumor board with the recommendation for robot-assisted distal pancreatectomy with preservation of the spleen and the splenic vessels. Endoscopic ultrasonography, possibly associated with fine needle aspiration of the cystic fluid, was not recommended because the results of this additional test were not deemed to possibly change the indication for surgery. After standard preoperative work-up the patient was then scheduled for surgery.
Operative procedure
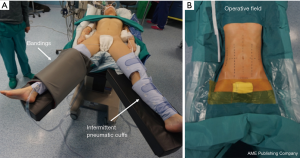
The patient was placed supine with the legs parted on an operating table equipped with a thermic blanket. She was secured to the table with wide bandings and pneumatic intermittent cuffs were placed around the legs to reduce the risk of venous thrombosis (Figure 3). The table was tilted on the patient’s right side (5–8°) and adjusted in reverse Trendelenburg position (15–20°).
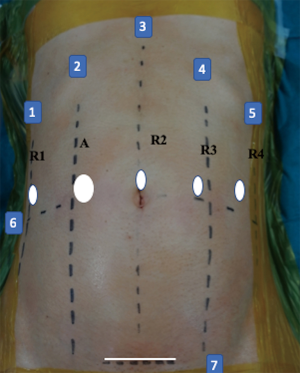
Using a Verres needle, pneumoperitoneum was created and maintained at 10 mmHg. A total of five ports were used, including four 8 mm robotic ports and one 12 mm assistant port (Figure 4). After docking of a da Vinci Xi robotic system (Intuitive Surgical, Inc., Sunnyvale, CA, USA), the liver was suspended by hanging the round ligament to the abdominal wall using a transparietal suture and suturing the anterior margin of liver segment three to the diaphragmatic dome (Figure 5).
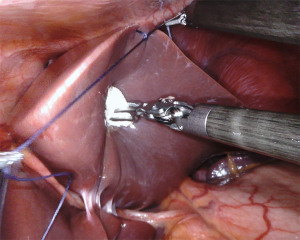
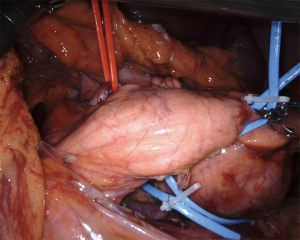
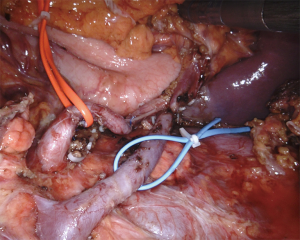
The operation began by opening the lesser sac dividing the reflection of colon and omentum. Next the inferior margin of the pancreas was identified and dissection proceeded to the left until the left colonic flexure was fully mobilized. Further mobilization of the pancreatic body-tail allowed visualization of landmark structures, such as the inferior mesenteric vein, the left renal vein and the left adrenal gland. The posteriorly located cystic tumor could also be visualized. After identification of the left gastric artery at the superior margin of the pancreatic body, dissection was further carried out posteriorly by detaching the splenic vein from the pancreas. Dissection employed bipolar Maryland forceps and electrified scissors. The splenic artery was then identified at the superior border of the pancreas and dissected off likewise with selective ligatures of pancreatic arteries using linen sutures (Figure 6). Both splenic vessels were encircled with vessel loops (Figure 7). Once a tunnel was developed behind the pancreatic neck, the gland was looped and a robotized stapler, using a reinforced stapler armed with a purple cartridge. After division of the pancreas, dissection was carried out along the splenic vessels until the splenic hilum. Lymph nodes around the pancreatic artery were also removed. Spurting bleeders were fixed using 5/0 polypropylene sutures. At the end of the procedure both splenic vessels were completely skeletonized (Figure 8). The specimen was placed in an endoscopic bag and retrieved from a small suprapubic transverse incision. The round ligament of the liver was mobilized and placed around the pancreatic stump. A 14 Fr pig-tail drain was placed close to the pancreatic stump and left to drain by gravity.
Post-operative course
The post-operative course was uneventful, and the patient was discharged on postoperative day 6.
Pathology
Histology demonstrated a mucinous cystadenoma with low grade dysplasia measuring 42 mm.
Discussion
The first robotic distal pancreatectomy was reported in 2003 (7). Several studies have shown that robotic distal pancreatectomy is not just feasible but also safe with respect to competing surgical approaches (8). While all types of minimally-invasive pancreatic resections have been performed using laparoscopic technique, the use of robotic assistance is believed to be rewarding when fine dissections and intracorporeal sutures are needed (9). Distal pancreatectomy with preservation of both spleen and splenic vessels is certainly a procedure that requires fine intracorporeal dissections. Indeed the use of robotic assistance, when compared to laparoscopy in the setting of robotic spleen-preserving distal pancreatectomy, was shown to increase the rate of spleen preservation (10-12), to reduce the rate of conversion to open surgery, and to shorten the length of hospital stay (12). Additionally, laparoscopic spleen-preserving distal pancreatectomy has been associated with high rates of post-operative thrombosis of the splenic vessels leading to the formation of gastric varices. Although no direct comparison is available, the robotic technique was associated with high rates of long-term patency of splenic vessels (13). The main argument against the use of robotic assistance, especially in procedures that can be performed laparoscopically, is costs (14). Current estimates, however, mostly take into account a quite rough evaluation of costs associated to the use of the robot. More sophisticated studies are clearly needed to define the economic sustainability of robotic assistance, as currently known, for procedures that can also per performed by conventional laparoscopic techniques (15).
As specifically regards the technique shown in this video, we have decided to remove the lymph nodes along the splenic artery. This is not standard and may not be required even in the context of mucinous cystadenocarcinoma due to the low rate of lymph node metastasis associated with this tumor type (16). We prefer to remove the lymph nodes along the splenic vessels in case of a different diagnosis at final pathology to have a complete staging of the tumor.
In conclusion in this video, we have demonstrated the technique for robotic spleen-preserving distal pancreatectomy, developed at a center having performed 380 robotic pancreatic resections.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/ales.2020.03.14
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2020.03.14). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this study and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Asbun HJ, Moekotte AL, Vissers FL, et al. The Miami International Evidence-based Guidelines on Minimally Invasive Pancreas Resection. Ann Surg 2020;271:1-14. [Crossref] [PubMed]
- Shoup M, Brennan MF, McWhite K, et al. The value of splenic preservation with distal pancreatectomy. Arch Surg 2002;137:164-8. [Crossref] [PubMed]
- Kimura W, Inoue T, Futakawa N, et al. Spleen-preserving distal pancreatectomy with conservation of the splenic artery and vein. Surgery 1996;120:885-90. [Crossref] [PubMed]
- Warshaw AL. Conservation of the spleen with distal pancreatectomy. Arch Surg 1988;123:550-3. [Crossref] [PubMed]
- Elabbasy F, Gadde R, Hanna MM, et al. Minimally invasive spleen-preserving distal pancreatectomy: Does splenic vessel preservation have better postoperative outcomes? A systematic review and meta-analysis. Hepatobiliary Pancreat Dis Int 2015;14:346-53. [Crossref] [PubMed]
- Chen S, Zhan Q, Chen JZ, et al. Robotic approach improves spleen-preserving rate and shortens postoperative hospital stay of laparoscopic distal pancreatectomy: a matched cohort study. Surg Endosc 2015;29:3507-18. [Crossref] [PubMed]
- Melvin WS, Needleman BJ, Krause KR, et al. Robotic resection of pancreatic neuroendocrine tumor. J Laparoendosc Adv Surg Tech A 2003;13:33-6. [Crossref] [PubMed]
- Cirocchi R, Partelli S, Coratti A, et al. Current status of robotic distal pancreatectomy: a systematic review. Surg Oncol 2013;22:201-7. [Crossref] [PubMed]
- Napoli N, Kauffmann EF, Menonna F, et al. Indications, technique, and results of robotic pancreatoduodenectomy. Updates Surg 2016;68:295-305. [Crossref] [PubMed]
- Kang CM, Kim DH, Lee WJ, et al. Conventional laparoscopic and robot-assisted spleen-preserving pancreatectomy: does da Vinci have clinical advantages? Surg Endosc 2011;25:2004-9. [Crossref] [PubMed]
- Zhou JY, Xin C, Mou YP, et al. Robotic versus Laparoscopic Distal Pancreatectomy: A Meta-Analysis of Short-Term Outcomes. PLoS One 2016;11:e0151189. [Crossref] [PubMed]
- Guerrini GP, Lauretta A, Belluco C, et al. Robotic versus laparoscopic distal pancreatectomy: an up-to-date meta-analysis. BMC Surg 2017;17:105. [Crossref] [PubMed]
- Hwang HK, Kang CM, Chung YE, et al. Robot-assisted spleen-preserving distal pancreatectomy: a single surgeon's experiences and proposal of clinical application. Surg Endosc 2013;27:774-81. [Crossref] [PubMed]
- Souche R, Herrero A, Bourel G, et al. Robotic versus laparoscopic distal pancreatectomy: a French prospective single-center experience and cost-effectiveness analysis. Surg Endosc 2018;32:3562-9. [Crossref] [PubMed]
- Patti JC, Ore AS, Barrows C, et al. Value-based assessment of robotic pancreas and liver surgery. Hepatobiliary Surg Nutr 2017;6:246-57. [Crossref] [PubMed]
- Doulamis IP, Mylonas KS, Kalfountzos CE, et al. Pancreatic mucinous cystadenocarcinoma: Epidemiology and outcomes. Int J Surg 2016;35:76-82. [Crossref] [PubMed]
Cite this article as: Kauffmann EF, Napoli N, Menonna F, Cacace C, Genovese V, Vistoli F, Boggi U. Robot-assisted spleen preserving distal pancreatectomy: case report. Ann Laparosc Endosc Surg 2021;6:13.