The relationship of hiatal hernia and gastroesophageal reflux symptoms—two-sphincter hypothesis: a review
Introduction
The esophagus, gastroesophageal junction (GEJ), diaphragm, and stomach are a set of highly complex and dynamic organs that must work in concert to ensure proper transit of consumed liquids and foods as well as the appropriate barrier protection against the reflux of gastric contents. As a result of this complexity there is a plethora of diseases relating to anatomical, muscular, neurological, and cellular abnormalities of these organs. These diseases range from asymptomatic reflux disease to primary malignancy. Gastroesophageal reflux disease (GERD) has not only plagued patients for hundreds of years but has been an area of highly contested research and debate amongst physicians for more than 60 years.
The anatomy and physiology of the GEJ, as it relates to the development and progression of GERD, has been the primary focus of much research beginning in the 1950s. Historically the GEJ was considered an anatomical barrier to reflux. As more became interested in this junction the pendulum swung in favor of a physiological barrier. With the improvements in imaging modalities and finally the ability to measure hollow organ pressures in real time, this barrier to reflux began to reveal its true nature. This paper aims to describe the concept of the “Two-Sphincter Hypothesis” by reviewing the components of the reflux barrier in health, disease and reconstruction.
Concept of the two-sphincter hypothesis
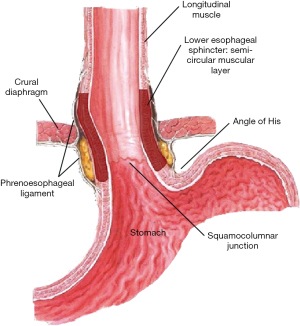
The “Two-Sphincter Hypothesis” is a concept that incorporates both the anatomy and the physiology of the GEJ to form the reflux barrier. The reflux barrier has four components—the lower esophageal sphincter (LES), the crural diaphragm, the angle of His, and phrenoesophageal membrane (Figure 1)—all of which must function together to establish a barrier against reflux (1). The components fall broadly into two categories: intrinsic sphincter which includes the LES and the angle of His and extrinsic sphincter which includes the crural diaphragm and phrenoesophageal ligament. Functionally, the intrinsic sphincter components contribute to the reflux barrier at rest; whereas, the extrinsic components actively contribute barrier function during respiration and changes in position and intra-abdominal pressure.
Intrinsic sphincter
The intrinsic sphincter is created by the LES and the angle of His. At rest these components, when intact, create the baseline anatomical barrier for reflux via radial compression and the acute insertion angle of the esophagus into the stomach.
LES
Anatomically, this area of the GEJ is composed of a variety of intertwined muscle layers that were mapped in 1995 by Stein et al. using 3D manometric pressure mapping to correlate an area of high pressure to meticulously dissected GEJs (2). They showed that medial to the external layer of longitudinal smooth muscles, that run lengthwise with the esophagus, was a semicircular internal muscle sheath that was perpendicular to the external layer. This internal layer diverged at the level of the GEJ into short and long bundles of muscle fibers that opposed each other (Figure 2). The short fibers, known as clasps, continue down the superior edge of the lesser curvature of the stomach and the long fibers or gastric sling fibers splay out over the angle of His and fundus of the stomach. Functionally, these muscle fibers maintain a tonic contraction to close the esophageal opening into the stomach as well as change the angle of His to make it more acute (3). Both sets of muscle fibers increase in thickness and concentration at the GEJ which correlates with the highest manometric pressures of the asymmetrical LES (4).
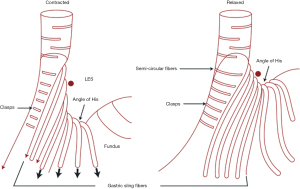
While the clasps and sling fibers give evidence of a muscular sphincter, it is the length of the LES and the subsequent pressure created by that length that allows the tonic contraction which contributes to the reflux barrier. The importance of LES length and pressure was demonstrated by studying patients with and without hiatal hernias who suffered from GERD. In 1971 Cohen et al. compared the LES pressures via manometry in 25 patients without reflux symptoms and no hiatal hernia, 25 patients without reflux symptoms but with a hiatal hernia, and 25 patients with severe gastroesophageal reflux (12 with a hiatal hernia and 13 without a hernia) (5). It was found that the first two groups had similar LES pressures and the third group with severe symptoms regardless of the presence of a hiatal hernia drastically reduced LES pressures.
With the addition of pH testing, DeMeester et al. studied 266 patients with a symptomology suggestive of GERD. By combining both manometry and 24-hour pH monitoring (6), they found that the competency of the LES varied with length such that short LES lengths required much higher pressures compared to longer sphincters which required lower pressures to maintain competence. In patients with a high-pressure zone (HPZ) of less than 5 mmHg and an abdominal esophageal length of less than 1 cm, the LES was grossly incompetent and thus significantly more at risk for GERD. In a subsequent canine study, it was determined that when LES pressure was 6 mmHg or less, regardless of overall LES length or the overall LES length was less than 2 cm that abnormal distal esophageal acid exposure was observed in 75–80% of subjects. In the case of a hiatal hernia and reflux, the characteristics are mostly absent, and reflux of gastric contents becomes more likely.
Angle of His
The angle of His creates a static anatomical barrier or flap valve at the GEJ (Figure 1) to contribute to the barrier protection at rest along with the LES. Animal models have been used to demonstrate the role of this flap valve. Marchand found that by removing the left hemi-diaphragm, the angle of insertion was accentuated and increased the competence of the flap-valve by allowing the fundus of the stomach to move superiorly. Similarly, by stapling off the fundus and removing the gastro-phrenic attachments, the angle of His is opened up, making it more obtuse, which decreased the pressure required for reflux (7). This concept has been observed in vitro when de novo GERD develops post-sleeve gastrectomy (8). Felsenreich et al. reported during long-term follow-up of 53 patients who had underwent a sleeve gastrectomy that 45% developed a hiatal hernia, 15% developed Barrett’s esophagus, and 38% developed GERD.
Using manometry, Thor et al. measured pressure gradients across the GEJ in cadavers while filling the stomach with water. Using open manometry catheters, the LES pressure was measured during stepwise filling of the stomach with 50ml boluses till reflux was observed. It was found that there was a pressure gradient of 4.6±1.6 cmH2O in the normal orientation of the GEJ. When the angle of His was made more acute via a valvuloplasty the pressure gradient increased to 12.5±3.1 cmH2O (9). These studies illustrate the importance of the angle of insertion of the esophagus into the stomach and establish the angle of His as a part of the resting reflux barrier.
Extrinsic sphincter
The crural diaphragm and the phrenoesophageal ligaments (Figure 1), in concert, play an extrinsic physiologic role in the barrier protection during respiration and periods of increased abdominal pressure.
Crural diaphragm
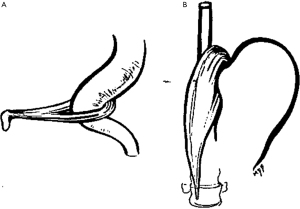
The crural diaphragm represents the canal through which the esophagus travels as is exits the low-pressure thoracic cavity to the higher-pressure abdominal cavity (1). In the absence of a hiatal hernia, the crura and the LES are superimposed on each other. In 1951 Allison noted that the right and left crura (right more dominate than left) create a sling, similar to the puborectalis muscle at the anorectal junction (Figure 3), around the GEJ anchoring it to the lumbar spine (10). When the right crus contracts, it creates not only external compression on the esophagus, but it pulls the esophagus inferior increasing the angle of His adding to the reflux barrier.
Independent of the intrinsic sphincter, the diaphragm sling provides an HPZ. When manometry is used to measure esophageal pressures in patients who had undergone an esophagogastrectomy (11), it was found that the crural diaphragm corresponded with an HPZ distal to the anastomosis. This HPZ fluctuated with respiratory cycles, increasing pressure with inhalation and decreasing pressure with exhalation. The crural diaphragm also partially relaxed with deglutition and contracted with increasing abdominal pressure via leg lifts or external abdominal compression.
Phrenoesophageal membrane
The final component of the reflux barrier and the second part of the extrinsic sphincter is the phrenoesophageal membrane or ligament (12). Bombeck et al. described the ligaments as membranous extensions of the transversalis fascia and endothoracic fascia bridging from the inferior side of the diaphragm to the left and right lateral edges of the lower esophagus (13). Each extension has two leaflets, a thicker upper leaflet and thinner lower leaflet. When positioned properly, the leaflets act in a suspensory fashion allowing the LES and diaphragm to move together during respiration. During periods of abnormal increases in intra-abdominal pressure, i.e., Valsalva maneuvers or coughing, the ligament maintains the LES intra-abdominal length and therefore alignment of the LES and crural canal and the competence of the barrier.
In cadavers, it was found that the insertion point of the upper leaflet of the phrenoesophageal ligament relative to the squamo-columnar epithelial junction was much lower in patients with esophagitis as compared to normal controls (1.13 versus 3.35 cm). In patients with hiatal hernia and no esophagitis the insertion point was 3.6 cm and in patients with hiatal hernia and esophagitis the insertion point was 0.5 cm above the junction. The importance of the insertion points relates to the position of the GEJ to the hiatus and the relative vector force on the GEJ. For example, if the insertion point of the upper leaflet is more proximal on the esophagus, it pulls the GEJ towards the hiatus thereby reinforcing the normal anatomical location and length of the LES. Comparatively, if the upper leaflet inserts more distal, the tension on the esophagus is more lateral and opens the GEJ counteracting the radial compression of the LES. The results from this study explain why, in the Hill repair, the median arcuate ligament anchoring suture reestablishes the function of the phrenoesophageal ligament (14).
Interactions between intrinsic and extrinsic sphincters
With the advent of manometry and other imaging techniques, it became possible to demonstrate the interactions between the two sphincters. In 1988 Mittal et al. provided evidence of the interaction between the diaphragm and LES while simultaneously recording diaphragmatic electrical activity via bipolar esophageal electrodes and LES pressures during spontaneous and voluntary diaphragmatic contraction in healthy individuals (15). During quiet inspiration, the electrical activity driving the diaphragmatic contraction correlated with increases in both LES and intra-abdominal pressures. During deglutition, the diaphragm relaxes and the electrical activity becomes negligible. There is a precipitous drop in LES pressure, but not to zero. This established the idea of an external sphincter that augments the intrinsic activity of the LES.
The presence of GERD and hiatal hernias compared to normal became a model to demonstrate the existence of these sphincters. Early on, several studies examined the relationship between GERD, the LES and hiatal hernias. They found similar pressures in normal asymptomatic individuals, with and without a hiatal hernia leading to the acceptance, at the time, that reflux is related to a weak intrinsic sphincter only and that the presence of a hiatal hernia was irrelevant (5,16). However, current day studies clearly demonstrate the role of the hiatal hernia in reflux development (17).
Sloan et al. studied esophageal emptying and GEJ competence in patients with hiatal hernia both during normal deglutition and increased intra-abdominal pressure (18,19). Using video fluoroscopy and manometry simultaneously impaired esophageal emptying was compared in healthy volunteers and patients with axial hiatal hernias. The patients with hiatal hernias were further sub-divided into reducible versus non-reducible hernias. Healthy participants experienced complete esophageal emptying in 86% of the barium swallows, patients with reducible hernias experienced complete emptying in 66%, and patients with non-reducible hernias experienced complete emptying in 35% which was statistically significant. The non-reducible group also experienced lower acid clearance times compared to the controls.
To expand upon these results Sloan et al. used the same investigative modalities to study the effects of eight maneuvers, aimed at provoking rapid increases in intra-abdominal pressure (19). Results showed that patients with hiatal hernias had shorter LESs and significantly lower LES pressures. All maneuvers, collectively, produced a significant increase in reflux in the hernia patients and most reflux occurred within two seconds of the instigation of the maneuver. These two studies demonstrate how the disruption of the two GEJ sphincters affects both the deglutition process and reflux prevention during times of increased intra-abdominal pressure.
Patti et al. studied 95 patients with GERD diagnosed using 24-hour pH monitoring. These patients were stratified using upper gastrointestinal series, endoscopy, and manometry based on the presence of a hiatal hernia (17). The hiatal hernia group was further sub-divided by size (<3.0, 3.0–5.0, and >5.0 cm). The patients with no hernia and a hiatal hernia <3.0 cm had similar LES length and pressure, but the patients with larger hiatal hernias had significantly shortened LESs with lower LES pressures including resting pressures. The two larger hiatal hernia groups also exhibited a greater number of reflux episodes over 5 minutes in length and longer periods of reflux clearance. This translated into significantly higher esophagitis scores. The lower resting pressures of the larger hernias represent the loss of the anatomical partnership between the LES and the crural diaphragm.
Two sphincters in action during anti-reflux surgery
Anti-reflux surgery has also been used to argue for the concept of a two-sphincter barrier by restoring LES length and aligning the crural diaphragm along with it. Mason et al. hypothesized that reconstruction of the GEJ via a Nissen fundoplication would completely abolish transient LES relaxations (tLESR) and therefore prevent reflux (20). This idea was based on previous research done by the same group that proved that tLESRs were a result of mechanical distention rather than some neurologic process (20). Using baboons, Mason evaluated the LES with esophageal manometry while filling the stomach with water causing distention before and after performing a Nissen fundoplication. Before the fundoplication progressive weakening and shortening of the LES was observed as a result of the distention. The fundoplication allowed the LES to maintain a consistent length, pressure, and reduced the number of tLESRs by limiting shortening of the LES during progressive gastric distention.
If the role of the fundoplication was to augment LES pressure, then what role does crural closure contribute to the reflux barrier? To answer the question of whether closing the hiatus was necessary for improved outcomes of a Nissen fundoplication, Louie et al. used high-resolution manometry during the repair to quantify the contributions of each part of the operation (21). Patients, undergoing laparoscopic Nissen fundoplication, were randomized to crural closure followed by fundoplication or fundoplication followed by crural closure while the esophagus was actively monitored with HRM. It was found that both the crural closure and the fundoplication contribute to the final length and pressure of the reconstructed GEJ but that LES pressure was driven by crural closure and not the fundoplication.
Additional evidence for the two-sphincter concept can be seen during magnetic sphincter augmentation (MSA) using the LINX Reflux Management System® (Torax Medical/Ethicon, Minneapolis, MN, USA). During a series of retrospective studies Warren et al. began to evaluate the factors for success with MSA and compared preoperative and postoperative manometric changes (22,23). In the first study, patients were stratified into three categories based on the structural integrity of their LES. There were patients with structurally normal LESs, patients with 1 structural defect, and patients with 2–3 defects. Defects were defined as resting pressure <6 mmHg, total LES length <2 cm, or intra-abdominal LES length <1 cm. They concluded that the sphincter could be augmented in 77% of the patients with a single structural defect restored and 56% of the patients with 2–3 defects restored to normal. But the conjectured surgeons controlled some of the restoration by restoring length to the abdomen. In the second study, they identified 3 factors that negatively impacted a good to excellent outcome: structurally defective sphincter, BMI >35 kg/m2 and elevated integrated relaxation pressure (IRP). In the univariate analysis hiatal hernia was also identified.
During this time, MSA was predominantly implanted with a minimal dissection (MD) technique and did not deliberately reconstruct a lax diaphragm or early hiatal hernia hoping to achieve augmentation through the device and maintenance of the natural ligaments—the phrenoesophageal membrane. This led to a study of the effects of hiatal dissection, restoring intra-abdominal LES length, and crural closure during MSA implantation (24). They compared the rate of normalization of DeMeester scores between patients who received MD of the hiatus, crural closure, formal crural repair (FC), and extensive dissection (ED) without closure. DeMeester score normalization occurred in 56%, 53%, 60%, and 38% respectively. The regression analysis of the techniques showed that FC was the most likely to normalize the acid exposure. The results of these studies support the two-sphincter hypothesis by demonstrating that a structurally intact LES and hiatal canal are necessary for an effective reflux barrier.
Conclusions
The GEJ is a complex region of the body that includes the LES, crural diaphragm, angle of His, and phrenoesophageal ligaments. These elements functionally and anatomically create a reflux barrier that, as evidence shows, is composed of two separate sphincters: an intrinsic one (LES and angle of His) and an extrinsic one (crural diaphragm and phrenoesophageal membrane). After more than a half century of curiosity and exploration, it has become clear that the distinct anatomic parts of the GEJ must work in unison to prevent reflux. These components come together as two-sphincters which has allowed for better understanding of GERD and the advancement of the treatment of GERD.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2020.04.01). Dr. Louie reports other from Intuitive Surgical, other from Torax medical/J and J, outside the submitted work. The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Schneider AM. Anatomy of the Reflux Barrier in Health, Disease, and Reconstruction. In: Aye RW, Hunter JG. editors. Fundoplication Surgery: A Clinical Guide to Optimizing Results. 1st edition. Springer International Publishing, 2016:1-17.
- Stein HJ, Liebermann-Meffert D, DeMeester TR, et al. Three-dimensional pressure image and muscular structure of the human lower esophageal sphincter. Surgery 1995;117:692-8. [Crossref] [PubMed]
- Kwiatek MA, Kahrilas PJ. Physiology of the LES. Dis Esophagus 2012;25:286-91. [Crossref] [PubMed]
- Korn O, Stein HJ, Richter TH, Liebermann-Meffert D. Gastroesophageal sphincter: a model. Dis Esophagus 1997;10:105-9. [Crossref] [PubMed]
- Cohen S, Harris LD. Does hiatus hernia affect competence of the gastroesophageal sphincter? N Engl J Med 1971;284:1053-6. [Crossref] [PubMed]
- DeMeester TR, Wernly JA, Bryant GH, et al. Clinical and in vitro analysis of determinants of gastroesophageal competence. A study of the principles of antireflux surgery. Am J Surg 1979;137:39-46. [Crossref] [PubMed]
- Marchand P. The gastro-oesophageal sphincter and the mechanism of regurgitation. Br J Surg 1955;42:504-13. [Crossref] [PubMed]
- Felsenreich DM, Kefurt R, Schermann M, et al. Reflux, Sleeve Dilation, and Barrett's Esophagus after Laparoscopic Sleeve Gastrectomy: Long-Term Follow-Up. Obes Surg 2017;27:3092-101. [Crossref] [PubMed]
- Thor KB, Hill LD, Mercer DD, et al. Reappraisal of the flap valve mechanism in the gastroesophageal junction. A study of a new valvuloplasty procedure in cadavers. Acta Chir Scand 1987;153:25-8. [PubMed]
- Allison PR. Reflux esophagitis, sliding hiatal hernia, and the anatomy of repair. Surg Gynecol Obstet 1951;92:419-31. [PubMed]
- Klein WA, Parkman HP, Dempsey DT, et al. Sphincterlike thoracoabdominal high pressure zone after esophagogastrectomy. Gastroenterology 1993;105:1362-9. [Crossref] [PubMed]
- Daniels BT. The phrenoesophageal membrane. Am J Surg 1965;110:814-7. [Crossref] [PubMed]
- Bombeck CT, Dillard DH, Nyhus LM. Muscular anatomy of the gastroesophageal junction and role of phrenoesophageal ligament; autopsy study of sphincter mechanism. Ann Surg 1966;164:643-54. [Crossref] [PubMed]
- Warren H. Alternatives to Nissen Fundoplication: The Hill Repair and the Nissen-Hill Hybrid. In: Aye RW, Hunter JG. editors. Fundoplication Surgery: A Clinical Guide to Optimizing Results. 1st edition. Springer International Publishing, 2016:71-89.
- Mittal RK, Rochester DF, McCallum RW. Electrical and mechanical activity in the human lower esophageal sphincter during diaphragmatic contraction. J Clin Invest 1988;81:1182-9. [Crossref] [PubMed]
- Atkinson M. Mechanisms protecting against gastro-oesophageal reflux: a review. Gut 1962;3:1-15. [Crossref] [PubMed]
- Patti MG, Goldberg HI, Arcerito M, et al. Hiatal hernia size affects lower esophageal sphincter function, esophageal acid exposure, and the degree of mucosal injury. Am J Surg 1996;171:182-6. [Crossref] [PubMed]
- Sloan S, Kahrilas PJ. Impairment of esophageal emptying with hiatal hernia. Gastroenterology 1991;100:596-605. [Crossref] [PubMed]
- Sloan S, Rademaker AW, Kahrilas PJ. Determinants of gastroesophageal junction incompetence: hiatal hernia, lower esophageal sphincter, or both?. Ann Intern Med 1992;117:977-82. [Crossref] [PubMed]
- Mason RJ, DeMeester TR, Lund RJ, et al. Nissen fundoplication prevents shortening of the sphincter during gastric distention. Arch Surg 1997;132:719-24; discussion 724-6. [Crossref] [PubMed]
- Louie BE, Kapur S, Blitz M, et al. Length and pressure of the reconstructed lower esophageal sphincter is determined by both crural closure and Nissen fundoplication. J Gastrointest Surg 2013;17:236-43. [Crossref] [PubMed]
- Warren HF, Louie BE, Farivar AS, et al. Manometric Changes to the Lower Esophageal Sphincter After Magnetic Sphincter Augmentation in Patients With Chronic Gastroesophageal Reflux Disease. Ann Surg 2017;266:99-104. [Crossref] [PubMed]
- Warren HF, Brown LM, Mihura M, et al. Factors influencing the outcome of magnetic sphincter augmentation for chronic gastroesophageal reflux disease. Surg Endosc 2018;32:405-12. [Crossref] [PubMed]
- Irribarra MM, Blitz S, Wilshire CL, et al. Does Treatment of the Hiatus Influence the Outcomes of Magnetic Sphincter Augmentation for Chronic GERD?. J Gastrointest Surg 2019;23:1104-12. [Crossref] [PubMed]
Cite this article as: Andrews WG, Louie BE. The relationship of hiatal hernia and gastroesophageal reflux symptoms—two-sphincter hypothesis: a review. Ann Laparosc Endosc Surg 2021;6:41.