
After the hiatal hernia repair: fundoplication, yes or no? Partial or complete?
Introduction
A hiatal hernia occurs when the esophageal hiatus enlarges and the phrenoesophageal ligament anchoring the gastroesophageal junction just inferior to the esophageal hiatus attenuates allowing for the gastroesophageal junction, stomach and, rarely, other intra-abdominal organs to migrate superior to the esophageal hiatus into the posterior mediastinum (1). Hiatal hernias are very common, especially in Western countries. The most common hiatal hernia is type I, accounting for up to 95% of all hiatal hernias (2). Paraesophageal hernias may account for up to 14% of all hiatal hernias, and the majority of paraesophageal hernias are of the type III variety (3). Their clinical manifestations can be completely asymptomatic to life-threatening incarceration and necrosis of the stomach or other organs (4). Therefore, a wide variety of treatment options may be appropriate, which will be addressed by other authors in this issue.
A basic question, nonetheless, is when a hiatal hernia is repaired, do they require a fundoplication? If the answer to this question is yes, then this answer begs the next question: Should we employ a complete, 360-degree fundoplication or a partial 270- or 180-degree fundoplication? If the answer to that question is a complete fundoplication, the next relevant question is should we employ a Nissen fundoplication using the native fundus, or should we use magnetic sphincter augmentation with the LinxTM device?
We believe the answers to these questions will be based on each individual patient’s presentation and knowledge of their physiology. The following sections will address these decision points.
Do all patients require a fundoplication after hiatal hernia repair?
Allison’s original approach to hiatal hernia was reduction of the stomach and gastroesophageal junction into the abdomen below the esophageal hiatus and repair of the hiatal defect by suture approximation of the diaphragmatic crura (5). His belief was that restoration of the gastroesophageal junction back into the abdomen and re-establishing the normal hiatal anatomy would lead to the reversal of gastroesophageal reflux. He famously presented his 20-year experience with this approach reporting that only 50% of patients achieved long-term symptomatic relief (6). This established the principle that for the management of gastroesophageal reflux disease (GERD), repairing the hiatal hernia alone is not adequate.
However, larger hiatal hernias and, especially, paraesophageal hernias (PEH) were considered to be a different entity and presenting a higher risk to patients. This was based on a publication by Skinner and Belsy in 1967 (7) describing PEH as having a high risk of incarceration, volvulus and strangulation, with a nearly 30% mortality rate. Therefore, the recommendation for decades was that PEH’s needed to be repaired when discovered, irrespective of their association with symptomatic GERD. Of course if one were repairing the hiatal hernia to prevent catastrophic gastric necrosis, reflux is not a concern. However, outcomes with this approached mimicked Allison’s with a significant number of patients developing GERD symptoms postoperatively and subsequent recommendations were to routinely add a fundoplication to PEH repair (8,9).
Nevertheless, it must be acknowledged that although fundoplication are desirable, the clinical condition of the patient may make them impractical. For example, the patient who presents with gastric necrosis requiring gastrectomy completely eliminates even the possibility of a fundoplication. The hemodynamically unstable patient who requires an expedited repair is clearly inappropriate for prolonging anesthesia time to add a fundoplication. The figure presents our decision flow chart in the overall management of hiatal hernia (Figure 1). On the other hand, it should be emphasized that in over 30 years of our combined surgical experience, we have encountered such circumstances only a handful of times. Therefore, in general, fundoplication should be used following all hiatal hernia repair.
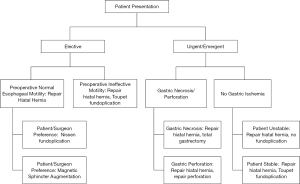
If a fundoplication is done, should it be partial or complete?
Now that it’s established that a fundoplication should be a routine part of hiatal hernia repair, with few exceptions, the next question to answer is what type of fundoplication. There is general consensus that the Nissen fundoplication is the fundoplication of choice for most patients with hiatal hernia and GERD symptoms (1). However, there is opinion among many surgeons that a partial fundoplication should be used to reduce the risk of postoperative dysphagia, especially in the presence of preoperative dysphagia or ineffective esophageal motility. Siegal et al. (10) studied the development of dysphagia in patients who underwent a fundoplication and found that high resolution manometry (HRM) does not always predict development of dysphagia; moreover, in patients with preoperative dysphagia and strong esophageal motility, the dysphagia would frequently improve. Further, they found that in patients with preoperative dysphagia and poor esophageal motility on HRM, dysphagia will persist. They reported that a distal contractile integral (DCI) greater than 1,000 was associated with dysphagia improvement in patients who underwent a Nissen and concluded that patients with weak esophageal contractility would be better served with a Toupet fundoplication (10). When one has the luxury of an elective case, the preoperative evaluation will help in the decision as to which fundoplication to use (11). The figure shows our decision factors in choosing between a complete or partial fundoplication (Figure 1). It is important to get this decision right. There have been several studies comparing partial and complete fundoplication. The type of fundoplication performed should be decided preoperatively and this decision should be linked to symptoms and the HRM findings.
There is consistent evidence that a Toupet fundoplication provides the same symptomatic relief as a Nissen fundoplication, at least initially. DeHaan et al. (12) used Endoflip™ impedance measurements to study the distensibility of the GE junction after Toupet and Nissen fundoplications. They found that the distensibility of the GE junction was greater with Toupet fundoplication and that this likely contributes to the increased gas bloat seen with Nissen as compared to Toupet fundoplications. Moreover, a recent meta-analysis compared dysphagia after fundoplication and found that the dysphagia rate following a Nissen was 12.5% as compared to 4.8% after a Toupet (12).
Although antireflux operations are widely performed with the same basic surgical principles as previously described, there are surgeon-specific differences in technique that may account for symptoms of dysphagia and gas bloat after fundoplication. When the fundoplication is described as “floppy”, there is significant imprecision and subjective surgical judgement that allows for wide variability in the actual fundoplication. Variable use and size of a bougie is another factor that can create variations in the quality of the fundoplication and resulting in variations of post-operative symptoms. Division of the short gastric vessels to allow for better mobilization of the fundus is another factor that creates large variations in practices and outcomes. Kinsey-Trotman et al. (13) found less gas bloating and a better preservation of the ability to vent the stomach in patients when the short gastric vessels were left intact.
Initially, partial and complete fundoplication have similar results in controlling GERD both subjectively and objectively. The potential problem of performing a partial fundoplication on a patient that otherwise by HRM findings would be a good candidate for a complete fundoplication is long-term recurrence of GERD. Several studies have shown that patients who undergo a partial fundoplication when compared to a complete fundoplication are at higher risk for developing recurrence of GERD over time (14). Horvath et al. (14) compared the recurrence of symptoms between Nissen and Toupet fundoplication. They found symptomatic failure rates of 20% and abnormal DeMeester scores on 59% of the patients after undergoing a partial fundoplication at a mean follow up time of 22 months. On the other hand, the symptomatic failure rate on long term follow up after a complete fundoplication was 4.5% (14). There is also correlation between the severity of reflux and the failure rates of a partial posterior fundoplication. Patients with a DeMeester score of 50 or higher also have an increased risk of failure if they undergo a Toupet compared to a Nissen Fundoplication (14).
With the advent of the magnetic sphincter augmentation (MSA) device, there now exists a choice between a fundoplication and MSA. In the elective setting in patients meeting physiological criteria for MSA, placement of the LinxTM instead of a fundoplication may be appropriate (15). Our purpose here is not to adjudicate the superiority of one approach over the other, but merely to acknowledge that both are viable options depending on the surgeon’s and patient’s preference in the elective setting.
MSA with the Linx™ device has become a widely-used alternative to fundoplication for GERD patients. There have been several studies that compare MSA to laparoscopic Nissen fundoplication. Skubleny et al. (16) performed a systematic review and meta-analysis and concluded that MSA is a good alternative with similar results in controlling reflux symptoms. It is also a less technically demanding procedure to perform with limited esophageal dissection and preservation of the short gastric vessels. Another perceived advantage is standardization of the MSA placement technique, whereby success rate with the fundoplication are correlated with surgeon expertise and experience performing these procedures.
There are patient factors that limit the use of MSA. It is currently not indicated in patients with esophageal dysmotility and severe erosive esophageal disease where fundoplication remains the standard of care. Additionally, some studies report increased early dysphagia with Linx™ as compared to Nissen fundoplication (16). Thus, dysphagia may require additional esophageal dilations to “stretch” the capsule that encases the device. Some studies report better results with bloating and ability to emesis compared to Nissen fundoplication (16). Although rare, there have been reports of the Linx™ device erosions into the esophagus. These erosions have generally occurred in the smaller devices (e.g., the 12 bead device), which is no longer on the market. Lastly, patients with some metal allergies, such as nickel and titanium, are not candidates for LinxTM as these metals are in the casings of the magnets.
Conclusions and recommendations
Under normal circumstances, most patients after hiatal hernia repair should have a fundoplication. The exceptions being the emergent cases associated the gastric necrosis or the patient in extremis. In patients with good motility, a complete fundoplication is preferable and MSA a viable alternative. Patients with preoperative symptoms of dysphagia and poor motility, should undergo a partial fundoplication, accepting the higher GERD recurrence rates.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2020.03.11). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bennett RD, Straughn DM, Velanovich V. Gastroesophageal reflux disease, hiatal hernia, and Barrett esophagus. In: Zinner MJ, Ashley SW, Hines OJ. editors. Maingot’s Abdominal Operations, 13 ed. New York: McGraw-Hill Education, 2019:393-422.
- Hyun JJ, Bak YT. Clinical Significance of Hiatal Hernia. Gut Liver 2011;5:267-77. [Crossref] [PubMed]
- Postlewaite RW. Surgery of the Esophagus. 2nd ed. Norwalk: Appleton Century-Crofts, 1986
- Vega JA Jr, Velanovich V. Paraesophageal hernia. Etiology, presentation, and indications for repair. In: Yeo CJ, DeMeester SR, McFadden DW, et al. editors. Shakelford’s Surgery of the Alimentary Tract, 8 ed. New York: Elsevier, 2018:279-283.
- Stylopoulos N, Rattner DW. The history of hiatal hernia surgery: From Bowditch to laparoscopy. Ann Surg 2005;241:185-93. [Crossref] [PubMed]
- Allison PR. Hiatus hernia (a 20 year retrospective survey). Ann Surg 1973;178:273-6. [Crossref] [PubMed]
- Skinner DB, Belsey RH. Surgical management of esophageal reflux and hiatus hernia. Long-term results with 1,030 patients. J Thorac Cardiovasc Surg 1967;53:33. [Crossref] [PubMed]
- Casabella F, Sinanan M, Horgan S, et al. Systematic use of gastric fundoplication in laparoscopic repair of paraesophageal hernias. Am J Surg 1996;171:485-9. [Crossref] [PubMed]
- Gantert WA, Patti MG, Arcerito M, et al. Laparoscopic repair of paraesophageal hernias. J Am Coll Surg 1998;186:428-32. [Crossref] [PubMed]
- Siegal S, Dunst CM, DeMeester SR, et al. Preoperative high resolution manometry criteria does not predict dysphagia after Nissen fundoplication. Gastroenterology 2017;152:S1284-5. [Crossref]
- Velanovich V. Secrets for successful laparoscopic antireflux surgery: Predictors. Ann Laparosc Endosc Surg 2017;2:27. [Crossref]
- DeHaan RK, Davila D, Frelich MJ, et al. Esophagogastric junction distensibility is greater following Toupet compared to Nissen fundoplication. Surg Endosc 2017;31:193-8. [Crossref] [PubMed]
- Kinsey-Trotman SP, Devitt PG, Bright T, et al. Randomized trial of division versus nondivision of short gastric vessels during Nissen fundoplication. Ann Surg 2018;268:228-32. [Crossref] [PubMed]
- Horvath KD, Jobe BA, Herron DM, et al. Laparoscopic Toupet fundoplication is an inadequate procedure for patients with severe reflux disease. J Gastrointest Surg 1999;3:583-91. [Crossref] [PubMed]
- Rabach L, Saad AR, Velanovich V. How to choose among fundoplication, magnetic sphincter augmentation or transoral incisionless fundoplication. Curr Opin Gastroenterol 2019;35:371-8. [Crossref] [PubMed]
- Skubleny D, Switzer NJ, Dang J, et al. LINX® magnetic esophageal sphincter augmentation versus Nissen fundoplication for gastroesophageal reflux disease: a systematic review and meta-analysis. Surg Endosc 2017;31:3078-84. [Crossref] [PubMed]
Cite this article as: Serrano L, Saad AR, DuCoin C, Velanovich V. After the hiatal hernia repair: fundoplication, yes or no? Partial or complete? Ann Laparosc Endosc Surg 2021;6:22.


