
Approaches to anti-reflux surgery: laparoscopic, robotic, and endoscopic
Introduction
Gastroesophageal reflux disease (GERD) is one of the most prevalent gastrointestinal (GI) diseases in the US with an estimated prevalence of 18.1–27.8% (1). The socioeconomic burden of GERD is tremendous with an estimated health care cost of $12.3 billion dollars annually. It is the most common reason for an outpatient GI appointment (8.9 million outpatient visits per year) and the primary reason for an upper endoscopy (2,3). In the US, it is estimated that approximately 40% of the entire population will experience heartburn at least monthly, 14% will experience weekly episodes, and 7–10% will have daily symptoms (4).
GERD was initially defined by the Montreal Consensus as “a condition which develops when reflux of stomach contents cause troublesome symptoms and/or complications” (5). Many underlying causes of GERD exist [defective lower esophageal sphincter (LES), impaired gastric emptying, failed esophageal peristalsis], and the disease is associated with a broad spectrum of symptoms, both typical esophageal symptoms (heartburn, regurgitation, and dysphagia) and extra-esophageal atypical symptoms (laryngitis, cough, asthma, and dental erosions) (4). While GERD itself is benign, disease progression can lead to harmful sequela including erosive gastritis, Barrett’s esophagus (BE), and esophageal malignancy. From a surgical perspective, GERD is considered a physiologic disorder of the gastroesophageal junction (GEJ), the natural anti-reflux barrier. When functioning properly, the LES, the crural diaphragm, and the geometry of the GEJ all aid in preventing the reflux of stomach acid into the esophagus.
The most common treatment for GERD is a step-up approach starting first with lifestyle modifications (i.e., weight loss; avoiding alcohol, spicy foods, and eating before lying down; and elevating the head of the bed) (6). After failing lifestyle modifications, patients are typically started on a H2 receptor antagonists (H2RA) or proton-pump inhibitors (PPI) with symptomatic relief expected in 60% and 83% of patients, respectively (4). However, an alternative to medical therapy, and often the next approach offered by clinicians, remains anti-reflux surgery. Patients with objective evidence of reflux disease may proceed with surgical intervention if they fail medical management (persistent or inappropriately controlled esophageal or extra-esophageal symptoms), prefer surgical management despite successful medical management, or suffer complications of GERD (BE or stricture) (5). A variety of surgical modalities are available today, including laparoscopic anti-reflux surgery (LARS), robotic assisted laparoscopic fundoplication (RALF), and endoscopic anti-reflux therapy. This review will provide a comprehensive assessment of each of these procedural interventions, assess their respective advantages and disadvantages, and appraise the current data available regarding each of the modalities effectiveness to treat GERD.
Laparoscopic treatment of reflux disease
Introduction of laparoscopic anti-reflux surgery
The first fundoplication, described as a complete 360º wrap of the stomach fundus around the esophagus, was performed by Dr. Rudolf Nissen in the 1950s. Other modifications soon followed and are named after their creators: Dor, Toupet, Belsey, Hill and Collis. These open techniques remained the standard of care until 1991 when Dr. Dallemagne published his first series utilizing the relatively new minimally invasive technique, laparoscopy, to perform a 360º wrap of the stomach (7). LARS soon became the standard of care with reduced short-term morbidity, shortened postoperative length of stay, and decreased incisional hernia rates (8). The minimally invasive approach made fundoplication a more acceptable treatment option for patients and contributed to the tendency to offer surgery earlier in the disease course (9). Multiple randomized clinical trials and meta-analyses have found LARS to be safe and effective with reduced perioperative morbidity as compared to its open counterpart. In 2009, a meta-analysis of 12 randomized clinical trials comparing open anti-reflux surgery to LARS found that patients who underwent LARS had a reduction in total length of hospital stay by 2.7 days, returned to normal activity an average of 7.8 days sooner, and had a 65% reduction in their postoperative complication rate (10). Similar results were found by Catarci et al. (11). In a pooled meta-analysis of multiple randomized control trials, the authors found that LARS was associated with a significantly lower operative morbidity rate, a shorter postoperative stay, and shorter patient sick leave. Importantly, there was no significant differences in the incidence of reflux recurrence, dysphagia, bloating and reoperation rates between the two approaches and no perioperative deaths were recorded (11). Additionally, laparoscopy has several non-GERD-related long-term advantages over open surgery. Incisional hernias can have a tremendous impact on patient’s quality of life, and in a 17-year follow-up study examining open fundoplication vs. LARS, Oor et al. found a significantly decreased rate of re-intervention for incisional hernias in the patients treated with laparoscopy (14% vs. 2%) (8).
While a step by step technical guide is outside the scope of this review, there are several technical approaches to a laparoscopic Nissen fundoplication that have been shown to improve postoperative outcomes. Based on a consensus of 40 expert foregut surgeons and published by the SAGES Guidelines Committee in 2010, the recommended surgical technique includes some of the following elements: (I) opening the phrenoesophageal ligament in a left-to-right fashion, (II) circumferential dissection of the hiatus, (III) sufficient transhiatal mobilization to allow approximately 3 cm of intraabdominal esophagus, (IV) short gastric vessel division to allow for a tension free wrap, (V) crural closure posteriorly with non-absorbable sutures, (VI) creation of a 1.5–2.0 cm wrap incorporating the anterior muscular wall of the esophagus, and (VII) bougie placement at the time of the fundoplication if a complete wrap is formed (5). A partial fundoplication has also been described as an effective and accepted alternative for anti-reflux surgery (12). Another laparoscopic anti-reflux procedure option, which has gained popularity for the treatment of reflux disease is the magnetic sphincter augmentation system, LINX® (Torax Medical LLC; Ethicon US, LLC) (12,13). Further details of these procedures, preoperative diagnosis and workup, intra-operative technical considerations and postoperative care and complications are discussed in other chapters of this edition.
Medical vs. laparoscopic anti-reflux surgery
Medical therapy has been successful in the treatment of GERD, and maintenance therapy with PPIs can be used to afford patients high rates of symptom resolution and esophageal healing. Developed in the late 1980’s, omeprazole (the first PPI) quickly became a mainstay for GERD treatment with usage rates doubling from 1999 to 2012 (14). GERD, however, is a chronic disease, and patients are often reluctant to take medications for their entire lifetime. Furthermore, since the implementation of PPIs, understanding of potential adverse effects of long-term PPI use has grown considerably and increased effort has been placed on finding potential alternatives to PPI therapy (15). Since this time, LARS has become established as an effective alternative to long-term PPI therapy. Multiple randomized control trials and meta-analyses have compared the results of medical and surgical therapy. Most have supported surgery as an effective alternative for both the treatment of GERD in patients with good symptom control and for those with only a partial response to medication. The LOTUS trial, published in 2011, was a 5-year randomized, multicenter control trial comparing optimized PPI therapy vs. standardized LARS in patients with chronic GERD (16). While the estimated remission rate in the PPI therapy group was 92% vs. 85% in the LARS group, prevalence and severity of heartburn and acid regurgitation was lower in the LARS group (16). However, there was significantly higher rates of dysphagia, bloating and flatulence with LARS. As the trial’s primary outcome measure was time to treatment failure, the authors concluded that both drug and surgically induced acid suppression allow patients to achieve and maintain satisfactory disease remission at 5 years (16).
Many studies have demonstrated objective evidence for LARS effectiveness. Based on manometric data and impedance measurements, fundoplication results in both less esophageal acid exposure and significantly increased LES pressures when compared with medical therapy. In a large, matched randomized clinical trial, Mahon et al. compared LARS to PPI therapy. At 3 months, mean DeMeester scores significantly improved from 42.7 to 8.6 in the LARS group and from 36.9 to 17.7 in the PPI group (17). There was also a significant increase in mean gastrointestinal symptom score and general well-being scores at 12-months in the LARS group as compared to patients randomized to PPI therapy (17). Additionally, a prospective, randomized open parallel-group, multicenter trial that compared the efficacy and safety of LARS to PPI therapy demonstrated that esophageal acid exposure was significantly reduced in the LARS group both at 6 months and 5 years (18).
Data also demonstrates that surgical treatment of GERD is effective and safe in the long- term. In a long-term follow-up study of a prospective randomized trial comparing medical and surgical treatment, Spechler et al. found that statistically fewer patients in the surgical group were using anti-reflux medications (62% for surgical treatment vs. 92% for medical treatment) at follow-up (9.1 years for surgical group and 10.6 years for medical treatment) (19). However, there was no significant difference in the grade of esophagitis, the frequency of esophageal stricture treatment, and the overall satisfaction with anti-reflux therapy between the two groups (19). The longest outcome data comes from Oor et al. (8). To compare conventional open surgery vs. LARS, they evaluated a total of 111 patients (60 LARS and 51 open fundoplication) 17 years after their initial operation. Both groups showed excellent long-term outcomes. However, they found that, compared with their preoperative symptom scores, patients in the LARS group continued to report significant improvement in general health and quality of life and 90% of patients reported continued symptom relief. While, at 17 years, 42% of LARS patients were dependent on the daily use of acid suppression medications, medication usage was significantly lower as compared with their preoperative dose (8).
LARS has been shown to be safe and effective relieving GERD symptoms and improving quality of life. However, it is still unclear if LARS is more effective than medical therapy at inducing remission of BE or esophageal dysplasia. BE occurs when the esophageal lining undergoes metaplastic change from the normal squamous cell epithelium to gastric columnar epithelium. BE significantly increases the risk of developing esophageal adenocarcinoma, and as a result, adequate treatment of BE is of utmost importance as GERD becomes more prevalent in the US. A non-randomized prospective study by Rossi et al. compared patients with low grade dysplasia (LGD) who were treated with high dose PPI therapy vs. LARS (20). They reported a significantly improved rate of regression of LGD to BE at 12 months in the surgical group (94%) vs. patients treated with high dose PPI therapy (63%). At 18 months, all LARS patients had continued confirmed absence of LGD (20). A matched cohort study out of Italy enrolled 33 patients with BE or LGD, 20 of whom underwent LARS and 13 who were treated medically. Not only was LARS associated with a better control of both acidic and weakly acidic reflux, it was also associated with a higher probability of LGD reversal (21). Smaller retrospective case series have also looked at the rate of regression of BE following LARS. A case series out of the Czech Republic examining 50 patients with BE demonstrated that 38% of patients following LARS had no detectable disease postoperatively. However, in their same series, 36% of patients had unchanged disease and 10% ultimately had disease progression (22). A larger retrospective case series by Morrow et al. showed a regression rate of 22% in patients who underwent LARS and a progression rate of 7% (23). The evidence of improved regression of BE following LARS or medical therapy remains inconclusive, and it remains clear that LARS does not alter the need for continued endoscopic surveillance in these patients. High-grade dysplasia should continue to be treated by endoscopic therapy to achieve complete histological eradication before anti-reflux surgery is attempted and esophageal adenocarcinoma should be treated by a multidisciplinary approach with esophagectomy, chemotherapy and radiation according to oncological standards (5).
Refractory GERD
While LARS is validated for patients who respond to PPI therapy, the data of treating PPI non-responders is mixed. Despite the high efficacy of PPI therapy, clinical failure remains common and can occur in around 20–45% of patients. Furthermore, poor response to PPI is associated with a negative impact on physical and mental-health quality of life (24). As such, treating refractory reflux can be challenging and frustrating for both the patient and clinician. For a substantial portion of these patients, anti-reflux surgery should be considered. The differential diagnosis for reflux disease is broad, however, and not all patients will be helped by surgical management. Clinicians should, therefore, carefully select patients for surgical therapy (25). Of note, while not specifically addressed in this review, there are several other indications for LARS including, but not limited to, bilious reflux disease, laryngopharyngeal reflux disease, and hiatal hernias.
Anvari and Allen were the first investigators to show that poor responders to PPI therapy can benefit from LARS and demonstrated that patients who underwent LARS had significant improvement in postoperative symptom scores and quality of life scores that correlated with lower esophageal acid exposure (26). They studied a group of 719 patients, all of whom had inadequate response to PPI therapy (<70% relief on visual analogue scale), and found a significantly improved postoperative quality of life for both physical health and mental health (26). Other groups have shown fewer promising outcomes. Wilkerson et al. found that while good and poor responders had a significant decrease in symptom score following LARS, poor responders had a lower percentage of “excellent” or “good” surgical outcomes, and many patients continued to report severe heartburn (27). In a prospective study of 370 patients who underwent LARS (296 PPI responders and 74 non-responders), Hamdy et al. found that good responders had a greater reduction of heartburn and regurgitation symptoms (24). Patient satisfaction with surgery was also significantly better in the good responder group. Postoperative assessment did not reveal any significant differences on esophageal manometric testing, LES pressures, or 24-h pH monitoring (24).
Laparoscopy challenges
The widespread acceptance of laparoscopy has offered multiple improvements for patient outcomes including shorter hospital stay, less postoperative pain, and improved long-term quality of life. Several disadvantages of laparoscopic surgery quickly became apparent including loss of 3D visualization, reduction in haptic feedback, mechanical constraint secondary to the “fulcrum effect,” instrument rigidity, tremor enhancement, and decreased range of motion (28,29). Laparoscopy has also been associated with new surgeon discomfort and ergonomic challenges. Previous studies have shown that even experienced laparoscopic surgeons suffer from significantly increased upper extremity muscle discomfort and physical work (30). Several factors contribute to the surgeon’s musculoskeletal stress, including prolonged static head and trunk posture, a greater amount of shoulder and upper arm movements while using laparoscopic instruments, and poor mechanical design of laparoscopic instruments. Patient characteristics have also contributed to differences in ergonomic stress. For example, a patient’s increased body mass index (BMI) adds increasing difficulty to a procedure. Additionally, laparoscopy relies on the experience and the knowledge of a surgical assistant and incorrect maneuvering can lead to poor visualization, increased operating room times and intraoperative complications (31). The challenges associated with laparoscopy paved the way for the development of surgical robotic systems, which seek to resolve some of these difficulties.
Robotic surgical treatment of reflux disease
History of robotic surgery
Robotic surgery was developed to address many of the previously identified challenges with laparoscopy, and its use is now becoming widespread in anti-reflux surgery. The robotic platform confers full control of the camera and instruments to the primary surgeon, improving surgeon ergonomics, visualization, and autonomy (31). While a number of early systems were developed, the predominant system for abdominal surgery at the time of this writing is the da Vinci Surgical System (dVSS) (Intuitive, Incorporated; Sunnyvale, CA) (Figure 1). Robotic technology has become pervasive throughout modern surgical practice and has been rapidly incorporated into many specialties such as gynecology, urology, colorectal, and bariatrics, and now ever increasingly, foregut surgery (32). In the year 2000, there were only 18 robotic surgery systems worldwide. As of March 2019, the number of available systems worldwide has skyrocketed to 5,114 and the total number of robotic-assisted procedures surpassed 5 million (33).
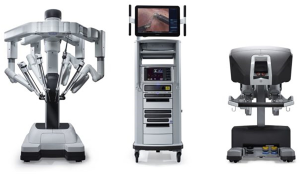
Commonly-used robotic surgical platforms are equipped with a variety of technologies that improve the precision and efficiency of complex surgical task performance compared to traditional laparoscopic surgery (34-38). Robotic platforms employ wristed instruments that provide surgeons with seven total degrees of freedom when performing surgical tasks, an increase compared to the four degrees of freedom provided by traditional rigid laparoscopic instrumentation (39). Commonly-used robotic platforms are paired with a high-definition three-dimensional camera that allows for improved precision and visualization of surgical targets when compared to the two-dimensional camera platforms used in laparoscopy (34-36). In addition, the surgeon is always in control of the camera, able to obtain the visualization required without added communication and guidance from a surgical assistant. The surgeon’s console allows for scalability of a robotic surgeon’s movements up to 3:1 to allow for finer movement at the instrument tip than traditional laparoscopic instrumentation and provides the added benefit of tremor elimination (39).
The laparoscopic operating room environment has many ergonomic challenges that robotic surgical systems seek to address (40-42): suboptimal operating table and monitor positioning, non-ergonomic instrument handles, and maintenance of awkward body positioning for extended periods of time (43,44). While using a robotic surgical platform, the operating surgeon is in a seated position viewing the procedure through a viewfinder on the console and manipulating instruments using lightweight masters. These features provide ergonomic benefits to operating surgeons and subjective improvements in ergonomic stress (45-47). In a quantitative comparison of ergonomic stress associated with laparoscopic and robotic assisted laparoscopic procedures, robotic platforms were associated with significantly decreased activation of multiple muscle groups: biceps, triceps, and deltoids bilaterally (48).
However, the robotic platform has several practical and technical limitations that affect their use. Robotic platforms provide operating surgeons with significantly less haptic feedback than is provided during conventional laparoscopy. Additionally, most robot-assisted surgical platforms restrict surgical activity to only part of the body cavity (although, the latest robotic platforms aim to address this restriction and are equipped for a wider field of surgery) (37). Furthermore, robotic surgery is associated with somewhat of a learning curve, such that surgeons less familiar with robotic surgery may initially experience a temporary slowing of their skills that can subsequently impact their clinical performance (49). Lastly, there is generally an increased upfront equipment cost associated with the robotic platform compared to conventional laparoscopic procedures, with a variable impact on length of hospital stay or operative time (50).
Robotic surgery vs. laparoscopic surgery—patient outcomes
Robotic-assisted laparoscopic fundoplication (RALF) has been demonstrated to be safe and feasible with similar short-term outcomes to LARS (51-55). In a randomized clinical trial by Draaisma et al. comparing 50 cases of RALF and LARS, the authors found no difference in postoperative pain scores, hospital stay, complication rates, reoperation rates, and self-rated quality of life improvement (55). A longer postoperative period was analyzed by Nakadi et al. who found that, after the 1st, 6th, and 12th postoperative months, patients reported a similar amount of postoperative complaints. Both groups had similar lengths of postoperative hospital stays (53). Yet another randomized control trial by Muller-Stich et al. found no difference in conversion to an open approach, mean length of hospital stay, or symptomatic outcomes at 30 days postoperatively between the two procedures (56). In a large analysis of anti-reflux procedures specifically examining readmission rates and patient outcomes, Owen et al. found a lower 30-day readmission rate after LARS, but no significant difference in mortality, morbidity, and length of stay between LARS and RALF (57).
RALF has also been shown to significantly decrease postoperative esophageal acid exposure time (EAET). Frazzoni et al. retrospectively examined postoperative manometric and acid reflux parameters between RALF and LARS and found that while there were no significant differences in manometric parameters, the median EAET was significantly lower for RALF than LARS. Additionally, they also found that abnormal EAET values were found in 0% (0/44) of patients who underwent RALF vs. 14% (6/44) of patients who underwent LARS. Furthermore, normal EAET was observed significantly less frequently after LARS as compared to RALF (86% vs. 100%) (58). This small, but significant, improvement can be clinically relevant in patients with challenging PPI-refractory reflux disease where any demonstrated therapeutic gain can be meaningful (58).
Robotic surgery vs. laparoscopic surgery—operative time and cost
While robotic platforms have slowly been introduced as an acceptable and safe option in anti-reflux surgery, operative times and the cost of the robotic platform are often cited as potential drawbacks. Most randomized clinical trials have reported a significant increase in operative times with RALF, especially in the earlier phases of adoption (51-53,59). Morino et al. examined 50 consecutive patients undergoing LARS or RALF and demonstrated that RALF required significantly longer total operative times (131 minutes for RALF vs. 91 minutes for LARS) (52). Similarly, in a retrospective cohort study, Jensen et al. compared 103 patients who underwent RALF or LARS and found significantly longer operative durations for robotic surgery (135 minutes for RALF vs. 86 minutes for LARS). While early experience with robotic surgery was associated with longer operating times, patients who are operated on by a single surgeon and a highly experienced operating room team have greatly decreased operative times (59). In a randomized prospective cohort trial, Muller-Stich et al. showed a shorter total operative time for RALF compared to conventional LARS (88 minutes for RALF vs. 103 minutes for LARS) and the authors attributed the results to the experience of the one operating surgeon and the experienced robotics team (56).
It is no surprise that there is often a higher upfront equipment cost of RALF when compared to LARS (59-61). A meta-analysis by Wang et al. found that total operative costs, while not reaching significance, were higher for RALF. The authors ultimately attributed this difference to expensive disposable materials and longer operative times (60). Similarly, a meta-analysis performed by Falkenback et al. of 33 publications on anti-reflux operations found significantly higher costs for the robotic method (61). In a large retrospective analysis of RALF and LARS, Owen et al. (57) compared 9,572 LARSs and 339 RALFs and found higher costs associated with RALF (LARS cost was $7,968±$6,969 compared to $10,644±$6,041 for RALF). At present, with expanding numbers of surgical teams utilizing RALF, there is an increased cost associated with the initial adoption of RALF and higher costs per individual procedure. However, overall costs of robotic surgery are evolving as we gain a better understanding of potential downstream savings in length of stay, readmissions, and complication rates. Furthermore, as additional robotic platforms emerge and competition between manufacturers ensue, costs will likely diminish.
Endoscopic therapy for the treatment of reflux disease
Introduction
The technological advancement of minimally invasive surgery is often driven by the desire to complete an operation with as few incisions as possible. Endoscopic anti-reflux therapy (EART) realizes this vision to become the holy grail of minimally invasive surgery: incisionless surgery through a natural orifice. Endoscopic anti-reflux therapy was developed in an attempt to further increase the number of patients with GERD who can be treated with surgical therapy. Lifestyle modifications and medical therapy remain the first line treatments, but around 20–40% of patients will experience only partial relief in their GERD symptoms. While LARS is the gold-standard for the surgical treatment of GERD, only a small percentage of patients with severe GERD opt for traditional surgical intervention. Minimally invasive endoscopic therapies have been developed to address many of the challenges faced with laparoscopy and robotics and to offer an alternative to medical therapy and major surgical intervention (62). While the currently available products all function differently, the main goal of the therapy is to endoscopically reduce lower esophageal sphincter compliance. They offer the potential for decreased postoperative pain and shorter hospital stay. Additionally, many seek to reduce some of the most common side-effects of LARS: inability to belch/vomit, increased postoperative dysphagia, and increased flatulence. Nevertheless, at this time, most endoscopic therapies have failed to consistently deliver adequate acid suppression in the majority of patients and more advanced alternatives would be needed before replacing LARS as the gold standard (9).
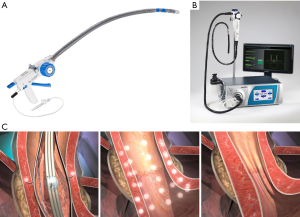
Today, there are four EART currently in use: three of which are executed with proprietary devices (Figure 2), and the fourth utilizing a traditional endoscopic set-up [anti-reflux Mucosectomy (ARMS)]. The three currently available proprietary options include the transoral incisionless fundoplication (TIF) (EsophyX device; EndoGastric Solutions, Redmond, Wash, USA), Stretta (Mederi Therapeutics, Restech, Houston, TX, USA), and Medigus Ultrasonic Surgical Endostapler (MUSE) (Medigus Ltd, Omer, Israel) (62). Several previous devices have been developed but have since been removed from the market either because of safety concerns or lack of efficacy (62). As the currently available devices are conceived now, EART should not be considered an alternative to traditional laparoscopic or robotic anti-reflux surgical therapy (62). These methods cannot adequately address the problem of a hiatal or paraesophageal hernia greater than 2 cm and should not be used in patients with significant anatomic abnormalities (9). These devices also have not been systematically studied in patients with active esophagitis, BE, and esophageal motility disorders and limited data exists in regards to treating patients with laryngopharyngeal reflux disease (62).
As with LARS and RALF, selecting the proper patient for EART is important. Many disease processes can mimic GERD and may not be relieved with traditional anti-reflux procedures. If a patient’s pH/impedance study is negative, the surgeon must consider other possible diagnoses, such as achalasia, ineffective motility, spastic esophageal dysmotility, gastroparesis, and eosinophilic esophagitis, and should counsel patients away from anti-reflux intervention. Data has shown that up to 44% of patients with esophageal dysmotility/achalasia, 57% of patients with eosinophilic esophagitis, and 73% of patients with gastroparesis may experience pathologic reflux symptoms that mimic reflux experienced by patients with GERD (63). As mentioned previously, patients with a hiatal hernia greater than 2 cm are not good candidates for endoscopic therapy and require a traditional surgical procedure. Lastly, in patients with morbid obesity, endoscopic therapy may not be the ideal option; the gold standard remains bariatric surgery, with Roux-en-Y gastric bypass favored amongst the many options. Of note, patients with a previous sleeve gastrectomy and new or recurrent reflux symptoms not responding to medical therapy may respond to endoscopic radiofrequency ablation with the Stretta (64). This could potentially be offered prior to the traditional therapy of conversion to Roux-en-Y gastric bypass.
Transoral incisionless fundoplication
Initially approved in September 2007 by the FDA, the TIF using the EsophyX device endoscopically reconstructs the LES and restores the angle of HIS. This procedure requires general anesthesia and offers the ability to reduce small hiatal hernias (<2 cm). It can create a 2–4 cm long valve with a >270º fundoplication (62). Two endoscopists are required for the procedure: one to operate the gastroscope and the second to manipulate the EsophyX device. The device is loaded over the shaft of a compatible gastroscope and both are advanced into the stomach. With the gastroscope retroflexed to view the gastric cardia, the EsophyX device creates the fundoplication. At the end of the procedure, 20 fasteners (placed 1–3 cm above the GEJ) create the fusion of the esophagus and fundus (62). A second generation of the device, EsophyX2, is now available and can be used to perform a slightly modified TIF 2.0 procedure.
Four randomized clinical trials have evaluated TIF 2.0 procedure with the EsophyX2 device for the treatment of GERD. The RESPECT (Randomized EsophyX2vs. Sham, Placebo-Controlled Transoral Fundoplication) study was a multicenter, blinded, randomized control trial that compared the TIF 2.0 procedure plus placebo medication vs. a sham operation and optimal PPI therapy for patients with >6 months of GERD and regurgitation symptoms despite PPI treatment. By an intention-to-treat analysis, TIF 2.0 eliminated troublesome regurgitation in 67% of patients vs. only 47% of patients with PPI therapy alone. Esophageal pH improved but did not normalize after TIF 2.0 and both groups had similar improvements in GERD symptom scores (65). A large systematic review and network meta-analysis comprising 1,128 patients by Richter et al. compared the efficacy of TIF, LARS, PPI therapy and a sham procedure. The authors found that while TIF had the highest probability of increasing patient’s health related quality of life (followed by LARS, sham procedure, and PPI), LARS was the best at controlling esophageal pH (followed by PPI, TIF, and sham) (66). Interestingly, other than the sham procedure, the TIF was associated with the greatest percentage of patients with persistent esophagitis (66). Ultimately, the authors concluded that TIF was not an adequate long-term alternative to LARS or PPI therapy (66).
Stretta
Originally cleared by the FDA in 2000, updated and cleared again in 2011, Stretta acts by delivering radiofrequency (RF) current to ablate the muscles of the LES (62). While the exact mechanism remains unclear, RF reduces the number of transient lower esophageal sphincter relaxations and decreases LES compliance (62). Stretta RF does not require general anesthesia and can be performed in a routine outpatient setting under sedation or monitored anesthesia care. Low power RF energy is delivered to the muscularis propria of the lower esophagus (5W per channel at 460 kHz frequency). Proper placement of the needle electrodes in the muscle is confirmed by impedance measurements (62). After performing an upper endoscopy to measure the distance from the bite block to the Z-line, a guidewire is introduced, the endoscope is retracted, and Stretta is advanced over the guidewire to about 1 cm proximal to the Z-line. Without endoscopic guidance, RF ablation is applied to a total of 56 treatment sites at 6 different treatment levels (4 antegrade and 2 retrograde in the proximal stomach) (62). Safety has been a major factor for the implementation of Stretta. Lipka et al. performed a Manufacturer and User Facility Device Experience search to assess the risks associated with the Stretta procedure. Since cleared by the FDA, multiple reported serious adverse events have occurred including pneumonia, gastroparesis, esophageal perforation, cardiac arrest and four reported deaths (67).
In total there have been four randomized clinical trials that have evaluated the Stretta device. The largest of which was a randomized control trial of 64 patients that compared Stretta (35 patients) to a sham procedure (29 patients) (68). At 6 months, there was no improvement in PPI usage or reduction in median esophageal acid exposure time. However, the group found a significant improvement in symptomatic relief (61% of Stretta patients vs. 33% in the sham group) and GERD-HRQL scores (68). A smaller randomized control trial of 22 patients randomized patients to a sham or Stretta procedure (69). Similarly to Corley et al., they found no difference in esophageal acid pH exposure or reduction in PPI use, but that Stretta significantly improved quality of life scores for bodily pain (69). Additionally, the authors examined the distensibility of the LES by using a barostat bag: a decrease in LES distensibility was noted after the Stretta procedure, but was found to be reversible on local administration of sildenafil (69). The authors concluded that the decreased compliance of the LES following the Stretta procedure is likely secondary to altered LES neuromuscular motility rather than LES fibrosis as was previously thought (69). A recent meta-analysis and systematic review by Fass et al. found more favorable results (70). In a review of 28 studies (4 randomized control trials) representing 2,468 patients, the authors found that Stretta was associated with improved health related quality of life scores and pooled heartburn standardized scores at a mean follow up of 25 months (70). Additionally, Stretta was associated with a significant reduction in the rate of erosive esophagitis by 24% (70). However, no change in LES basal pressure was found (70).
Medigus ultrasonic surgical endostapler
The Medigus Ultrasonic Surgical Endostapler (MUSE®), cleared for use by the FDA in January 2015, is an endoscopic stapling system that creates a partial fundoplication. The MUSE endoscope is advanced into the stomach through a previously placed overtube, retroflexed and pulled back to the correct level above the GEJ (usually around 3 cm) using a built-in ultrasonographic gap finder (62). The anvil engages with the rigid section of the endoscope shaft to clamp the fundus against the distal esophagus. A staple is delivered and the procedure is repeated to form a 180º fundoplication.
The clinical efficacy of the MUSE was assessed in a multicenter, prospective trial. Sixty-nine (69) patients underwent MUSE endoscopic stapling: GERD-HRQL scores improved by >50% off PPI therapy in 73% of patients and 65% of patients were no longer using daily PPIs at 6 months (71). Long-term follow-up of the data showed that at four years, 69% were off their daily PPIs and their GERD-HRQL score had significantly decreased from baseline (71). Another smaller, non-randomized study was performed to assess the product’s safety in which endoscopic stapling (11 patients) was compared with laparoscopic fundoplication (16 patients) (72). The authors did not find a significant difference in GERD-HRQL scores: scores decreased by 87% in the LARS group vs. 64% in the endoscopic stapling subset. PPI use was found to be higher in the endoscopic stapling group, but the results were not significant (72).
Safety has been the main concern with the MUSE device: 8 serious adverse events, including pain, fever, viral infection and mediastinitis, were reported in the first 24 patients in a multicenter trial. In the same series, two severe adverse events were also reported: esophageal leak (resulting in pneumothorax, empyema and a 22 day hospital stay) and severe upper GI bleeding (requiring two units of blood) (72). These early adverse events led to changes in the procedure protocol (prophylactic antiemetic therapy and an additional stapling site) and the routine use of a post-procedure chest radiograph to exclude a postoperative esophageal leak (62,72). Limited adverse events have been reported following these changes.
Anti-reflux mucosectomy
ARMS is the newest endoscopic technique designed to treat reflux disease. It is unique amongst the endoscopic options as it does not require special or proprietary equipment. The procedure consists of a hemi-circumferential endoscopic mucosal resection of the gastric cardia around the GEJ (73). With the scope in a retroflexed position, the mucosa is marked with the snare 270º around the GE valve. The mucosa of the cardia is raised with a combination of saline, methylene blue and epinephrine; the tissue is banded; and subsequently transected with forced coagulation (73). This process is repeated, rotating the scope around the GEJ. As the mucosectomy bed heals and scars, the tissue contracts and tightens to augment the natural anti-reflux valve of GEJ (73).
Limited long-term data exists on the ARMS procedure. An early pilot study to assess ARMS’ efficacy to treat reflux disease followed 10 patients after undergoing the procedure. The authors found significantly decreased total DeMeester scores, mean regurgitation scores, and mean heartburn scores at 2 months (74). 24-hour esophageal pH monitoring revealed that the fraction of time at pH <4 improved from 29% to 3%, but was ultimately not statistically significant. In all 10 patients, PPI therapy was discontinued. However, in the cohort, two patients required repeat balloon dilation to control post-procedural esophageal stenosis (74). A second case series by Hedberg et al. detailed 19 patients who underwent ARMS. In this series, 68% of patients showed significant symptom improvement scores and significant rates of PPI discontinuation. However, three patients, post-procedure, had troubling dysphagia and required balloon dilation (73). Additionally, of the six patients who did not have symptom relief following ARMS, 3 (50%) ultimately underwent LARS. More data and future randomized studies are needed to accurately compare the efficacy and safety of ARMS to mainstay PPI therapy and LARS.
Endoscopic anti-reflux therapy conclusion
Endoscopic anti-reflux therapy offers a truly minimally invasive option for select patients. While randomized trials and case series have reported mixed efficacy with these techniques, EART potentially expands the treatment options available for troubling reflux disease. Although most trials have not shown consistent improvement in objective measurements, such as normalization of pH values and augmentation of LES pressures, many patients do report subjective clinical improvement following EART and many can ultimately wean from PPI therapy. The complexity of GERD and the many underlying causes of the disease dictates that a thorough, multidisciplinary diagnostic evaluation occur prior to decision making and that patient selection must be done carefully. Each of the aforementioned EART modalities have unique features and their selection needs to be tailored to the individual patient’s history and clinical presentation. As these and other minimally invasive techniques arrive on the market and long-term data becomes available, these devices should be limited to centers specializing in reflux disease (65,75). Future work should be geared to testing these devices against both laparoscopic and robotic anti-reflux procedures and should examine their long-term efficacy.
Conclusions
Today, patients and physicians have a multitude of anti-reflux therapy options for treating GERD. As with any interventional procedure, proper patient selection is key. Each of the three main modalities, LARS, RALF, and EART, come with their own set of advantages and disadvantages. Foregut surgeons and gastroenterologists should be familiar with each of these options to be able to provide their patients with customizable therapy to best treat their disease. Further work with large well-designed studies are needed to continue to evaluate the effectiveness of each of these three therapies and determine which patients may benefit most from each specific procedure.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2020.03.01). Dr. Awad has the following conflicts of interest- Education grants from Intuitive, Incorporated; Sunnyvale, CA. The other authors have no conflicts of interest to disclose.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- El-Serag HB, Sweet S, Winchester CC, et al. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut 2014;63:871-80. [Crossref] [PubMed]
- Friedenberg FK, Hanlon A, Vanar V, et al. Trends in gastroesophageal reflux disease as measured by the National Ambulatory Medical Care Survey. Dig Dis Sci 2010;55:1911-7. [Crossref] [PubMed]
- Peery AF, Dellon ES, Lund J, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology 2012;143:1179-87.e3. [Crossref] [PubMed]
- Tolboom RC, Broeders IA, Draaisma WA. Robot-assisted laparoscopic hiatal hernia and antireflux surgery. J Surg Oncol 2015;112:266-70. [Crossref] [PubMed]
- Stefanidis D, Hope WW, Kohn GP, et al. Guidelines for surgical treatment of gastroesophageal reflux disease. Surg Endosc 2010;24:2647-69. [Crossref] [PubMed]
- Nowak M, Buttner P, Raasch B, et al. Lifestyle changes as a treatment of gastroesophageal reflux disease: a survey of general practitioners in North Queensland, Australia. Ther Clin Risk Manag 2005;1:219-24. [PubMed]
- Dallemagne B, Weerts JM, Jehaes C, et al. Laparoscopic Nissen fundoplication: preliminary report. Surg Laparosc Endosc 1991;1:138-43. [PubMed]
- Oor JE, Roks DJ, Broeders JA, et al. Seventeen-year outcome of a randomized clinical trial comparing laparoscopic and conventional nissen fundoplication: a plea for patient counseling and clarification. Ann Surg 2017;266:23-8. [Crossref] [PubMed]
- Watson DI. Laparoscopic treatment of gastro-oesophageal reflux disease. Best Pract Res Clin Gastroenterol 2004;18:19-35. [Crossref] [PubMed]
- Peters MJ, Mukhtar A, Yunus RM, et al. Meta-analysis of randomized clinical trials comparing open and laparoscopic anti-reflux surgery. Am J Gastroenterol 2009;104:1548-61; quiz 7, 62.
- Catarci M, Gentileschi P, Papi C, et al. Evidence-based appraisal of antireflux fundoplication. Ann Surg 2004;239:325-37. [Crossref] [PubMed]
- Shaw JM, Bornman PC, Callanan MD, et al. Long-term outcome of laparoscopic Nissen and laparoscopic Toupet fundoplication for gastroesophageal reflux disease: a prospective, randomized trial. Surg Endosc 2010;24:924-32. [Crossref] [PubMed]
- Aiolfi A, Asti E, Bernardi D, et al. Early results of magnetic sphincter augmentation versus fundoplication for gastroesophageal reflux disease: Systematic review and meta-analysis. Int J Surg 2018;52:82-8. [Crossref] [PubMed]
- Freedberg DE, Kim LS, Yang YX. The risks and benefits of long-term use of proton pump inhibitors: expert review and best practice advice from the american gastroenterological association. Gastroenterology 2017;152:706-15. [Crossref] [PubMed]
- Maes ML, Fixen DR, Linnebur SA. Adverse effects of proton-pump inhibitor use in older adults: a review of the evidence. Ther Adv Drug Saf 2017;8:273-97. [Crossref] [PubMed]
- Galmiche JP, Hatlebakk J, Attwood S, et al. Laparoscopic antireflux surgery vs esomeprazole treatment for chronic GERD: the LOTUS randomized clinical trial. JAMA 2011;305:1969-77. [Crossref] [PubMed]
- Mahon D, Rhodes M, Decadt B, et al. Randomized clinical trial of laparoscopic Nissen fundoplication compared with proton-pump inhibitors for treatment of chronic gastro-oesophageal reflux. Br J Surg 2005;92:695-9. [Crossref] [PubMed]
- Hatlebakk JG, Zerbib F, Bruley des Varannes S, et al. Gastroesophageal acid reflux control 5 years after antireflux surgery, compared with long-term esomeprazole therapy. Clin Gastroenterol Hepatol 2016;14:678-85.e3. [Crossref] [PubMed]
- Spechler SJ, Lee E, Ahnen D, et al. Long-term outcome of medical and surgical therapies for gastroesophageal reflux disease: follow-up of a randomized controlled trial. JAMA 2001;285:2331-8. [Crossref] [PubMed]
- Rossi M, Barreca M, de Bortoli N, et al. Efficacy of Nissen fundoplication versus medical therapy in the regression of low-grade dysplasia in patients with Barrett esophagus: a prospective study. Ann Surg 2006;243:58-63. [Crossref] [PubMed]
- Tolone S, Limongelli P, Romano M, et al. The patterns of reflux can affect regression of non-dysplastic and low-grade dysplastic Barrett's esophagus after medical and surgical treatment: a prospective case-control study. Surg Endosc 2015;29:648-57. [Crossref] [PubMed]
- Aujeský R, Neoral C, Vrba R, et al. The effect of laparoscopic fundoplication in therapy of Barrett's esophagus. Wideochir Inne Tech Maloinwazyjne 2014;9:213-8. [Crossref] [PubMed]
- Morrow E, Bushyhead D, Wassenaar E, et al. The impact of laparoscopic anti-reflux surgery in patients with Barrett's esophagus. Surg Endosc 2014;28:3279-84. [Crossref] [PubMed]
- Hamdy E, El Nakeeb A, Hamed H, et al. Outcome of laparoscopic Nissen fundoplication for gastroesophageal reflux disease in non-responders to proton pump inhibitors. J Gastrointest Surg 2014;18:1557-62. [Crossref] [PubMed]
- Seo HS, Choi M, Son SY, et al. Evidence-Based Practice Guideline for Surgical Treatment of Gastroesophageal Reflux Disease 2018. J Gastric Cancer 2018;18:313-27. [Crossref] [PubMed]
- Anvari M, Allen C. Surgical outcome in gastro-esophageal reflux disease patients with inadequate response to proton pump inhibitors. Surg Endosc 2003;17:1029-35. [Crossref] [PubMed]
- Wilkerson PM, Stratford J, Jones L, et al. A poor response to proton pump inhibition is not a contraindication for laparoscopic antireflux surgery for gastro esophageal reflux disease. Surg Endosc 2005;19:1272-7. [Crossref] [PubMed]
- Supe AN, Kulkarni GV, Supe PA. Ergonomics in laparoscopic surgery. J Minim Access Surg 2010;6:31-6. [Crossref] [PubMed]
- Nisky I, Huang F, Milstein A, et al. Perception of stiffness in laparoscopy - the fulcrum effect. Stud Health Technol Inform 2012;173:313-9. [PubMed]
- Park AE, Zahiri HR, Hallbeck MS, et al. Intraoperative "Micro Breaks" With Targeted Stretching Enhance Surgeon Physical Function and Mental Focus: A Multicenter Cohort Study. Ann Surg 2017;265:340-6. [Crossref] [PubMed]
- Moore MD, Afaneh C, Gray KD, et al. The impact of the robotic platform on assistant variability in complex gastrointestinal surgery. J Surg Res 2017;219:98-102. [Crossref] [PubMed]
- Brody F, Richards NG. Review of robotic versus conventional laparoscopic surgery. Surg Endosc 2014;28:1413-24. [Crossref] [PubMed]
- Cole AP, Trinh QD, Sood A, et al. The rise of robotic surgery in the new millennium. J Urol 2017;197:S213-5. [Crossref] [PubMed]
- Maier-Hein L, Groch A, Bartoli A, et al. Comparative validation of single-shot optical techniques for laparoscopic 3-D surface reconstruction. IEEE Trans Med Imaging 2014;33:1913-30. [Crossref] [PubMed]
- Wilhelm D, Reiser S, Kohn N, et al. Comparative evaluation of HD 2D/3D laparoscopic monitors and benchmarking to a theoretically ideal 3D pseudodisplay: even well-experienced laparoscopists perform better with 3D. Surg Endosc 2014;28:2387-97. [Crossref] [PubMed]
- Lusch A, Bucur PL, Menhadji AD, et al. Evaluation of the impact of three-dimensional vision on laparoscopic performance. J Endourol 2014;28:261-6. [Crossref] [PubMed]
- Protyniak B, Jorden J, Farmer R. Multiquadrant robotic colorectal surgery: the da Vinci Xi vs Si comparison. J Robot Surg 2018;12:67-74. [Crossref] [PubMed]
- Chandra V, Nehra D, Parent R, et al. A comparison of laparoscopic and robotic assisted suturing performance by experts and novices. Surgery 2010;147:830-9. [Crossref] [PubMed]
- Zihni A, Gerull WD, Cavallo JA, et al. Comparison of precision and speed in laparoscopic and robot-assisted surgical task performance. J Surg Res 2018;223:29-33. [Crossref] [PubMed]
- Berguer R, Chen J, Smith WD. A comparison of the physical effort required for laparoscopic and open surgical techniques. Arch Surg 2003;138:967-70. [Crossref] [PubMed]
- Berguer R. Surgical technology and the ergonomics of laparoscopic instruments. Surg Endosc 1998;12:458-62. [Crossref] [PubMed]
- Lee G, Lee T, Dexter D, et al. Ergonomic risk associated with assisting in minimally invasive surgery. Surg Endosc 2009;23:182-8. [Crossref] [PubMed]
- O'Sullivan OE, O'Reilly BA. Robot-assisted surgery:--impact on gynaecological and pelvic floor reconstructive surgery. Int Urogynecol J 2012;23:1163-73. [Crossref] [PubMed]
- Lunca S, Bouras G, Stanescu AC. Gastrointestinal robot-assisted surgery. A current perspective. Rom J Gastroenterol 2005;14:385-91. [PubMed]
- Wright AS, Gould JC, Melvin WS. Computer-assisted robotic antireflux surgery. Minerva Gastroenterol Dietol 2004;50:253-60. [PubMed]
- Frick AC, Falcone T. Robotics in gynecologic surgery. Minerva Ginecol 2009;61:187-99. [PubMed]
- Xia T, Baird C, Jallo G, et al. An integrated system for planning, navigation and robotic assistance for skull base surgery. Int J Med Robot 2008;4:321-30. [Crossref] [PubMed]
- Zihni AM, Ohu I, Cavallo JA, et al. Ergonomic analysis of robot-assisted and traditional laparoscopic procedures. Surg Endosc 2014;28:3379-84. [Crossref] [PubMed]
- Zihni A, Ge T, Ray S, et al. Transfer and priming of surgical skills across minimally invasive surgical platforms. J Surg Res 2016;206:48-52. [Crossref] [PubMed]
- Higgins RM, Frelich MJ, Bosler ME, et al. Cost analysis of robotic versus laparoscopic general surgery procedures. Surg Endosc 2017;31:185-92. [Crossref] [PubMed]
- Cadière GB, Himpens J, Vertruyen M, et al. Evaluation of telesurgical (robotic) NISSEN fundoplication. Surg Endosc 2001;15:918-23. [Crossref] [PubMed]
- Morino M, Pellegrino L, Giaccone C, et al. Randomized clinical trial of robot-assisted versus laparoscopic Nissen fundoplication. Br J Surg 2006;93:553-8. [Crossref] [PubMed]
- Nakadi IE, Melot C, Closset J, et al. Evaluation of da Vinci Nissen fundoplication clinical results and cost minimization. World J Surg 2006;30:1050-4. [Crossref] [PubMed]
- Wykypiel H, Bodner J, Wetscher G, et al. Robot-assisted versus conventional laparoscopic fundoplication: short-term outcome of a pilot randomized controlled study. Surg Endosc 2008;22:1407. [Crossref] [PubMed]
- Draaisma WA, Ruurda JP, Scheffer RC, et al. Randomized clinical trial of standard laparoscopic versus robot-assisted laparoscopic Nissen fundoplication for gastro-oesophageal reflux disease. Br J Surg 2006;93:1351-9. [Crossref] [PubMed]
- Müller-Stich BP, Reiter MA, Wente MN, et al. Robot-assisted versus conventional laparoscopic fundoplication: short-term outcome of a pilot randomized controlled trial. Surg Endosc 2007;21:1800-5. [Crossref] [PubMed]
- Owen B, Simorov A, Siref A, et al. How does robotic anti-reflux surgery compare with traditional open and laparoscopic techniques: a cost and outcomes analysis. Surg Endosc 2014;28:1686-90. [Crossref] [PubMed]
- Frazzoni M, Conigliaro R, Colli G, et al. Conventional versus robot-assisted laparoscopic Nissen fundoplication: a comparison of postoperative acid reflux parameters. Surg Endosc 2012;26:1675-81. [Crossref] [PubMed]
- Jensen JS, Antonsen HK, Durup J. Two years of experience with robot-assisted anti-reflux surgery: A retrospective cohort study. Int J Surg 2017;39:260-6. [Crossref] [PubMed]
- Wang Z, Zheng Q, Jin Z. Meta-analysis of robot-assisted versus conventional laparoscopic Nissen fundoplication for gastro-oesophageal reflux disease. ANZ J Surg 2012;82:112-7. [Crossref] [PubMed]
- Falkenback D, Lehane CW, Lord RV. Robot-assisted oesophageal and gastric surgery for benign disease: antireflux operations and Heller's myotomy. ANZ J Surg 2015;85:113-20. [Crossref] [PubMed]
- ASGE Technology Committee , Thosani N, Goodman A, et al. Endoscopic anti-reflux devices (with videos). Gastrointest Endosc 2017;86:931-48. [Crossref] [PubMed]
- Galindo G, Vassalle J, Marcus SN, et al. Multimodality evaluation of patients with gastroesophageal reflux disease symptoms who have failed empiric proton pump inhibitor therapy. Dis Esophagus 2013;26:443-50. [Crossref] [PubMed]
- Mattar SG, Qureshi F, Taylor D, et al. Treatment of refractory gastroesophageal reflux disease with radiofrequency energy (Stretta) in patients after Roux-en-Y gastric bypass. Surg Endosc 2006;20:850-4. [Crossref] [PubMed]
- Triadafilopoulos G. Endoscopic options for gastroesophageal reflux: where are we now and what does the future hold? Curr Gastroenterol Rep 2016;18:47. [Crossref] [PubMed]
- Richter JE, Kumar A, Lipka S, et al. Efficacy of laparoscopic nissen fundoplication vs transoral incisionless fundoplication or proton pump inhibitors in patients with gastroesophageal reflux disease: a systematic review and network meta-analysis. Gastroenterology 2018;154:1298-308 e7.
- Lipka S, Kumar A, Richter JE. No evidence for efficacy of radiofrequency ablation for treatment of gastroesophageal reflux disease: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2015;13:1058-67 e1.
- Corley DA, Katz P, Wo JM, et al. Improvement of gastroesophageal reflux symptoms after radiofrequency energy: a randomized, sham-controlled trial. Gastroenterology 2003;125:668-76. [Crossref] [PubMed]
- Arts J, Bisschops R, Blondeau K, et al. A double-blind sham-controlled study of the effect of radiofrequency energy on symptoms and distensibility of the gastro-esophageal junction in GERD. Am J Gastroenterol 2012;107:222-30. [Crossref] [PubMed]
- Fass R, Cahn F, Scotti DJ, et al. Systematic review and meta-analysis of controlled and prospective cohort efficacy studies of endoscopic radiofrequency for treatment of gastroesophageal reflux disease. Surg Endosc 2017;31:4865-82. [Crossref] [PubMed]
- Kim HJ, Kwon CI, Kessler WR, et al. Long-term follow-up results of endoscopic treatment of gastroesophageal reflux disease with the MUSE endoscopic stapling device. Surg Endosc 2016;30:3402-8. [Crossref] [PubMed]
- Danalioglu A, Cipe G, Toydemir T, et al. Endoscopic stapling in comparison to laparoscopic fundoplication for the treatment of gastroesophageal reflux disease. Dig Endosc 2014;26:37-42. [Crossref] [PubMed]
- Hedberg HM, Kuchta K, Ujiki MB. First Experience with Banded Anti-reflux Mucosectomy (ARMS) for GERD: Feasibility, Safety, and Technique (with Video). J Gastrointest Surg 2019;23:1274-8. [Crossref] [PubMed]
- Inoue H, Ito H, Ikeda H, et al. Anti-reflux mucosectomy for gastroesophageal reflux disease in the absence of hiatus hernia: a pilot study. Ann Gastroenterol 2014;27:346-51. [PubMed]
- Triadafilopoulos G, Akiyama J. Emerging endoscopic techniques for the identification of esophageal disease. Expert Rev Gastroenterol Hepatol 2016;10:605-13. [Crossref] [PubMed]
Cite this article as: Kushner BS, Gerull WD, Smith ER, Awad MM. Approaches to anti-reflux surgery: laparoscopic, robotic, and endoscopic. Ann Laparosc Endosc Surg 2021;6:19.


