Techniques for closing the hiatus: mesh, pledgets and suture techniques
Introduction
The Montreal Consensus report defines gastroesophageal reflux disease (GERD) as “a condition in which reflux of stomach contents cause troublesome symptoms and/or complications” (1). There are several factors that contribute to GERD including a defective lower esophageal sphincter, impaired gastric emptying, failed esophageal peristalsis, or the presence of a hiatal hernia. A hiatal hernia occurs when portions of the stomach and/or other abdominal contents herniate cephalad into the mediastinum through a defect in the esophageal hiatus. When these hernias are present, reduction of the hiatal hernia and repair of the diaphragmatic crus are paramount to the success of any antireflux operation and increasingly to bariatric surgery procedures as well. In the early laparoscopic era, unacceptably high recurrence rates were encountered (2). At the same time awareness of high recurrence rates of hiatal repairs were gaining notice, synthetic mesh in the use of abdominal wall hernias was gaining widespread acceptance due to its effectiveness and safety. These findings paved the groundwork for some practitioners to advocate for use of mesh at the hiatus.
The idea of using mesh in a hiatal hernia repair is similar to the principles of a groin hernia repair, in that it is used to minimize the tension of the repair with prosthetic reinforcement. Early reports using mesh at the hiatus were associated with disastrous complications with some requiring esophagogastric resections (3). These complications were thought to be caused by problems with the use of synthetic mesh materials. Synthetic mesh was prone to erosion or infection that could lead to life-limiting dysphagia, chronic abdominal pain, and recurrent GERD (4,5). As a result, bioprosthetic mesh was advocated by many as an alternative prosthetic to create a tension-free crural repair.
With modern advancements in minimally invasive surgery (i.e., improved visualization, better instrumentation, and more experience), laparoscopy has become the standard of care in hiatal hernia repair (6-8). The tenets of repair shared by most high-volume centers include complete mediastinal sac reduction, mobilization of 2–3 cm of intra-abdominal esophagus and tension-free hiatal closure (9). All of these are associated with improved outcomes. The goal of this chapter is to review the advantages and disadvantages of using mesh and other adjuncts such a pledgets when closing the hiatus.
Suture cruroplasty
Technique
There is no consensus regarding the optimal technique to close the diaphragmatic hiatus during a hiatal hernia repair. Most commonly, the crural defect at the esophageal hiatus is closed using intracorporeally placed interrupted sutures. The configuration of these sutures varies, as horizontal mattress, figure-of-eight and single interrupted sutures have all been described. Although the suture configuration varies, there are several key steps of a laparoscopic hiatal hernia repair that are required to achieve a successful outcome. This includes complete dissection and excision of the hernia sac, mobilization of the esophagus to achieve at least 3 cm of intra-abdominal length, preservation of the peritoneal covering of the crura and closure of the diaphragmatic hiatus using non-absorbable suture. Like abdominal wall hernias, the repair must be as tension free as possible. Sutures are typically placed at 1cm intervals. If the hiatus is still not approximated after 2–3 posterior sutures, additional sutures are placed anterior to the esophagus. This is done to avoid sigmoid distortion of the distal esophageal body (10). Although interrupted sutures are typically used, a running suture technique has also been described using barbed suture (V-Loc, Covidien, Norwalk, CT, USA), with proponents suggesting that this method increases tissue apposition (11).
Recurrence rates
Early laparoscopic repair with primary closure resulted in good clinical outcomes and success rates approaching 90% (12,13). In order to more accurately assess recurrence, subsequent studies utilized radiographic studies including solid and liquid phase esophagram. In 2004, Andujar et al. reported a 25% radiographic recurrence rate at 15-months in a study of 166 patients (14). In a series of 100 consecutive patients, Gibson et al. reported early recurrence rate of 9%, with only 2% being symptomatic (15). At 24-month follow up, the same group reported a 25% recurrence rate, with 3% of patients requiring reoperation for dysphagia. This study also showed persistent improvement in overall quality of, even in the subgroup with recurrence (16). To assess long-term outcomes, Nason et al. reviewed 187 patients from 1997 to 2003, with a median follow-up of 77 months. This study reported a 15% radiographic recurrence rate, a high degree of satisfaction and preservation of GERD-related quality of life (GERD-HRQL) at a median follow up of over 6 years (17). These studies suggest that even if a small radiological recurrence is present, only a small percentage of these patients actually develop recurrent symptoms. The significance of asymptomatic recurrences remains unknown; however, there is concern that these patients may progressively develop symptoms over time.
Pledgeted cruroplasty
Technique
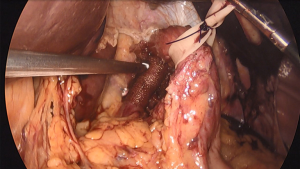
During the hiatal dissection, care should be taken to preserve the peritoneal covering over the edges of the diaphragm. This covering provides support for the sutures used to close the hiatus (12). If these peritoneal coverings are violated, the sutures can tear through the crura leading to early recurrence. To better prevent this, some surgeons prefer a pledgeted horizontal mattress suture technique. Pledgets are used to buttress the hiatal closure and aid in preventing the sutures from cutting through the crural tissue. This is particularly helpful when the diaphragm is thin or has been damaged during the mediastinal dissection. Standardized polytetrafluoroethylene (PTFE) pledgets measuring 15 mm × 10 mm × 1.6 mm are often used, with two pledgets per stitch (Figure 1).

Recurrence rates with pledgets
In 2014, Kang et al. described their experience with primary crural closure with pledgets in 89 patients. At a mean 161-day follow up, the radiographic recurrence rate was 6.7% and 5 patients with recurrence were symptomatic. When assessing GERD-HRQL, 82% of respondents were satisfied or very satisfied (18). A recent study by Weitzendorfer et al. describes the results of pledgeted sutures for hiatal closure in 41 patients who were evaluated for radiologic recurrence with barium swallow at 3 months and 1 year. Postoperative recurrence was diagnosed in 6.8% of patients at 3 months and in 10.8% of patients at 1 year, with only 1 patient symptomatic at 1 year after surgery (19). These studies suggest that the use of pledgets to reinforce hiatal sutures is safe and is may be associated with a lower early recurrence rate though we lack prospective comparative data to truly define the advantage.
Mesh cruroplasty
Mesh type
In the beginning, synthetic mesh was employed for hiatal reinforcement. This idea was a direct extension in our use of these meshes with good success with inguinal and ventral hernia repairs. PFTE was the first mesh documented in the literature in 2002 (20). The use of polypropylene was later described (21). When compared to inguinal and ventral hernia defects, hiatal defects are more dynamic due to continuous diaphragmatic motion that creates friction at the esophageal and stomach interface. This friction when combined with synthetic mesh resulted in mesh erosion into the esophagus and migration into the stomach (22). In 2009, a study of 28 patients brought light to such complications, with intraluminal mesh erosion in 17 patients, esophageal stenosis in 6 patients, and resultant esophagectomy in 6 patients (23). Due to these safety concerns with synthetic mesh, attention was turned to absorbable mesh to use as reinforcement of the hiatal closure.
The ideal material for mesh cruroplasty should provide enough strength to reinforce the hiatus and reduce the risk of recurrent herniation. At this same time, however, it must also avoid visceral erosions and postoperative dysphagia (24). The goal of absorbable mesh is to provide scaffolding for ingrowth of tissue for persistent reinforcement (22). Many types of absorbable mesh have been reported and are available for use. Biologic mesh options include porcine submucosa (Surgisis, Cook Medical, Bloomington, Indiana), bovine pericardium (Varitas, Baxter International, Deerfield, Illinois), human acellular dermis (AlloDerm, LifeCell Corporation, Branchburg, New Jersey), and porcine dermal collagen (Permacol, Medtronic, Dublin, Ireland). More recently, synthetic bio-absorbable meshes have gained acceptance including Bio-A (Gore Medical, Flagstaff, Arizona) and Phasix ST (Bard Medical, Warwick, Rhode Island). Due to their risk of mesh-related complications, the use of nonabsorbable mesh is largely discouraged.
Mesh configuration
Multiple configurations of various mesh types and sizes have been described and are too numerous to list individually. In general, the mesh is placed either as a bridge to cover a gap in the crural defect or as an overlay to reinforce the primary closure. Some surgeons utilize relaxing incisions in the diaphragm to allow for primary crus closure and then use the mesh to bridge the created defect (25). Circumferential mesh placement was related with several complications including erosion and stricture and is generally avoided. Common mesh shapes include keyhole, U-shaped, and butterfly-shaped (Figure 2). Securing the mesh to the hiatus can be done in several ways including suture, absorbable tacks, and bio-glues. A recent metanalysis of nine studies showed no differences in outcomes between configuration and types of mesh (24). A completed mesh cruroplasty is shown in Figure 3.

Recurrence rates
Several early studies reported reduced recurrence rates with mesh cruroplasty (3,20,26). A systemic review by Tam et al. sought to summarize the literature regarding the use of mesh for crural reinforcement. This review included 13 studies, consisting of 3 randomized controlled trials and 10 observational studies (4 prospective, 4 retrospective and 2 with design not specific). Unfortunately, the review was limited due to differences in surgical technique, mesh type, duration of follow up, and definition of recurrence. All but one study used contrast esophagram to assess the presence of a hiatal hernia recurrence following laparoscopic repair. The overall recurrence rate after mesh cruroplasty was 13% (46/354) and the rate of reoperation was 3.7%. In comparison, the recurrence rate after suture cruroplasty was 24% (91/382) and the reoperation rate was 6%. An analysis was performed to determine the odds for both recurrence and reoperation using both techniques. The odds of hernia recurrence was 49% less and the odds of reoperation was 58% less with mesh cruroplasty compared with suture cruroplasty. Despite evidence of radiographic recurrence, the majority of these studies reported excellent symptomatic results after both mesh and suture repairs (27).
Four randomized control trials have been conducted comparing the use of mesh cruroplasty (both absorbable and nonabsorbable) to suture cruroplasty. The results of these studies are shown in Table 1. To date only one of these studies provided long term follow-up at 5 years (28). In this study, recurrence was defined as a herniation of the stomach into the mediastinum of 2 cm of more on contrast study. At 5 years, the rate of recurrence was found to be alarmingly high, with 59% recurrence in the suture repair group and 54% in the mesh group. These findings suggest that the durability of repair with bioprosthetic mesh and suture cruroplasty decays over time and that objective recurrence may not be as important as a quality metric as has previously been assumed.
Table 1
| Study | Patients (n) | Randomization/mesh | Findings |
|---|---|---|---|
| Fantzides et al. [2002] | 72 | 36 PTFE | No recurrence in mesh |
| 36 suture | 22% recurrence in no-mesh | ||
| Granderath et al. [2005] | 100 | 50 prolene | 8% recurrence in mesh |
| 50 suture | 26% recurrence in no-mesh | ||
| Oelschlager et al. [2011] | 108 | 51 bio mesh (SIS) | 54% recurrence in mesh (5 yr) |
| 57 suture | 59% recurrence in no-mesh (5 yr) | ||
| Watson et al. [2015] | 126 | 41 absorbable | 30.8% recurrence in absorbable mesh |
| 42 Non-absorbable | 12.8% in non-absorbable mesh | ||
| 43 suture | 23.1% no mesh |
The most recent meta-analysis looking into mesh versus no mesh for cruroplasty was performed in 2019 (24). This meta-analysis included nine studies and showed a significant reduction in recurrence with mesh cruroplasty compared to suture cruroplasty. In this meta-analysis, the overall recurrence rate of primary suture closure was 19 of 327 (5.8%); with mesh reinforcement, the rate was 6 of 338 (1.8%). The rate of reoperation in the same cohort did not differ between suture cruroplasty and mesh closure, although most of the studies lacked long-term follow up.
Conclusions
Hiatal hernia recurrence rates appear to be lower in patients repaired with mesh at least in the short term. Only one study has produced long-term data (5 years) on the use of absorbable mesh with high and equal recurrence rates in both the mesh and non-mesh groups. There appears to be significant improvement in quality-of-life following hiatal hernia repair regardless of whether mesh was used or not. The paucity of long-term outcomes regarding hiatal hernia repair with and without the use of mesh has made it difficult to determine how best to treat these patients. In addition, the constant introduction of new technology, new mesh products, and new operative strategies has made developing a standard of care in this field a moving target that has been difficult to establish.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Steven G. Leeds and Lee L. Swanström) for the series “Hiatal Hernia” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2020.03.04). The series “Hiatal Hernia” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Stefanidis D, Hope WW, Kohn GP, et al. Guidelines for surgical treatment of gastroesophageal reflux disease. Surg Endosc 2010;24:2647-69. [Crossref] [PubMed]
- Hashemi M, Peters JH, DeMeester TR, et al. Laparoscopic repair of large type III hiatal hernia: objective followup reveals high recurrence rate. J Am Coll Surg 2000;190:553-60; discussion 560-551.
- Carlson MA, Condon RE, Ludwig KA, et al. Management of intrathoracic stomach with polypropylene mesh prosthesis reinforced transabdominal hiatus hernia repair. J Am Coll Surg 1998;187:227-30. [Crossref] [PubMed]
- Nandipati K, Bye M, Yamamoto SR, et al. Reoperative intervention in patients with mesh at the hiatus is associated with high incidence of esophageal resection--a single-center experience. J Gastrointest Surg 2013;17:2039-44. [Crossref] [PubMed]
- Parker M, Bowers SP, Bray JM, et al. Hiatal mesh is associated with major resection at revisional operation. Surg Endosc 2010;24:3095-101. [Crossref] [PubMed]
- Athanasakis H, Tzortzinis A, Tsiaoussis J, et al. Laparoscopic repair of paraesophageal hernia. Endoscopy 2001;33:590-4. [Crossref] [PubMed]
- Davis SS Jr. Current controversies in paraesophageal hernia repair. Surg Clin North Am 2008;88:959-78. vi. [Crossref] [PubMed]
- Draaisma WA, Gooszen HG, Tournoij E, et al. Controversies in paraesophageal hernia repair: a review of literature. Surg Endosc 2005;19:1300-8. [Crossref] [PubMed]
- Nason KS, Luketich JD, Witteman BP, et al. The laparoscopic approach to paraesophageal hernia repair. J Gastrointest Surg 2012;16:417-26. [Crossref] [PubMed]
- Gouvas N, Tsiaoussis J, Athanasakis E, et al. Simple suture or prosthesis hiatal closure in laparoscopic repair of paraesophageal hernia: a retrospective cohort study. Dis Esophagus 2011;24:69-78. [Crossref] [PubMed]
- Wade A, Dugan A, Plymale MA, et al. Hiatal Hernia Cruroplasty with a Running Barbed Suture Compared to Interrupted Suture Repair. Am Surg 2016;82:e271-4. [PubMed]
- Watson DI, Davies N, Devitt PG, et al. Importance of dissection of the hernial sac in laparoscopic surgery for large hiatal hernias. Arch Surg 1999;134:1069-73. [Crossref] [PubMed]
- Edye M, Salky B, Posner A, et al. Sac excision is essential to adequate laparoscopic repair of paraesophageal hernia. Surg Endosc 1998;12:1259-63. [Crossref] [PubMed]
- Andujar JJ, Papasavas PK, Birdas T, et al. Laparoscopic repair of large paraesophageal hernia is associated with a low incidence of recurrence and reoperation. Surg Endosc 2004;18:444-7. [Crossref] [PubMed]
- Gibson SC, Wong SC, Dixon AC, et al. Laparoscopic repair of giant hiatus hernia: prosthesis is not required for successful outcome. Surg Endosc 2013;27:618-23. [Crossref] [PubMed]
- Furtado RV, Vivian SJ, van der Wall H, et al. Medium-term durability of giant hiatus hernia repair without mesh. Ann R Coll Surg Engl 2016;98:450-5. [Crossref] [PubMed]
- Nason KS, Luketich JD, Qureshi I, et al. Laparoscopic repair of giant paraesophageal hernia results in long-term patient satisfaction and a durable repair. J Gastrointest Surg 2008;12:2066-75; discussion 2075-67.
- Kang T, Urrego H, Gridley A, et al. Pledgeted repair of giant hiatal hernia provides excellent long-term results. J Laparoendosc Adv Surg Tech A 2014;24:684-7. [Crossref] [PubMed]
- Weitzendorfer M, Pfandner R, Antoniou SA, et al. Short-term results after laparoscopic repair of giant hiatal hernias with pledgeted sutures: a retrospective analysis. Hernia 2019;23:397-401. [Crossref] [PubMed]
- Frantzides CT, Madan AK, Carlson MA, et al. A prospective, randomized trial of laparoscopic polytetrafluoroethylene (PTFE) patch repair vs simple cruroplasty for large hiatal hernia. Arch Surg 2002;137:649-52. [Crossref] [PubMed]
- Müller-Stich BP, Holzinger F, Kapp T, et al. Laparoscopic hiatal hernia repair: long-term outcome with the focus on the influence of mesh reinforcement. Surg Endosc 2006;20:380-4. [Crossref] [PubMed]
- Ringley CD, Bochkarev V, Ahmed SI, et al. Laparoscopic hiatal hernia repair with human acellular dermal matrix patch: our initial experience. Am J Surg 2006;192:767-72. [Crossref] [PubMed]
- Stadlhuber RJ, Sherif AE, Mittal SK, et al. Mesh complications after prosthetic reinforcement of hiatal closure: a 28-case series. Surg Endosc 2009;23:1219-26. [Crossref] [PubMed]
- Sathasivam R, Bussa G, Viswanath Y, et al. 'Mesh hiatal hernioplasty' versus 'suture cruroplasty' in laparoscopic para-oesophageal hernia surgery; a systematic review and meta-analysis. Asian J Surg 2019;42:53-60. [Crossref] [PubMed]
- DeMeester SR. Laparoscopic paraesophageal hernia repair: critical steps and adjunct techniques to minimize recurrence. Surg Laparosc Endosc Percutan Tech 2013;23:429-35. [Crossref] [PubMed]
- Oelschlager BK, Pellegrini CA, Hunter J, et al. Biologic prosthesis reduces recurrence after laparoscopic paraesophageal hernia repair: a multicenter, prospective, randomized trial. Ann Surg 2006;244:481-90. [PubMed]
- Tam V, Winger DG, Nason KS. A systematic review and meta-analysis of mesh vs suture cruroplasty in laparoscopic large hiatal hernia repair. Am J Surg 2016;211:226-38. [Crossref] [PubMed]
- Oelschlager BK, Pellegrini CA, Hunter JG, et al. Biologic prosthesis to prevent recurrence after laparoscopic paraesophageal hernia repair: long-term follow-up from a multicenter, prospective, randomized trial. J Am Coll Surg 2011;213:461-8. [Crossref] [PubMed]
Cite this article as: Westcott LZ, Ward MA. Techniques for closing the hiatus: mesh, pledgets and suture techniques. Ann Laparosc Endosc Surg 2020;5:16.