
D3 lymphadenectomy for right colon cancer
Introduction
Recently several important clinical studies have reported high-quality evidence of the relationship between the overall survival and the number of harvested lymph nodes in colon cancers. It is reported that about 4% of right colon cancers metastasize to central mesocolic lymph nodes (1). Therefore, in efforts to improve the number of resected lymph nodes and the completeness of right colon cancer resection, numerous of surgical units implemented the complete mesocolic dissection with central vessel ligation (CME-CVL) or D3 lymphadenectomy in right hemicolectomy for right-sided colon cancer. In this review, we focus on the detailed description of the surgical technique for CME-CVL or D3 lymphadenectomy in the right-sided colon cancer patient as well as provide a critical appraisal of the quality of the available data in the current literature.
Biology of lymphatic spread in colon cancer
The classification by the Japanese Society for Cancer of the Colon and Rectum (JSCCR) categorizes in colon cancer the mesenteric lymph nodes into three groups: main lymph nodes, intermediate lymph nodes, and pericolic lymph nodes (2). The main lymph nodes are located at the origin of the main feeding artery. Intermediate lymph nodes are located between the first and the terminal branch of the main feeding artery, while the pericolic lymph nodes are located between the terminal branch of the main feeding artery and the colon. Grouping of involved lymph nodes according to their relationship with local vessel in right-sided colon cancer proposed by JSCCR is presented in Figure 1 (3).
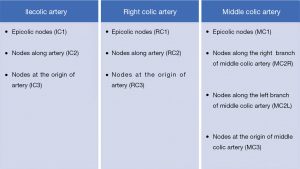
Recently the metastatic pattern of lymphatic node spread in right-sided colon cancer has been analyzed in several studies. Cecum location of tumor was mostly linked with ileocolic nodes metastases. However metastases were also observed along right colic artery and right branch of the middle colic artery (4).
In patients with ascending colon cancer and in similar pattern in patients with hepatic flexure tumors, lymph node metastases beside the artery, which supplies the right side of the colon were most common, nonetheless metastases at the origin of the middle colic artery were also present. In case of ascending colon tumors, similar number of metastases in the peripheral part of the right colic artery and in the peripheral part of the ileocolic artery was observed, whereas in patients with hepatic flexure tumors, there are observed a similar number of metastasis along the ascending and middle colic arteries. These metastatic patterns suggest that metastases may occur along lymph nodes having a marginal artery, therefore, progressing to the origin of the main supplying artery, with the relative extent of spreading to central and peripheral parts dependent on the colon localization of the cancer (5).
The mechanisms that drive lymphatic metastasis in colon cancer still remain not fully understood. Currently, the two biological models of the metastatic pathway are proposed. The first model involves lymphatic spread by the progressive and anatomical pathway, from the colon tumor to adjacent lymph nodes, henceforth to intermediate nodes, afterwards to central nodes, and finally to distant organs. This model suggests that progression of cancer cells along the metastatic pathway may only appear when the former lymphatic nodal barrier is breached and efforts to resect all lymph nodes involved by cancer cells. It may improve surgical outcomes related to improvement of overall survival (6).
The second one, supported by numerous preclinical and clinical studies, assumes that lymphatic spread in colon cancer may occur immediate and at random way (7). In this model, metastatic lymph nodes are seen as a marker of the biological behavior of cancer and malignant capability of the disease, therefore it is suggested that efforts to remove affected nodes will not affect overall survival. Firstly, circulating tumor cells (CTC) may have been found in the patients’ peripheral blood at every stage of the colorectal cancer (CRC), independently of the laboratory techniques and marker used (8). In study be Bork et al., the detection rate of CTC of 4.9%, 10.5%, and 8.3% in CRC patients with I, II, and III disease stages have been reported respectively (9). In the recent meta-analysis, it was found that the molecular detection of cancer cells in the regional lymph nodes allows prediction of disease recurrence rate and impaired survival rate in node-negative and in stage III CRC (10).
The findings from postoperative pathology suggest that systemic spread of cancer cells may occur independently from involvement of locoregional lymph node. In the study by Knijn et al. comparable incidence rate of liver and lung metastases between node-negative and node-positive patients were found, what suggested that dissemination to distant organs may occur independently of lymphatic spread (11).
When cancer progression through lymphatic system was stepwise, the patients with involvement of intermediate nodes would have more advanced disease stage related to worse prognosis in comparison to those with only pericolic nodes involvement. In fact, multiple studies from the Far East revealed any difference in term of overall survival between D1 and D2 nodes invaded in stage III colon cancer (12,13). Mentioned data implies that lymphatic spread of cancer cells in a stochastic manner is more plausible than in stepwise process and may proceed from the beginnings of cancerogenesis.
Definition of D3 lymphadenectomy for right colon cancer
During the latest years, the western concept of complete mesocolic excision with central vascular ligation (CME-CVL) and the eastern D3 lymphadenectomy for right hemicolectomy for right-sided colon cancer are gaining popularity among surgeons. Proper understanding and adequate knowledge about the complex anatomy of main vessels (SMV and SMA) is necessary in terms of iatrogenic complications avoidance during mentioned right-sided colon cancer resections (14,15).
The CME-CVL or D3 lymphadenectomy for right-sided colon cancer require ligation of the ileocolic vein, right colic vein, Henle trunk, and middle colic vein on their appearance from the SMV, and of the ileocolic artery, right colic artery, and middle colic artery on their emergence from the SMA. In recent meta-analysis, a wide variation of anatomical structures of the major vessels was presented and the right hemicolectomy with CME-CVL is quite complicated and demanding procedure. It was proposed that the surgery should be done carefully, following the embryological planes. The area of D3 lymphadenectomy has the following anatomical borders: (I) cranially—5 mm proximal to the horizontal line through the Henle trunk and middle colic artery origins; (II) caudally—5 mm distal to the horizontal line through the origin of the ileocolic artery; (III) medially—the left edge of the SMA; (IV) laterally—10 mm from the right edge of the SMV (16).
The JSCCR defines D3 lymphadenectomy as dissection of the main lymph nodes, intermediate lymph nodes, and pericolic lymph nodes and D2 lymphadenectomy as the removal of the pericolic lymph nodes and intermediate lymph nodes. The D3 lymphadenectomy according to the JSCCR is comparable in conception to the Western CME-CVL in which all lymphatic, vascular, and neural tissue in the drainage area of the colon tumor is excised as a complete mesocolic tissue specimen. Right hemicolectomy with CME and D3 lymphadenectomy involves complete resection of regional lymph nodes, including pericolic nodes (N1 region), intermediate nodes (N2 region), and main nodes along superior mesenteric vessels (N3 region) as defined by the JSCCR (2).
Theory of central vessel ligation or D3 lymphadenectomy is based on a resection along the anterior aspect of superior mesenteric artery (SMA), superior mesenteric vein (SMV) and ligation of colonic vessel high. This may enhance the rate of complete removal of lymph nodes that may harbor the cancer cells. In general, Japanese D3 dissection is considered to be similar to CME-CVL. However, there are some discrepancies which involve variation in definitions D3 vascular ligation and length of bowel that are resected, which are lower in the D3 lymphadenectomy technique (2,17).
The initial CME-CVL proposed by Hohenberger et al. involve kocherization the duodenum, and takedown of the mesenteric attachments to the duodenum, and en bloc resection of the affected colonic segment, excision of the corresponding mesocolon in a compact fascial layer and a high ligation of the tributary vessels along with all the perivascular lymph nodes developed on vertical direction. In procedure suggested by Hohenberger et al. the resection of lymph nodes along the gastroepiploic artery including the infrapyloric area is only done when cancer is located in the hepatic flexure or transverse colon, but not in coecal or ascending colon cancer (18). The JSCCR D3 dissection in definition omits duodenal kocherization, and removal of the gastroepiploic and infrapyloric lymph nodes (17).
The Guidelines by the JSCCR for the Treatment of CRC recommend D3 lymph node dissection for T3/4 or N+ disease and D2 lymph node dissection for T1N0 disease (17). Meanwhile, either D3 or D2 lymph node dissection can be performed for T2N0 disease. Thus, D3 lymph node dissection is basically recommended for stage II or III colon cancer in tertiary surgical centers.
Right hemicolectomy with D3 extended mesenterectomy for right-sided colon cancer is probably the most radical lymphadenectomy removing all nodes that lie both anterior and posterior to the superior mesenteric vessels as well as dividing the colonic arteries and veins at their origin with complete resection of these lymph nodes from the level of the middle colic artery to that of the ileocolic artery origin. All soft tissue in the area of the superior mesenteric vessels at this level should be resected (19,20).
Toyota et al. suggested the D3 resection technique for right-sided colon cancer should consist of harvesting the lymph node-bearing tissue along the main trunk feeding artery to the main nodes situated anterior to the surgical trunk with resection of the intestine 10 cm proximal and distal to the tumor. Authors also underline that the additional dissection of the suspected infrapyloric lymph node metastasis must be always performed (21).
Outcomes after D3 lymphadenectomy in right-sided CRC
Nowadays, one of the most important predictive factors in CRC is involvement of lymph nodes. The presence of lymph node metastasis determines patients to receive adjuvant chemotherapy and predict outcomes related to disease-free and overall survival (22). The small amount of harvested lymph nodes may influence on the underestimation of cancer cells involvement. Findings from recent studies suggest that survival rate improves when more lymph nodes are gathered, regardless of current node status (23,24). Adequate lymphadenectomy, including pericolic, intermediate, and main node dissection (D3 lymphadenectomy), is underline by surgeons as an important issue for accurate staging and therapy and implementation of accurate therapy with the best oncological outcomes. This approach is a “standard” surgical protocol for Japanese surgeons in right-sided colon cancer treatment (17).
Proponents of CME with D3 lymphadenectomy underline the currently solid clinical findings in oncological principles for the approach, which include positive correlation of greater amount of harvested lymph nodes, even cancer negative nodes, with better overall survival (25,26). On the other hand, another study stated that relationship between lymph nodes and survival is difficult to define due to several confounding variables and suggested that the beneficial role of extended dissection of mesenteric nodes may be not relevant (27). It is explained by the stochastic phenomenon of cancer lymph node spread, which is more likely than stepwise process as it was believed earlier. Therefore, lymph node metastases, according to their biological behavior, may occur early and simultaneously with distant metastases, thus resecting them seems to have minimal impact on overall survival (28).
In the large retrospective study by Hashiguchi et al. T2-T4 colon cancer patients’ lymph nodes were anatomically mapped and classified into three groups (paracolic, mesocolic and main trunk nodes). Results revealed that extended dissection of the main arterial trunk nodes had no impact on overall survival or staging accuracy improvement compared to resection of mesocolic or pericolic nodes (29). In another study, where Ikeda et al. showed similar survival rate at stage II and III CRC regardless of whether the main arterial trunk (“apical”) nodes were dissected (30).
In a study by Kanemitsu et al., concerning D3 lymphadenectomy in right hemicolectomy, authors revealed that the mean number of harvested lymph nodes per patient was 31.0 (31). A mean number of lymph nodes obtained per patient yields of 13.6 from the pericolic node level, 8.4 from the intermediate node level, and 6.1 from the main node level. They observed that metastases to main nodes occurred in 3% of patients, which, represents the frequency of residual nodes typically left behind in D2 dissection. Of these 3% of patients, 54.5% had no cancer deposits in the intermediate nodes (skipped metastases). In this study a steady increase in the proportion of positivity in the main nodes with increasing depth of invasion was observed, regardless of the tumor location. However, no metastases have been observed in patients with pT1 or pT2 cancer. Of all 370 patients enrolled in this study who underwent D3 lymphadenectomy 56 patients had a recurrence. 81.8% (9/11) of patients who had positive main nodal metastases developed a recurrence of cancer (31).
In multivariate survival analysis, by Kotake et al., of the 10,098 patients with T3 and T4 colon cancer the estimated hazard ratio for overall survival of patient with D3 lymphadenectomy versus D2 was 0.827 (95% confidence interval, 0.757 to 0.904) (25). This large study reported that D3 lymph node dissection for pT3 and pT4 colon cancer have been associated with a significant improvement of survival rate, even after adjusting potential confounders of lymphadenectomy, with a relative reduction in the risk of death by up to 18%.
In recent systematic review it has been reported that the frequency of detectable primary lymph node metastases for right-sided colon cancers varies between 1% and 22%, while it is below the 12% for left-sided colon cancers (32). These results indicate that lymphadenectomy in right-sided colon cancers need specific attention.
The extended lymphadenectomy compared to traditional surgery is reasoned by several arguments. Firstly, more radical lymphadenectomy offers better chance to acquire complete resection and control of the lymphatic metastases (33,34). Secondly, several studies have revealed that greater number of lymph nodes collected after colonic resection correlate with increased survival, while higher negative lymph node yields is beneficial to survival in more advanced colon cancer (35-38). The ratio of metastatic lymph nodes to the total number of resected lymph nodes could be used as prognostic factor, which several studies reported to be better than number of positive lymph nodes or pN status (39-41). The greater the ratio of negative to metastatic lymph node is, the better prognosis is expected. Moreover, metastases may occur as “skip lesions”, known as metastatic lymph nodes from central or apical nodes (D3), whereas those harvested from pericolic or intermediate (D1 and D2) nodes were free from metastases (1,42). Couple possible explanations have been suggested for the beneficial role of higher number of resected negative lymph nodes during extensive lymphadenectomy, such as increased likelihood of discovering occult metastatic lymph node and therefore acquiring proper staging. Another proposed argument is that the specimens from extended resection may involve undetected micrometastases or isolated tumor cells which would, if left in situ, significantly alter patients’ survival (33,34,43).
However, for many authors the question of a survival benefit from a greater number of harvested lymph nodes is still a matter of debate. The association of the higher number of harvested lymph nodes with overall survival is not fully understood as there are a multitude of possible influential factors (27,44). Based on previous studies it is known that many other factors may significantly influence the number of harvested lymph node including patient age, immune status, tumor location, tumor characteristics and institutional factors (28,34,45,46). Several recent studies suggested that there is no or minimal survival benefit in a higher number of harvested lymph nodes greater than 12, especially if good quality surgery and lymph node examination is achieved. Moreover there are difficulties in defining a threshold number for lymph node retrieval (47-50). Also, couple of non-CME studies have failed to show a relationship between greater lymph node yield, harvested during high vascular tie surgeries, and higher number of metastatic lymph nodes or improved survival (29,51,52). Some of the authors propose the CME-CVL approach for all stages of colon cancer, whereas there is evidence that in early cancer (T1–T2) and more advanced (T3 and T4) cancers located at specified distances from the feeding artery, the incidence of D3 metastatic nodes is insignificant and therefore CME-CVL or D3 lymphadenectomy may constitutes over-treatment (29,53).
Extended and more radical dissection may result in an increased number of surgical complications. In the study by Prevost et al. increased rate of vascular injuries was noted, compared to conventional right hemicolectomy (54). The urinary retention was observed in 1.8% of patients, which was self-limiting and may be related to the temporary disturbance of the autonomic nerve around the mesenteric root during the surgical procedure. Bertelsen et al. revealed an increased risk of postoperative morbidity in patients undergoing CME-CVL or lymphadenectomy D3, related to a higher rate of postoperative sepsis and a higher rate of intraoperative injury including splenic and superior mesenteric vein injuries (55). In the other studies regarding more radical dissection of lymph nodes the morbidity rates were acceptable and similar to the conventional approach (18,56,57).
Apart from surgical complications, extended D3 lymphadenectomy may be related to non-surgical complications, like pneumonia with respiratory failure in connection with D2 resection for right-sided colonic cancer (58).
In case of proper approach to lymphadenectomy D3 in right-sided colon cancer, there is a trial which suggests that open surgery should be the primary selection, but also the laparoscopic one may be also acceptable (59). Another study underlines the beneficial role of laparoscopic approach on short-term wound complications, including incisional hernia and wound dehiscence (60). However, no differences were found in the latest complications. In terms of overall survival at stage II or III right-sided colon cancer the laparoscopy with D3 lymphadenectomy was on the same level as open surgery. Based on results of this study, overall survival in both groups was similar and better than expected, laparoscopic surgery with D3 lymphadenectomy according to Japanese definition seems to be an appropriate option in the treatment for patients with stage II or III right-sided colon cancer (60).
Currently, there are a couple of ongoing trials regarding proper surgical treatment of right-sided colon cancer. The Chinese Laparoscopic Right Colectomy for right-sided Colon Cancer trial (RELARC, NCT02619942) is set to compare 3 years disease-free survival between laparoscopic CME and standard (D2) resection. Similar randomized trials are being conducted in Egypt (NCT02526836) and Ukraine (RICON trial, NCT03200834).
Conclusions
In conclusion, the conception of D3 lymphadenectomy for right-sided colon cancer was proposed to improve surgical and oncological outcomes. Recently, many surgeons have used the principles and conducted clinical studies to verify the effect of CME-CVL in right hemicolectomy. However, it still remains very controversial whether D3 lymphadenectomy should be implemented as a standard surgical technique. Some of the recent studies indicated that the D3 lymphadenectomy for right colon cancer was associated with the higher risk of morbidity. The CME-CVL in right hemicolectomy has shown the improvement in the survival rate, however, there are numerous limitations in regard to adjustment of the technique, histopathological reporting and complication rates. Although, there are reasonable anatomical and oncological basis for right hemicolectomy with CME-CVL and D3 lymphadenectomy, there is still lack of randomized controlled trials and there is no consistent high-quality evidence. Recently presented evidence was taken from studies with large amount of heterogeneity in research methodology, populations and outcomes. The latest systematic reviews on right hemicolectomy with CME-CVL with D3 lymphadenectomy summarized that the currently presented evidence has many significant limitations that enable the implementation. Moreover, the latest outcomes in improvement in long-term survival have not been clearly proved. The current evidence of the advantages and associated morbidity of the right hemicolectomy with CME-CVL and D3 dissection have been insufficient and further studies are warranted to confirm or reject this approach as standard of care in the future.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Roberto Bergamaschi and Mahir Gachabayov) for the series “Right Colon Cancer Surgery: Current State” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2019.09.01). The series “Right Colon Cancer Surgery: Current State” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Liang JT, Lai HS, Huang J, et al. Long-term oncologic results of laparoscopic D3 lymphadenectomy with complete mesocolic excision for right-sided colon cancer with clinically positive lymph nodes. Surg Endosc 2015;29:2394-401. [Crossref] [PubMed]
- Watanabe T, Muro K, Ajioka Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. Int J Clin Oncol 2018;23:1-34. [Crossref] [PubMed]
- Japanese Society for Cancer of the Colon and Rectum. Japanese Classification of Colorectal Carcinoma. Tokyo: Kanehara, 1999.
- Yada H, Sawai K, Taniguchi H, et al. Analysis of vascular anatomy and lymph node metastases warrants radical segmental bowel resection for colon cancer. World J Surg 1997;21:109-15. [Crossref] [PubMed]
- Park IJ, Choi GS, Kang BM, et al. Lymph node metastasis patterns in right-sided colon cancers: is segmental resection of these tumors oncologically safe? Ann Surg Oncol 2009;16:1501-6. [Crossref] [PubMed]
- Klein CA. Parallel progression of primary tumours and metastases. Nat Rev Cancer 2009;9:302-12. [Crossref] [PubMed]
- Fisher B. Biological Research in the Evolution of Cancer Surgery: A Personal Perspective. Cancer Res 2008;68:10007-20. [Crossref] [PubMed]
- Torino F, Bonmassar E, Bonmassar L, et al. Circulating tumor cells in colorectal cancer patients. Cancer Treat Rev 2013;39:759-72. [Crossref] [PubMed]
- Bork U, Rahbari NN, Schölch S, et al. Circulating tumour cells and outcome in non-metastatic colorectal cancer: a prospective study. Br J Cancer 2015;112:1306-13. [Crossref] [PubMed]
- Rahbari NN, Bork U, Motschall E, et al. Molecular Detection of Tumor Cells in Regional Lymph Nodes Is Associated With Disease Recurrence and Poor Survival in Node-Negative colorectal cancer: A Systematic Review and Meta-Analysis. J Clin Oncol 2012;30:60-70. [Crossref] [PubMed]
- Knijn N, van Erning FN, Overbeek LIH, et al. Limited effect of lymph node status on the metastatic pattern in colorectal cancer. Oncotarget 2016;7:31699-707. [Crossref] [PubMed]
- Kim CH, Huh JW, Kim HR, et al. Prognostic Comparison Between Number and Distribution of Lymph Node Metastases in Patients with Right-Sided Colon Cancer. Ann Surg Oncol 2014;21:1361-8. [Crossref] [PubMed]
- Kataoka K, Debrie E, Shiozawa M, et al. Prognostic significance of number versus location of positive mesenteric nodes in node positive colon cancer. J Clin Oncol 2018;36:abstr 3587.
- O'Sullivan AW, Heaton N, Rela M. Cancer of the uncinate process of the pancreas: surgical anatomy and clinicopathological features. Hepatobiliary Pancreat Dis Int 2009;8:569-74. [PubMed]
- Negoi I, Beuran M, Hostiuc S, et al. Surgical Anatomy of the Superior Mesenteric Vessels Related to Colon and Pancreatic Surgery: A Systematic Review and Meta-Analysis. Sci Rep 2018;8:4184. [Crossref] [PubMed]
- Spasojevic M, Stimec BV, Dyrbekk AP, et al. Lymph node distribution in the d3 area of the right mesocolon: implications for an anatomically correct cancer resection. A postmortem study. Dis Colon Rectum 2013;56:1381-7. [Crossref] [PubMed]
- Watanabe T, Itabashi M, Shimada Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2010 for the treatment of colorectal cancer. Int J Clin Oncol 2012;17:1-29. [Crossref] [PubMed]
- Hohenberger W, Weber K, Matzel K, et al. Standardized surgery for colonic cancer: complete mesocolic excision and central ligation - technical notes and outcome. Colorectal Dis 2009;11:354-64. [Crossref] [PubMed]
- Nesgaard JM, Stimec BV, Bakka AO, et al. Right Colectomy with Extended D3 Mesenterectomy: Anterior and Posterior to the Mesenteric Vessels. Surg Technol Int 2019;35. [Epub ahead of print]. [PubMed]
- Gaupset R, Nesgaard JM, Kazaryan AM, et al. Introducing Anatomically Correct CT-Guided Laparoscopic Right Colectomy with D3 Anterior Posterior Extended Mesenterectomy: Initial Experience and Technical Pitfalls. J Laparoendosc Adv Surg Tech A 2018;28:1174-82. [Crossref] [PubMed]
- Toyota S, Ohta H, Anazawa S. Rationale for extent of lymph node dissection for right colon cancer. Dis Colon Rectum 1995;38:705-11. [Crossref] [PubMed]
- Omenn GS. Prognostic Factors in Cancer, 3rd edition. In: Gospodarowicz BK, O’Sullivan B, Sobin LH. editors. Proteomics 2006;6:6385-6.
- Baxter NN, Virnig DJ, Rothenberger DA, et al. Lymph Node Evaluation in colorectal cancer Patients: A Population-Based Study. JNCI J Natl Cancer Inst 2005;97:219-25. [Crossref] [PubMed]
- Kotake K, Honjo S, Sugihara K, et al. Number of Lymph Nodes Retrieved is an Important Determinant of Survival of Patients with Stage II and Stage III colorectal cancer. Jpn J Clin Oncol 2012;42:29-35. [Crossref] [PubMed]
- Kotake K, Mizuguchi T, Moritani K, et al. Impact of D3 lymph node dissection on survival for patients with T3 and T4 colon cancer. Int J Colorectal Dis 2014;29:847-52. [Crossref] [PubMed]
- Tsai HL, Lu CY, Hsieh JS, et al. The Prognostic Significance of Total Lymph Node Harvest in Patients with T2-4N0M0 colorectal cancer. J Gastrointest Surg 2007;11:660-5. [Crossref] [PubMed]
- Willaert W, Ceelen W. Extent of surgery in cancer of the colon: is more better? World J Gastroenterol 2015;21:132-8. [Crossref] [PubMed]
- Willaert W, Mareel M, Van De Putte D, et al. Lymphatic spread, nodal count and the extent of lymphadenectomy in cancer of the colon. Cancer Treat Rev 2014;40:405-13. [Crossref] [PubMed]
- Hashiguchi Y, Hase K, Ueno H, et al. Optimal margins and lymphadenectomy in colonic cancer surgery. Br J Surg 2011;98:1171-8. [Crossref] [PubMed]
- Ikeda Y, Shimabukuro R, Saitsu H, et al. Influence of prophylactic apical node dissection of the inferior mesenteric artery on prognosis of colorectal cancer. Hepatogastroenterology 2007;54:1985-7. [PubMed]
- Kanemitsu Y, Komori K, Kimura K, et al. D3 lymph node dissection in right hemicolectomy with a no-touch isolation technique in patients with colon cancer. Dis Colon Rectum 2013;56:815-24. [Crossref] [PubMed]
- Bertelsen CA, Neuenschwander AU, Jansen JE, et al. Short-term outcomes after complete mesocolic excision compared with “conventional” colonic cancer surgery. Br J Surg 2016;103:581-9. [Crossref] [PubMed]
- Søndenaa K, Quirke P, Hohenberger W, et al. The rationale behind complete mesocolic excision (CME) and a central vascular ligation for colon cancer in open and laparoscopic surgery. Int J Colorectal Dis 2014;29:419-28. [Crossref] [PubMed]
- Kessler H, Hohenberger W. Extended Lymphadenectomy in Colon Cancer is Crucial. World J Surg 2013;37:1789-98. [Crossref] [PubMed]
- Le Voyer TE, Sigurdson ER, Hanlon AL, et al. Colon Cancer Survival Is Associated With Increasing Number of Lymph Nodes Analyzed: A Secondary Survey of Intergroup Trial INT-0089. J Clin Oncol 2003;21:2912-9. [Crossref] [PubMed]
- Chang GJ, Rodriguez-Bigas MA, Skibber JM, et al. Lymph Node Evaluation and Survival After Curative Resection of Colon Cancer: Systematic Review. J Natl Cancer Inst 2007;99:433-41. [Crossref] [PubMed]
- Chen SL, Bilchik AJ. More Extensive Nodal Dissection Improves Survival for Stages I to III of Colon Cancer. Trans. Meet Am Surg Assoc 2006;124:267-75.
- Johnson PM, Porter GA, Ricciardi R, et al. Increasing Negative Lymph Node Count Is Independently Associated With Improved Long-Term Survival in Stage IIIB and IIIC Colon Cancer. J Clin Oncol 2006;24:3570-5. [Crossref] [PubMed]
- Parnaby CN, Scott NW, Ramsay G, et al. Prognostic value of lymph node ratio and extramural vascular invasion on survival for patients undergoing curative colon cancer resection. Br J Cancer 2015;113:212-9. [Crossref] [PubMed]
- Lykke J, Roikjaer O, Jess P. Danish colorectal cancer Group. The relation between lymph node status and survival in Stage I-III colon cancer: results from a prospective nationwide cohort study. Colorectal Dis 2013;15:559-65. [Crossref] [PubMed]
- Rosenberg R, Friederichs J, Schuster T, et al. Prognosis of Patients With colorectal cancer Is Associated With Lymph Node Ratio. Ann Surg 2008;248:968-78. [Crossref] [PubMed]
- Liang JT, Huang KC, Lai HS, et al. Oncologic Results of Laparoscopic D3 Lymphadenectomy for Male Sigmoid and Upper Rectal Cancer with Clinically Positive Lymph Nodes. Ann Surg Oncol 2007;14:1980-90. [Crossref] [PubMed]
- Faerden AE, Sjo OH, Bukholm IRK, et al. Lymph Node Micrometastases and Isolated Tumor Cells Influence Survival in Stage I and II Colon Cancer. Dis Colon Rectum 2011;54:200-6. [Crossref] [PubMed]
- Murphy J, Young-Fadok T. Extended Lymphadenectomy in Colon Cancer Is Debatable. World J Surg 2013;37:1799-807. [Crossref] [PubMed]
- Kang J, Kim I, Kang SI, et al. Laparoscopic right hemicolectomy with complete mesocolic excision. Surg Endosc 2014;28:2747-51. [Crossref] [PubMed]
- Nash GM, Row D, Weiss A, et al. A Predictive Model for Lymph Node Yield in Colon Cancer Resection Specimens. Ann Surg 2011;253:318-22. [Crossref] [PubMed]
- Bläker H, Hildebrandt B, Riess H, et al. Lymph node count and prognosis in colorectal cancer: The influence of examination quality. Int J Cancer 2015;136:1957-66. [Crossref] [PubMed]
- Moro-Valdezate D, Pla-Martí V, Martín-Arévalo J, et al. Factors related to lymph node harvest: does a recovery of more than 12 improve the outcome of colorectal cancer? Colorectal Dis 2013;15:1257-66. [Crossref] [PubMed]
- Tsikitis VL, Larson DL, Wolff BG, et al. Survival in Stage III Colon Cancer Is Independent of the Total Number of Lymph Nodes Retrieved. J Am Coll Surg 2009;208:42-7. [Crossref] [PubMed]
- Gleisner AL, Mogal H, Dodson R, et al. Nodal Status, Number of Lymph Nodes Examined, and Lymph Node Ratio: What Defines Prognosis after Resection of Colon Adenocarcinoma? J Am Coll Surg 2013;217:1090-100. [Crossref] [PubMed]
- Cirocchi R, Trastulli S, Farinella E, et al. High tie versus low tie of the inferior mesenteric artery in colorectal cancer: A RCT is needed. Surg Oncol 2012;21:e111-23. [Crossref] [PubMed]
- Hida J, Okuno K. High ligation of the inferior mesenteric artery in rectal cancer surgery. Surg Today 2013;43:8-19. [Crossref] [PubMed]
- Hida J, Okuno K, Yasutomi M, et al. Optimal Ligation Level of the Primary Feeding Artery and Bowel Resection Margin in Colon Cancer Surgery: The Influence of the Site of the Primary Feeding Artery. Dis Colon Rectum 2005;48:2232-7. [Crossref] [PubMed]
- Prevost GA, Odermatt M, Furrer M, et al. Postoperative morbidity of complete mesocolic excision and central vascular ligation in right colectomy: a retrospective comparative cohort study. World J Surg Oncol 2018;16:214. [Crossref] [PubMed]
- Bertelsen CA, Neuenschwander AU, Jansen JE, et al. Short-term outcomes after complete mesocolic excision compared with ‘conventional’ colonic cancer surgery. Br J Surg 2016;103:581-9. [Crossref] [PubMed]
- Pramateftakis MG. Optimizing colonic cancer surgery: high ligation and complete mesocolic excision during right hemicolectomy. Tech Coloproctol 2010;14:S49-51. [Crossref] [PubMed]
- Shin JW, Amar AHY, Kim SH, et al. Complete mesocolic excision with D3 lymph node dissection in laparoscopic colectomy for stages II and III colon cancer: long-term oncologic outcomes in 168 patients. Tech Coloproctol 2014;18:795-803. [Crossref] [PubMed]
- Numata M, Sawazaki S, Aoyama T, et al. D3 lymph node dissection reduces recurrence after primary resection for elderly patients with colon cancer. Int J Colorectal Dis 2019;34:621-8. [Crossref] [PubMed]
- Kitano S, Inomata M, Mizusawa J, et al. Survival outcomes following laparoscopic versus open D3 dissection for stage II or III colon cancer (JCOG0404): a phase 3, randomised controlled trial. Lancet Gastroenterol Hepatol 2017;2:261-8. [Crossref] [PubMed]
- Yamamoto S, Inomata M, Katayama H, et al. Short-Term Surgical Outcomes From a Randomized Controlled Trial to Evaluate Laparoscopic and Open D3 Dissection for Stage II/III Colon Cancer. Ann Surg 2014;260:23-30. [Crossref] [PubMed]
Cite this article as: Włodarczyk M, Włodarczyk J, Trzciński R, Mik M, Dziki Ł, Dziki A. D3 lymphadenectomy for right colon cancer. Ann Laparosc Endosc Surg 2019;4:96.


