Endoscopy in the bariatric patient
Introduction
Obesity is a worldwide problem. The number of overweight and morbidly obese individuals has risen dramatically in last few decades. According to data from the National Health and Nutrition Examination Survey (NHANES) 2013–2014, the number of overweight or obese Americans combined is over 70%.
Of these, more than 37% of individuals are obese and around 7.7% of individuals suffer from extreme obesity (1). Obesity is associated with a higher risk of all-cause mortality and is associated with numerous comorbid conditions including diabetes mellitus, cardiovascular disorders, cancers, musculoskeletal and mental health disorders along with many others comorbid conditions (2). The treatment of obesity is complex. There is no single modality which can cure obesity. A treatment plan usually starts with life style change, diet and exercise plans with the aim of limiting caloric intake and creating a balanced healthy lifestyle. The chances of significant and maintained weight loss with these measures are statistically rather small unfortunately. Pharmacotherapy can be an adjunct and can achieve a modest weight loss which usually wears off once medications are stopped. Surgical weight loss seems to be the most successful way of losing significant weight and can be maintained with higher success rate if proper diet and exercise recommendations are followed.
Surgical procedures are invasive, expensive and not available to majority of morbidly obese individuals for various reasons including availability, insurance coverage, patient preference, and risk of complications (3,4). There is a growing interest in endoscopic and other less invasive weight loss procedures. Various endoscopic interventions like intra gastric balloon insertion, endoscopic sleeve gastroplasty and various devices to limit the food ingestion and or food absorption have been introduced or are being developed (5). In addition, endoscopic techniques offer a diagnostic as well as less invasive therapeutic role in the obesity treatment paradigm. It is an important tool in the management of the complications arising after bariatric surgical interventions. This manuscript highlights the role of endoscopy in the care of the bariatric patient in the pre-, intra- and post-operative periods.
Preoperative endoscopy in bariatric patients
The routine use of endoscopy prior to surgery has been controversial. Many surgeons consider endoscopic evaluation of the upper gastrointestinal tract prior to surgical alteration with a bariatric procedure a requirement. On the other hand other surgeons advocate selective endoscopy as some surgeons feel it can delay the surgical procedure, cause additional expense and rarely alter the medical management or the choice of the surgical procedure (<10%) (6).
There are many papers supporting the routine use of preoperative endoscopy. Saarinen et al., in their review of 1,275 preoperative endoscopy reports, identified significant endoscopic findings in almost half of the patients. Twenty three percent of patients had clinically significant findings like hiatal hernia, esophagitis, Barrett’s esophagus (BE), and/or esophageal dysplasia which could be relevant for procedure choice. Over half of these patients were asymptomatic. These findings are significant if being considered for a laparoscopic sleeve gastrectomy (LSG). Thirteen (3.2%) patients had their planned sleeve gastrectomy switched to a laparoscopic gastric bypass procedure (LGBP) because of findings of gastroesophageal reflux disease (GERD) (7). Parikh et al. reviewed 28 studies including 6616 patients divided the patients into two groups. Group 1 were those with preoperative endoscopic findings which did not significantly change the management plan for their bariatric surgery (e.g., mild to moderate duodenitis, esophagitis or gastritis, HPylori infection or hiatal hernia <2 cm). Group 2 had patients with endoscopic findings which were clinically significant and delayed, altered, or cancelled the planned surgery (e.g., severe duodenitis, esophagitis, gastric varices, hiatal hernia >2 cm, mass or cancer). Overall 92.4% (n=6,112) had a normal EGD or findings that did not change clinical management, however 7.6% patients had findings that delayed or altered surgery. The revised estimate noted that 20.6% of all those with esophagitis (regardless of grade) were re-categorized into Group 2 (8). They support routine preoperative endoscopy. Salama et al. in their retrospective review of 232 patients who underwent preoperative EGD identified significant endoscopic findings in 143 patients (61.6%). Fifteen percent of patients had medical management altered and 1.7% of patients had their surgical management altered based on these findings. Age >55 years and the presence of GERD in the history were associated with an abnormal finding on screening upper endoscopy (9).
The Standards of Practice Committee of the American Society for Gastrointestinal Endoscopy (ASGE) in conjunction with representatives from the Society of Gastrointestinal and Endoscopic Surgeons (SAGES) and the American Society for Metabolic and Bariatric Surgery (ASMBS published guidelines on this topic in 2015. This multi-society recommendation is that the decision for performing preoperative screening endoscopy should be tailored according to the individual patient’s needs and should be based on a detailed discussion between patient and the surgeon; keeping in mind the particular type of bariatric surgical procedure being planned (10).
Sleeve gastrectomy has become the most common bariatric surgical procedure performed across the globe and there are rising concerns about the long-term effects of sleeve gastrectomy. Concerns surround the new onset of GERD and potential development of Barrett’s esophagus. Genco et al. in their study of 110 patients (follow-up rate 69%, 58 months) noticed an increase in the incidence of GERD (68.1% vs. 33.6%), VAS mean score (3 vs. 1.8) and PPI intake (57.2% from 19.1%). They also noted an upward migration of the “Z” line and a biliary-like esophageal reflux was found in 73.6% and 74.5% of cases, respectively. A significant increase in the incidence and in the severity of erosive esophagitis (EE) was evidenced. In addition, non-dysplastic Barrett’s esophagus (BE) was newly diagnosed in 19 (17.2%) patients (11). Sebastianelli et al. in review of their 90 patients with a mean follow-up of 78±15 months, reported a post sleeve gastrectomy prevalence of BE around 18.8% with no significant difference among the centers included in the study. The prevalence of GERD symptoms, erosive esophagitis, and the usage of PPIs all were noted to have respectively increased from 22%, 10%, and 22% pre sleeve gastrectomy to 76%, 41%, and 52% after the sleeve gastrectomy (12). These reports are concerning. It is known that obesity is associated with a higher risk of GERD to begin with. There are several papers suggesting an improvement in GERD after sleeve, but there appears to be more data supporting the worsening of GERD in many patients after sleeve gastrectomy. The authors recommend performing a diagnostic endoscopy in patients and before any surgical intervention which will alter the foregut anatomy. There are very few operative procedures in which anatomy is altered and a thorough evaluation of those organs is not undertaken preoperatively. This preoperative evaluation may help avoid GERD related problems and complications after any bariatric procedure.
Intra-operative endoscopy in bariatrics
The use of endoscopy in the operating room at the time of surgery is also controversial. Many surgeons do not use endoscopy due to lack of access or even politics within their institution. Others consider the endoscope to be a necessary tool when performing any foregut procedures. Intraoperative endoscopy is used by many bariatric surgeons to perform an intraoperative leak test, to assess anatomy during revisional bariatric procedures, to identify and control bleeding at the time of surgery and also to size the sleeve lumen or the diameter of the surgical anastomosis (13). Champion et al. identified intraoperative technical errors in 34 patients (4.1%) including 29 suture and staple line leaks, 2 bougie perforations, 2 inadvertent stoma closures, and 1 mucosal perforation in a gastric pacemaker. All of the technical issues were successfully repaired at the time of discovery in operating room, and avoided a postoperative complication. Unfortunately, 3 patients developed leaks later on (0.36%): 1 leak occurred in the patients who had findings repaired (2.9%) and 2 in the remaining 791 patients (0.25%). They concluded that intraoperative endoscopy potentially reduced the post-operative morbidity by identifying these issues at the time of the index surgery (14). Stricture or narrowing at the incisura is a dreaded complication during any sleeve gastrectomy. The resulting high pressure results in a higher risk of leak at proximal end of the staple line and also higher risk of post- sleeve GERD and it sequalae. Intra-op endoscopy may help reduce this complication by avoiding, identifying and correcting this problem. Nimeri and colleagues in their experience of 310 LSG cases reported a leak rate of 0.3%. None of the patients had positive intraoperative leak test however intra operative endoscopy showed stenosis in 10 patients (3.2%), which was corrected after removing over-sewing sutures thus concluding that the use of intraoperative endoscopy may decrease postoperative stenosis in laparoscopic sleeve gastrectomy (15). In a recent analysis of data from the American College of Surgeons National Surgical Quality Improvement Program database (2011–2016), done by Minhem and colleagues; 17.9% of LSG, and 19.7% of RNY patients had intraoperative endoscopy. Endoscopy-assisted LSG was associated with a decrease in sepsis (0.37% vs. 0.21%), unplanned reoperation (0.58% vs. 0.38%), prolonged hospital-stay (14.9% vs. 14.0%) and composite complications (1.43% vs. 1.17%). Outcomes after LRYGB were similar in both groups, except for decreased prolonged hospital stay with intraoperative endoscopy (22.4% vs. 20.6%) thus concluding that endoscopy at the time of procedure is associated with a decreased risk of postoperative complications particularly sepsis, unplanned reoperations, prolonged hospital stay, and composite complications after LSG; and hospital stay after LGBP (16). The authors advocate for the use of intraoperative endoscopy for the reasons mentioned.
Post bariatric surgery endoscopy diagnostic and therapeutic roles
After bariatric surgery, the use of endoscopy has really expanded our ability to care for many problems the patients have. Endoscopy used to be only for diagnostics. Now with the advent of improved clipping devices and suturing devices, many complications and problems after bariatric surgery can now be managed endoscopically and help avoid an operation. Some of those applications are reviewed here.
Endoscopy can help with the diagnosis of abdominal pain, nausea and vomiting. Endoscopy can be of vital importance in identifying problems like anastomotic stricture and marginal ulcer in gastric bypass patients (17). Post-operative endoscopy not only helps identify the altered anatomy in patients who have undergone bariatric operations but it also is an important tool in management of post bariatric surgical complications like bleeding, leak, strictures, fistula etc. (18,19).
Reporting endoscopic findings in bariatric patients
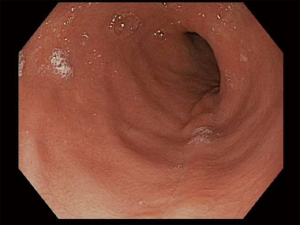
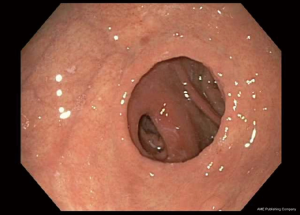
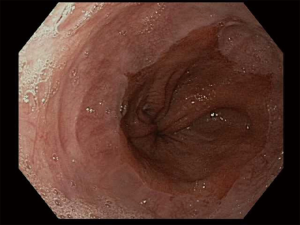
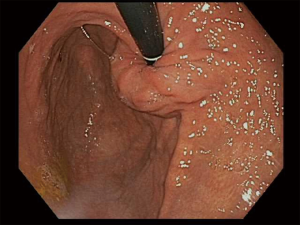
There is no set pattern to document endoscopic findings in a bariatric patient. The authors recommend documenting landmarks carefully. In a sleeve gastrectomy patient, commenting on the presence and extent of esophagitis, Barrett’s esophagus, hiatal hernia, retained fundus, diameter of sleeve (Figure 1) and narrowing, angulation or spiraling at incisura level should be described in detail. In a gastric bypass, the endoscopy report should comment on the distance of Z line and anastomosis from the incisors, diameter of the stoma (Figure 2), and the presence and size of a hiatal hernia. Special attention should be paid to describe the length of the blind limb of the Roux limb (i.e., candy-cane limb) and an ulcer should be ruled out on the jejunal side immediately next to the staple line. These ulcers can be hidden and easily missed if this area is not thoroughly examined. In gastric banding (Figure 3), again, a hiatal hernia (Figure 4) should be looked for, the appearance of the gastroesophageal junction should be described along with lumen of the aperture through the band and whether a band erosion or ulcer was noted. Knowledge of historical surgical procedures including gastric stapling, vertical banded gastroplasty or less commonly performed surgical procedures like duodenal switch, is very helpful in identifying the anatomical findings.
Endoscopic management of leak in bariatric patient
Leak is a dreaded complication after sleeve gastrectomy and gastric bypass. Various endoscopic modalities are now in the surgical endoscopist’s armamentarium for controlling a leak after bariatric surgery. Eubanks and colleagues described their experience with endoscopic stenting for the management of post bariatric surgery complications in 2008. They reported an 84% success rate with endoscopic stenting done for leak or stricture after bariatric procedures. The mean time for successful leak healing was 33 days. Three of the nineteen stents needed surgical retrieval after migration, and minor migration was reported in more than half patients (20). Blackmon and colleagues reported their results of successful management of esophageal and gastric leaks using covered self-expanding covered metal stents (cSEMS) in 25 patients. Ten patients who developed leaks after bariatric procedures were successfully managed with application of cSEMS (21). In another study, a stent migration rate of 23% and restenting rate of 20% was reported (22). Migration is one of the most challenging issues with the use of stents to manage leaks. Various methods of fixing the stents have been described including bridling of the stent to the nose, clipping in place and suturing in place. The clips appear to be ineffective. Endoscopic suturing is challenging in the small space but can be useful. Our most successful approach is surgical suturing externally with prolene sutures through the stent when exploring the patient to place drains. Bhayani and Swantsrom in described the role of endoscopic stenting in leaks presenting later than 48 hours (23). They had mixed results. They noted stents available in United States are not long enough to traverse entire length of a gastric sleeve. A longer stent can potentially decrease risk of the stent migration in sleeves. Others have placed 2 stents to mitigate this problem. Wezenbeek described their results of using a specifically designed long stent for bariatric leaks. Of 1,702 patients, 12 developed leaks. Seven had a leak after LSG and five patients had leak after LGBP. An average of 2.4 endoscopic procedures were performed and an average of 1.25 stents were used per patient. Seventy five percent of patients responded successfully with resolution of leak. Two thirds of the patients had stent migration (24). Aryaie et al. reported their outcomes of 20 consecutive endoscopic stents placed for various supper GI leaks including 4 after LSG. A success rate of 90% was ultimately achieved with 40% incidence of stent migration and one death due to stent erosion causing aortoenteric fistula (25).
In a more recent study, migration remained a problem in about 25% of patients and stent management was only successful about two thirds of the time (26).
With the technology currently available, the authors recommend using a fully covered stent for the management of leaks. The fully covered stent will avoid tissue ingrowth into the stent wall for easy stent removal. We also suggest removing or exchanging the stents within 6 weeks. If a partially covered stent is used, we recommend an earlier removal or exchange of stent. These are less likely to migrate but get more ingrowth. Patients often do require significant medical management of nausea and should be supported through this with medication. One should also have low suspicion for stent migration risk and a weekly X-ray is a good strategy to monitor the position of the stent. Pigtails can also help with internal drainage (27).
Clips
Over the Scope Clips (OTSC) have also been used for management of post-surgical GI tract leaks. The first multicenter study in the United States utilized OTSC for various GI indications including chronic fistula closure, iatrogenic perforation, anastomotic leaks after esophagectomy, hemostasis, iatrogenic GI tract perforation, closure of defects after endomucosal resection. The overall success rate was 71%. The results varied greatly depending on the indication (hemostasis, 100%; iatrogenic perforation, 75%; fistula closure, 65%) (28). Following that the use of OTSC has been described by numerous authors to successfully managing leaks after bariatric procedures (29). In one retrospective study, leaks post LSG were managed with OTSC in 26 patients. They reported a success rate of 80.76%. Success was defined as tolerance of complete oral nutrition by the patients without any further evidence of ongoing leakage. The majority of leaks were proximal at the gastroesophageal junction (84%) and the remaining were more distal along the staple line. Each patient required 2 to 7 (median 3) endoscopic procedures to achieve these results. Successful outcomes could not be obtained in 5 patients (two with distal and three with proximal staple line leaks). Median time for successful closure of leaks was 32 days (30). In a recent review of literature performed by Saber and colleagues, use of OTSC was analyzed in patients with leak/fistula after sleeve gastrectomy. Ten eligible studies with 195 patients with post-LSG leak or fistula were included in the review. Seventy-three of the patients in the study were treated with OTSC for leak/fistula after sleeve gastrectomy and 63 patients were reported to have obtained successful closure (86.3%). Most of the mural defects were located proximally closer to GEJ along the staple line, and they ranged from 3 to 20 mm in size. The duration between diagnosis of the leak and application of OTSC ranged between 0 to 271 days. More than half patients only required one clip application for closure of the defect. A leak occurred in five patients (9.3%). OTSC migration, stenosis, and tear were reported to occur in one patient each at a low rate of 1.8% (31). Overall, OTSC management of holes works well when the edges are not too friable. The clips come in various sizes and the appropriate size should be chosen. These are easily loaded on the scope and applied by grasping the tissue or suction. It is a great method to have in the toolbox.
Endoscopic vacuum assisted closure (e-VAC)
e-VAC, utilizes the negative suction therapy used for wound Management to treat leaks after bariatric surgery. A sponge connected to a negative suction apparatus via a tube is inserted endoscopically and placed inside the cavity outside the lumen at the leak site. Nagle and Holt described the procedure for management of colorectal leaks in 2006 (32). The concept has been adopted by experts for management for leaks after intestinal surgery and has been successfully applied in the management of leaks after esophagogastric surgery (33). Leeds and colleagues first described their experience with managing anastomotic leaks using E-Vac therapy in 35 patients. Nine of these patients were identified with a staple line leak after LSG. Eight of 9 patients were admitted from outside hospitals with a mean of 61 days (5–233 days) after LSG. Each of the patients required about 10 procedures over 50 days, and all had confirmed resolution of the leak. Two thirds of these patients had laparoscopy prior and 5 of 9 patients had endoscopic stenting done during their admission with treatment failure, but then responded to endo-vac therapy (34). Another study noted successful closure of staple line defects after sleeve using this Evac approach as well. The authors exchanged the sponge every 4 days and needed to do it 18 times over a 72-day period to obtain successful results without any procedure related complications (35).
Internal drainage
Another technique for managing leaks involves placing pigtail drains through the leak site into the extramural abscess cavity for drainage into the intestinal lumen, thereby improving healing. Donatelli and colleagues reported their experience with endoscopic internal drainage for a leak after sleeve gastrectomy in 2014. They later reported their results in 67 patients managed successfully with endoscopic internal drainage (98.5%). Six patients (9%) developed late stenosis which required further endoscopic management (36,37).
Chronic leaks pose a new problem and require other methods of management. An endoscopic septotomy was described by Campos and colleagues for management of chronic leak after gastric bypass using an endoscopic technique which initially was named stricturotomy (38). A detailed description of the technique was published by the authors later on in 2016. In principle, the technique involves identifying the septum between the perigastric collection and the intestinal lumen and then the septum is divided using sequential incisions with argon plasma coagulation (APC). This allows free communication between the perigastric cavity and the gastric lumen. Along with septotomy, Balloon dilation of the incisura area is often performed using 30-mm achalasia balloon which reduces the intraluminal pressure inside the stomach to reduce the outflow obstruction (39). Shnell and colleagues have reported their outcomes in 10 patients. Eight patients had a distal sleeve stricture along with proximal leak. The stricture was dilated with an achalasia balloon along with septotomy. On average five sessions were needed over the period of 43 days for successful treatment. Through the scope dilation of the fistula alone was performed in 2 patients who did not have sleeve stricture and had small perigastric collection only. No adverse events were noted (40). Similar results were reported in other small studies (41).
Endoscopic management of stricture after bariatric procedures
Stricture formation at the gastrojejunal anastomosis after gastric bypass is a well-known complication. Endoscopic balloon dilation has been performed for correction since early 2000s. Ahmad et al. in 2003, reported endoscopic dilation with a hydrostatic balloon in 14 patients with a success rate of 58% in 12 patients who underwent dilation with 15 mm Balloon. The remaining 5 of the 12 patients required additional dilations. Two patients who underwent dilation with 18 mm balloon did not require additional dilation (42). Peifer and colleagues reported their experience with strictures after bypass (43/801 of 5.4%) and most of the patients responded to one dilation with a 15 mm balloon. Weight loss at one year was not different between patients who underwent dilation compared with that of the patients who did not (43). Rosenthal and colleagues reported a 6% incidence of stricture in 1,012 gastric bypass patients. 61 patients with strictures underwent 128 dilations. Twenty-eight percent of patients needed single dilation, 33% patients needed two, 26% patients needed 3, 11.5% patients needed 4, 1.5% of patients needed 5 dilations respectively (44). Numerous studies reported similar results in managing stricture after gastric bypass.
Sleeve gastrectomy is most common performed bariatric procedure now. Stricture at incisura is a dreaded complication and results in causing leak and GERD issues. Successful endoscopic interventions have been reported along with surgical measures to manage this complication. Rebibo and colleagues reported their experience e of 1,210 patients who LSG. 17 patients developed gastric stenosis (1.4%); 11 patients had an organic stricture and six patients had functional strictures. Thirteen patients underwent pneumatic balloon dilation and 2 patients underwent endoscopic stenting. Endoscopic treatment was successful in 15 (88.2%) patients. The remaining 2 patients were converted to RYGB (45). Manos et al. reported 18 cases of post LSG stricture managed with endoscopic balloon dilation with success rate of 94.4%. Mean number of endoscopic procedures was 1.3 (46). Kumbhari and colleagues recommended an algorithmic approach in 2017 for managing stricture after sleeve gastrectomy. Their predefined treatment algorithm consisted of serial dilations using achalasia balloons, followed by a fully covered self-expanding metal stent (FCSEMS) if dilations were not successful. Nonresponders or those who did not want endoscopic intervention were recommended to have gastric bypass (47). More recently Cottam and colleagues have reported 93.3% success rate in managing post SG stricture using achalasia balloon in 33 patients (48). The risk of using the pneumatic balloon is perforation and in all of these series, perforation rates were extremely low. This may be a good way to manage strictures at the incisura after sleeve gastrectomy.
Endoscopic management of weight regain after gastric bypass
Weight regain is a common presentation for patients many years after bariatric surgery. A thorough endoscopic evaluation of the anatomy can be helpful in managing these patients. Often the weight gain is due to poor patient habits and can be managed with lifestyle and diet modification. Sometimes anatomical findings after the procedures are implicated in the weight gain and can be managed endoscopically. One of these is dilation of the gastric outlet after gastric bypass. Trans-oral outlet reduction (TORe) with endoscopic sutures has been applied with success. In a review of 13 patients who underwent TORe for reduction of the diameter of their dilated gastrojejunostomy (>15 mm), a mean weight loss at six months was 12.29 kg, (weight loss of 56.85% of the weight regained after RYGB initially). The mean GJ stoma diameter was 36 mm (20–45 mm) which was reduced by 75% to 9 mm (range, 5–12) with an average of 2.5 sutures. The mean pouch size was noted to be 7.2 cm (2–10 cm), which decreased to by 35% to 4.7 cm (range 4–5 cm) with using an average of 2.7 sutures (49). A recent review of literature by Thompson and colleagues concluded that with “proliferation of endoluminal therapies with evidence showing safety and efficacy in the treatment of weight regain, it is likely that endoscopic revision will be the gold standard to treat weight regain in patients with gastric bypass” (50). Weight regain after the sleeve is more challenging to manage endoscopically and often requires surgical modification.
Primary endoscopic bariatric procedures
With the alarming increase in the incidence of obesity, there is a growing need for endoscopic procedures for the management of obesity. Surgical procedures limited by expense, complication risk and are only being sought by a small portion of the population who qualify. Primary endoscopic bariatric and metabolic procedures can fill the void which exists between nonsurgical and surgical obesity management. Endoscopic procedures hypothetically can be less invasive, cheaper and are applicable to a larger patient population. Endoscopic procedures also have less serious and relatively easier to manage complications and are mostly be reversible. Some of the available and under investigation endoscopic bariatric devices are described in section below.
Intragastric space occupying devices
The original Garren-Edward Gastric Bubble (GEGB, 1985) was a gas filled cylindrical balloon made of polyurethane, with volume of 200–220 mL. The device did not meet expectations and was removed from the market due to migrations, bowel obstruction and such serious complications (51).
Orbera™ Intragastric Balloon
Orbera™ Intragastric Balloon (Apollo Endosurgery, Austin, TX, USA), approved by the FDA since 2015, is a silicone balloon with a fill volume of 400–700 cc. It requires endoscopy for placement and removal and remains in place for 6 months. The volume cannot be changed after device has been placed. Side effects including nausea, vomiting, abdominal pain, reflux and device intolerance usually happen early after placement and improves fairly quickly. While in place the patient has a feeling of satiety and after it is removed the patient receives ongoing counseling on diet. The Orbera US post-approval multi-center study reported outcomes in 321 subjects with average total body weight loss (TBWL) around 11.8% at six months. A decent improvement in comorbid conditions was also reported (52). Worldwide over 200,000 have been place with weight loss of 30–50 lbs on average with a good safety profile.
ReShape Duo®
ReShape Duo® (ReShape Medical, San Clemente, CA, USA) was approved by FDA in 2015. The device had two interconnected intragastric balloons. Placement and removal required endoscopy. Volume of each balloon was 450 mL, usually filled with methylene blue-mixed saline. The device claimed to reduce deflation-associated risk of migration and intestinal obstruction due to its unique individually sealed balloon design. The REDUCE pivotal trial reported a 25.1% EWL at 24 weeks in the patients who had the device placed in comparison to 11.3% EWL in patients who were managed with diet modification (53).
The device was recently pulled out of market after the company was acquired by Apollo.
The Obalon Gastric Balloon®
The Obalon Gastric Balloon® (Obalon Therapeutics Inc, Carlsbad, CA, United States) is the most recently FDA approved balloon device. The device is ingestible, comes covered inside a gelatin capsule which dissolves after balloon is ingested. The balloon is filled with 250 cc of Nitrogen through a connecting tubing after its placement inside stomach is confirmed. Up to three balloons can be placed at 6–12 weeks intervals. This staggered placement reduces the nausea associated with space filling intragastric devices. The balloons are endoscopically removed after 6 months. A modest 5 kg weight loss after 12 weeks has been reported (54). Weight loss is reportedly less than fluid filled balloons but with more desirable side effects profile.
The Spatz adjustable balloon system
The Spatz adjustable balloon system (Spatz Medical, NY, USA) is currently being tested in multicenter trials for use in the United States and is not yet FDA approved. It is a silicone balloon, filled with 400–800 cc of saline. Its unique feature is that the volume can be adjusted through a tether system. The Spatz can stay inside the patient for 12 months thus making it the longest implantable space-filling device. Total body weight loss of 19% over 12 months had been reported in ¾ of the patients in previous studies. Most of the issues reported in patients with the first generation device were claimed to be design related and was difficult to grasp and adjust. The new device design (Spatz 3) is thought to have minimized those problems (55).
Endoscopic aspiration therapy for obesity
The Aspire Assist™ device (Aspire Bariatrics, King of Prussia, PA, USA) is a modified percutaneous endoscopic gastrostomy (PEG) tube and has a larger Silicone made aspiration tube which is endoscopically placed. This is connected to a skin port component which has a valve that allows the gastric contents to be aspirated using gravity and a siphon effect. A third of a patient’s ingested food is aspirated 20 minutes after meal consumption. The patient also observes mindful eating, otherwise the food will not drain. Outcomes similar to space filling devices have been reported with advantage of long duration the device can remain in the patient. Both in a European trial and US trial patients have lost about 8–12% of their body weight. The weight loss is respectable, but due to the nature of the device, its use is not widespread yet (56).
Endoscopic sleeve gastroplasty (ESG)
Endoscopic Sleeve Gastroplasty (ESG) consists of suturing the greater curvature of the stomach with an endoscopic suturing device (Overstitch™; Apollo Endosurgery, Austin, TX), thus creating a lumen equivalent to a surgical vertical sleeve gastrectomy. A multicenter study with 242 patients showed 18.2%±11.6% TBWL at 12 months (57). Another recent study comprising 112 patients across 3 centers showed a change in weight of 16.4±10.7 kg (TBWL 14.9%±6.1%) at 6 months (58). This procedure shows promise, however, some concerns have been raised regarding bleeding if the stitches go too deep and the durability of the procedure. Finally the clips used to secure the sutures may impede later stapling procedures. In some patients this may be an option as a bridging procedure.
The BAROnova TransPyloric Shuttle* (TPS®)
TransPyloric Shuttle TPS™ [TPS (BAROnova, Goleta, CA, USA)] is comprised of a larger spherical silicone orb attached to a smaller cylindrical silicone bulb with a flexible cord. The device is inserted into the stomach endoscopically. This device causes an intermittent gastric outlet obstruction by virtue of its design, thus delaying gastric emptying. BAROnova received its FDA approval in April 2019 based on the results of the ENDObesity® II study presented at the obesity week meeting in November 2018. Three hundred and two patients from nine investigational centers across the United States were enrolled in a randomized, double-blinded and sham-controlled study. Patients in the TPS group lost 3.4x more weight when compared to the sham-control group; (9.5% total weight loss for the TPS group and 2.8% TWL for the Control group, P<0.0001) at the 12-month follow up. Five percent or more weight loss was reported in approximately 67% of people treated with TPS. Forty percent of people in the TPS group lost 10% or more weight (vs. 14% in sham-treated control group).
The EndoBarrier™
The EndoBarrier™ (GI Dynamics, Lexington, MA, USA) is Teflon-coated sleeve which is 65 CM long and is endoscopically deployed in the proximal duodenum. It bypasses the proximal jejunum and works as a Duodeno-Jejunal Bypass Sleeve (DJBS). A phase 2 US clinical trial was discontinued because of unexpected complications including a 3.5% rate of hepatic abscess formation. Initial studies reported a 12% EWL after 12 months and reduction in severity of diabetes (59). This design included no dietary counseling and noted just the effect of the sleeve alone. A redesign is reportedly in process as this device did show promise.
ValenTx
ValenTx (ValenTx, Inc., Hopkins, MN, USA) is another endoscopic liner device which mimics gastroduodenojejunal bypass. It is 120 CM long and is deployed in the distal esophagus. A 53% excess weight loss after 12 months has been shown in a single small study (60). Previous devices required a combined endoscopic and laparoscopic approach for deployment. A modified design which can be fully deployed endoscopically is in progress but is not yet FDA approved or commercially available yet.
Endoscopic anastomosis technologies
Magnets are being used to create endoscopic anastomoses. The principle applied in these devices is tissue approximation and forced pressure ischemia causing two segments of the intestine to fuse to each other thus creating anastomosis. This is accomplished by inserting self-assembling magnets and usually requires an upper and lower endoscopy to deploy these devices into 2 segments of bowel that will then attract to each other. Magnamosis and GI Window are two companies investigating endoscopic anastomosis technology. The first in-human pilot for partial jejunal diversion in 10 patients reported total body weight loss of 14.6% at 12 months in a web release by the company (61). The technology is promising and future application can be widespread not only for weight loss but also for bypassing obstructions in malignant conditions.
Conclusions
The number of patients having bariatric procedures or looking for alternative weight loss methods is rising. There is a great need for new endoscopic technologies to meet the needs of this bariatric patient population. Endoscopy has an important role at each step in the management of bariatric patients. Prior to surgery, endoscopy is helpful in identifying conditions which may affect any procedure which alters the upper gastrointestinal tract. After surgery, endoscopy is critical in identifying problems and may even be used to manage the postoperative complication in a minimally invasive way. The role of therapeutic endoscopy as a primary weight loss intervention is on the rise as well and newer technologies have lots of promise. These endoscopic interventions may one day bridge the gap between medical and surgical approaches to obesity. This also highlights the need for establishing proper training and certification programs to train and verify skills of the endoscopists who are interested in practicing bariatric endoscopy. National organizations including SAGES and ASMBS are actively implementing such programs to improve the widespread use of endoscopy in the field of bariatrics.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Jeffrey M. Marks and Ryan M. Juza) for the series “Surgical Endoscopy: Essential Skills in Gastrointestinal Surgery” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2019.08.05). The series “Surgical Endoscopy: Essential Skills in Gastrointestinal Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Finkelstein EA, Trogdon JG, Cohen JW, et al. Annual medical spending attributable to obesity: payer-and service-specific estimates. Health Aff (Millwood) 2009;28:w822-31. [Crossref] [PubMed]
- Bhaskaran K, Douglas I, Forbes H, et al. Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5·24 million UK adults. Lancet 2014;384:755-65. [Crossref] [PubMed]
- Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation 2014;129:S102-38. [Crossref] [PubMed]
- Kurian M, Kroh M, Chand B, et al. SAGES review of endoscopic and minimally invasive bariatric interventions: a review of endoscopic and non-surgical bariatric interventions. Surg Endosc 2018;32:4063-7. [Crossref] [PubMed]
- Huang S, Zhang J, Dong Z, et al. Efficacy and future of endoscopic bariatric surgery in the treatment of obesity and metabolic diseases. Zhonghua Wei Chang Wai Ke Za Zhi 2017;20:383-7. [PubMed]
- D'Silva M, Bhasker AG, Kantharia NS, et al. High-Percentage Pathological Findings in Obese Patients Suggest that Esophago-gastro-duodenoscopy Should Be Made Mandatory Prior to Bariatric Surgery. Obes Surg 2018;28:2753-9. [Crossref] [PubMed]
- Saarinen T, Kettunen U, Pietiläinen KH, et al. Is preoperative gastroscopy necessary before sleeve gastrectomy and Roux-en-Y gastric bypass? Surg Obes Relat Dis 2018;14:757-62. [Crossref] [PubMed]
- Parikh M, Liu J, Vieira D, et al. Preoperative Endoscopy Prior to Bariatric Surgery: a Systematic Review and Meta-Analysis of the Literature. Obes Surg 2016;26:2961-6. [Crossref] [PubMed]
- Salama A, Saafan T, El Ansari W, et al. Is Routine Preoperative Esophagogastroduodenoscopy Screening Necessary Prior to Laparoscopic Sleeve Gastrectomy? Review of 1555 Cases and Comparison with Current Literature. Obes Surg 2018;28:52-60. [Crossref] [PubMed]
- ASGE STANDARDS OF PRACTICE COMMITTEE. The role of endoscopy in the bariatric surgery patient. Surg Obes Relat Dis 2015;11:507-17. [Crossref] [PubMed]
- Genco A, Soricelli E, Casella G, et al. Gastroesophageal reflux disease and Barrett's esophagus after laparoscopic sleeve gastrectomy: a possible, underestimated long-term complication. Surg Obes Relat Dis 2017;13:568-74. [Crossref] [PubMed]
- Sebastianelli L, Benois M, Vanbiervliet G, et al. Systematic Endoscopy 5 Years After Sleeve Gastrectomy Results in a High Rate of Barrett's Esophagus: Results of a Multicenter Study. Obes Surg 2019;29:1462-9. [Crossref] [PubMed]
- Haddad A, Tapazoglou N, Singh K, et al. Role of intraoperative esophagogastroenteroscopy in minimizing gastrojejunostomy-related morbidity: experience with 2,311 laparoscopic gastric bypasses with linear stapler anastomosis. Obes Surg 2012;22:1928-33. [Crossref] [PubMed]
- Champion JK, Hunt T, DeLisle N. Role of routine intraoperative endoscopy in laparoscopic bariatric surgery. Surg Endosc 2002;16:1663-5. [Crossref] [PubMed]
- Nimeri A, Maasher A, Salim E, et al. The Use of Intraoperative Endoscopy May Decrease Postoperative Stenosis in Laparoscopic Sleeve Gastrectomy. Obes Surg 2016;26:1398-401. [Crossref] [PubMed]
- Minhem MA, Safadi BY, Tamim H, et al. Does intraoperative endoscopy decrease complications after bariatric surgery? Analysis of American College of Surgeons National Surgical Quality Improvement Program database. Surg Endosc 2019; [Epub ahead of print]. [Crossref] [PubMed]
- Blom-Høgestøl IK, Stubhaug A, Kristinsson JA, et al. Diagnosis and treatment of chronic abdominal pain 5 years after Roux-en-Y gastric bypass. Surg Obes Relat Dis 2018;14:1544-51. [Crossref] [PubMed]
- Docimo S Jr, Svestka M. Endoscopic Evaluation and Treatment of Postoperative Bariatric Surgery Complications. Surg Innov 2017;24:616-24. [Crossref] [PubMed]
- Schulman AR, Thompson CC. Endoscopic Evaluation/Management of Bariatric Surgery Complications. Curr Treat Options Gastroenterol 2017;15:701-16. [Crossref] [PubMed]
- Eubanks S, Edwards CA, Fearing NM, et al. Use of endoscopic stents to treat anastomotic complications after bariatric surgery. J Am Coll Surg 2008;206:935-8; discussion 938-9. [Crossref] [PubMed]
- Blackmon SH, Santora R, Schwarz P, et al. Utility of removable esophageal covered self-expanding metal stents for leak and fistula management. Ann Thorac Surg 2010;89:931-6; discussion 936-7. [Crossref] [PubMed]
- Freedman J, Jonas E, Näslund E, et al. Treatment of leaking gastrojejunostomy after gastric bypass surgery with special emphasis on stenting. Surg Obes Relat Dis 2013;9:554-8. [Crossref] [PubMed]
- Bhayani NH, Swanström LL. Endoscopic therapies for leaks and fistulas after bariatric surgery. Surg Innov 2014;21:90-7. [Crossref] [PubMed]
- van Wezenbeek MR, de Milliano MM, Nienhuijs SW, et al. A Specifically Designed Stent for Anastomotic Leaks after Bariatric Surgery: Experiences in a Tertiary Referral Hospital. Obes Surg 2016;26:1875-80. [Crossref] [PubMed]
- Aryaie AH, Singer JL, Fayezizadeh M, et al. Efficacy of endoscopic management of leak after foregut surgery with endoscopic covered self-expanding metal stents (SEMS). Surg Endosc 2017;31:612-7. [Crossref] [PubMed]
- Martin Del Campo SE, Mikami DJ, Needleman BJ, et al. Endoscopic stent placement for treatment of sleeve gastrectomy leak: a single institution experience with fully covered stents. Surg Obes Relat Dis 2018;14:453-61. [Crossref] [PubMed]
- Shehab H. Enteral stents in the management of post-bariatric surgery leaks. Surg Obes Relat Dis 2018;14:393-403. [Crossref] [PubMed]
- Baron TH, Song LM, Ross A, et al. Use of an over-the-scope clipping device: multicenter retrospective results of the first U.S. experience (with videos). Gastrointest Endosc 2012;76:202-8. [Crossref] [PubMed]
- Shehab H, Abdallah E, Gawdat K, et al. Large Bariatric-Specific Stents and Over-the-Scope Clips in the Management of Post-Bariatric Surgery Leaks. Obes Surg 2018;28:15-24. [Crossref] [PubMed]
- Keren D, Eyal O, Sroka G, et al. Over-the-Scope Clip (OTSC) System for Sleeve Gastrectomy Leaks. Obes Surg 2015;25:1358-63. [Crossref] [PubMed]
- Shoar S, Poliakin L, Khorgami Z, et al. Efficacy and Safety of the Over-the-Scope Clip (OTSC) System in the Management of Leak and Fistula After Laparoscopic Sleeve Gastrectomy: a Systematic Review. Obes Surg 2017;27:2410-8. [Crossref] [PubMed]
- Nagell CF, Holte K. Treatment of anastomotic leakage after rectal resection with transrectal vacuum-assisted drainage (VAC). A method for rapid control of pelvic sepsis and healing. Int J Colorectal Dis 2006;21:657-60. [Crossref] [PubMed]
- Pournaras DJ, Hardwick RH, Safranek PM, et al. Endoluminal Vacuum Therapy (E-Vac): A Treatment Option in Oesophagogastric Surgery. World J Surg 2018;42:2507-11. [Crossref] [PubMed]
- Leeds SG, Burdick JS. Management of gastric leaks after sleeve gastrectomy with endoluminal vacuum (E-Vac) therapy. Surg Obes Relat Dis 2016;12:1278-85. [Crossref] [PubMed]
- Schmidt F, Mennigen R, Vowinkel T, et al. Endoscopic Vacuum Therapy (EVT)-a New Concept for Complication Management in Bariatric Surgery. Obes Surg 2017;27:2499-505. [Crossref] [PubMed]
- Donatelli G, Dumont JL, Cereatti F, et al. Treatment of Leaks Following Sleeve Gastrectomy by Endoscopic Internal Drainage (EID). Obes Surg 2015;25:1293-301. [Crossref] [PubMed]
- Donatelli G, Ferretti S, Vergeau BM, et al. Endoscopic Internal Drainage with Enteral Nutrition (EDEN) for treatment of leaks following sleeve gastrectomy. Obes Surg. Obes Surg 2014;24:1400-7. [Crossref] [PubMed]
- Campos JM, Siqueira LT, Ferraz AA, et al. Gastrobronchial fistula after obesity surgery. J Am Coll Surg 2007;204:711. [Crossref] [PubMed]
- Campos JM, Ferreira FC, Teixeira AF, et al. Septotomy and Balloon Dilation to Treat Chronic Leak After Sleeve Gastrectomy: Technical Principles. Obes Surg 2016;26:1992-3. [Crossref] [PubMed]
- Shnell M, Gluck N, Abu-Abeid S, et al. Use of endoscopic septotomy for the treatment of late staple-line leaks after laparoscopic sleeve gastrectomy. Endoscopy 2017;49:59-63. [PubMed]
- Mahadev S, Kumbhari V, Campos JM, et al. Endoscopic septotomy: an effective approach for internal drainage of sleeve gastrectomy-associated collections. Endoscopy 2017;49:504-8. [Crossref] [PubMed]
- Ahmad J, Martin J, Ikramuddin S, et al. Endoscopic balloon dilation of gastroenteric anastomotic stricture after laparoscopic gastric bypass. Endoscopy 2003;35:725-8. [Crossref] [PubMed]
- Peifer KJ, Shiels AJ, Azar R, et al. Successful endoscopic management of gastrojejunal anastomotic strictures after Roux-en-Y gastric bypass. Gastrointest Endosc 2007;66:248-52. [Crossref] [PubMed]
- Ukleja A, Afonso BB, Pimentel R, et al. Outcome of endoscopic balloon dilation of strictures after laparoscopic gastric bypass. Surg Endosc 2008;22:1746-50. [Crossref] [PubMed]
- Rebibo L, Hakim S, Dhahri A, et al. Gastric Stenosis After Laparoscopic Sleeve Gastrectomy: Diagnosis and Management. Obes Surg 2016;26:995-1001. [Crossref] [PubMed]
- Manos T, Nedelcu M, Cotirlet A, et al. How to treat stenosis after sleeve gastrectomy?. Surg Obes Relat Dis 2017;13:150-4. [Crossref] [PubMed]
- Agnihotri A, Barola S, Hill C, et al. An Algorithmic Approach to the Management of Gastric Stenosis Following Laparoscopic Sleeve Gastrectomy. Obes Surg 2017;27:2628-36. [Crossref] [PubMed]
- Dhorepatil AS, Cottam D, Surve A, et al. Is pneumatic balloon dilation safe and effective primary modality of treatment for post-sleeve gastrectomy strictures? A retrospective study. BMC Surg 2018;18:52. [Crossref] [PubMed]
- Espinet Coll E, Nebreda Durán J, López-Nava Breviere G, et al. Efficacy and safety of transoral outlet reduction via endoscopic suturing in patients with weight regain after a surgical Roux-en-Y gastric bypass. Rev Esp Enferm Dig 2018;110:551-6. [Crossref] [PubMed]
- Hourneaux De Moura DT, Thompson CC. Endoscopic management of weight regain following Roux-en-Y gastric bypass. Expert Rev Endocrinol Metab 2019;97-110. [Crossref] [PubMed]
- Gleysteen JJ. A history of intragastric balloons. Surg Obes Relat Dis 2016;430-5. [Crossref] [PubMed]
- Vargas EJ, Pesta CM, Bali A, et al. Single Fluid-Filled Intragastric Balloon Safe and Effective for Inducing Weight Loss in a Real-World Population. Clin Gastroenterol Hepatol 2018;16:1073-80.e1. [Crossref] [PubMed]
- Ponce J, Woodman G, Swain J, et al. The REDUCE pivotal trial: a prospective, randomized controlled pivotal trial of a dual intragastric balloon for the treatment of obesity. Surg Obes Relat Dis 2015;11:874-81. [Crossref] [PubMed]
- Mion F, Ibrahim M, Marjoux S, et al. Swallowable Obalon® gastric balloons as an aid for weight loss: a pilot feasibility study. Obes Surg 2013;23:730-3. [Crossref] [PubMed]
- Brooks J, Srivastava ED, Mathus-Vliegen EM. One-year adjustable intragastric balloons: results in 73 consecutive patients in the U.K. Obes Surg 2014;24:813-9. [Crossref] [PubMed]
- Nyström M, Machytka E, Norén E, et al. Aspiration Therapy As a Tool to Treat Obesity: 1- to 4-Year Results in a 201-Patient Multi-Center Post-Market European Registry Study. Obes Surg 2018;28:1860-8. [Crossref] [PubMed]
- Lopez-Nava G, Sharaiha RZ, Vargas EJ, et al. Endoscopic Sleeve Gastroplasty for Obesity: a Multicenter Study of 248 Patients with 24 Months Follow-Up. Obes Surg 2017;27:2649-55. [Crossref] [PubMed]
- Sartoretto A, Sui Z, Hill C, et al. Endoscopic Sleeve Gastroplasty (ESG) Is a Reproducible and Effective Endoscopic Bariatric Therapy Suitable for Widespread Clinical Adoption: a Large, International Multicenter Study. Obes Surg 2018;28:1812-21. [Crossref] [PubMed]
- Bazerbachi F, Vargas Valls EJ, Abu Dayyeh BK. Recent Clinical Results of Endoscopic Bariatric Therapies as an Obesity Intervention. Clin Endosc 2017;50:42-50. [Crossref] [PubMed]
- Riedel N, Laubner K, Lautenbach A, et al. Longitudinal evaluation of efficacy, safety and nutritional status during one-year treatment with the duodenal-jejunal bypass liner. Surg Obes Relat Dis 2018;14:769-79. [Crossref] [PubMed]
- Machytka E, Bužga M, Zonca P, et al. Partial jejunal diversion using an incisionless magnetic anastomosis system: 1-year interim results in patients with obesity and diabetes. Gastrointest Endosc 2017;86:904-12. [Crossref] [PubMed]
Cite this article as: Abbas M, Khaitan L. Endoscopy in the bariatric patient. Ann Laparosc Endosc Surg 2019;4:93.