
The smart approach to surgical treatment of submucosal tumors based on preoperative EUS-typing
Introduction
Currently, different types of surgeries have been proposed for the treatment of patients with non-epithelial tumors of the stomach and duodenum, including both laparoscopic and thoracoscopic resections of the organ, and endoscopic tunneling dissections. To select a modern organ-preserving surgical technique we need criteria, which will determine the best type of surgery in each particular case, especially for tumors at anatomically narrow sites—the esophagogastric junction, pylorus and duodenum. We have analyzed the growth pattern, localization, structure of tumors, and the possibility of performing various types of surgeries on the esophagus, stomach and duodenum. Based on the results obtained, a working classification of tumors based on EUS-typing was developed to determine the optimal surgical technique.
Methods
From 2005 to 2017, 168 patients with non-epithelial tumors of the esophagus, stomach, and duodenum were examined, and 80 patients with non-epithelial tumors were operated at the A.V. Vishnevsky Institute of Surgery, Moscow, Russia. Among patients who underwent surgical procedures there were 46 females and 34 males with median age of 43.8±16.2 [28–72] years. All patients underwent an endoscopic examination of the upper gastrointestinal tract, and CT with bolus tracking. The key method to examine patients was an endoscopic ultrasound (EUS) to determine the putative non-epithelial tumor type, as well as the source wall layer and the size of the tumor base.
All patients underwent an endoscopic examination of the upper gastrointestinal tract, and CT with bolus tracking. The key method to examine patients was an EUS to determine the putative non-epithelial tumor type, as well as the source wall layer and the size of the tumor base. Intraoperative laparoscopic ultrasound was used only for surgical navigation.
Laparoscopic atypical stomach resections were performed in 62 cases with laparoscopic ultrasound and/or endoscopic navigation. In 7 cases, endoscopic tunnel dissections with and without laparoscopic assistance were performed, in 11—removal of the tumor with the mucosal covering by the submucosal dissection.
Based on an analysis of the data from preoperative endosonography, intraoperative ultrasound and histological examination of the resected specimen, a working classification of non-epithelial tumors was developed that determines what type of surgical intervention will be the most effective with maximum organ preservation.
Results
Median operating time was 64±12.6 (45–150 minutes) with minimal blood loss (<30–40 mL) and with R0 resections on pathology of specimens in all cases. Recovery was uneventful with no major complications (1 patient with wound seroma), no re-interventions and median post-op hospital stay was 6±2.4 (from 2 to 9) days. Post-operative assessment includes evaluation of the complaints, the results of upper GI endoscopy and CT.
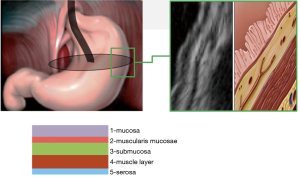
Based on the results in 69 cases, the tumor originated from the muscular layer of the stomach wall (fourth echo layer), in five—from the submucosal layer (third echo layer), in four—from the muscularis mucosa (second echo layer).
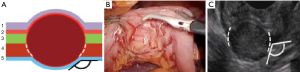
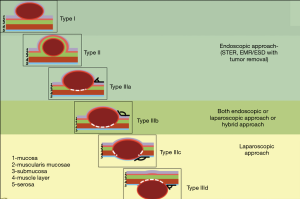
The classification involves three types of tumors depending on wall layer of the hollow organ where the tumor base is localized (Figure 1).
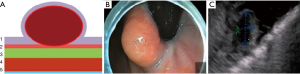
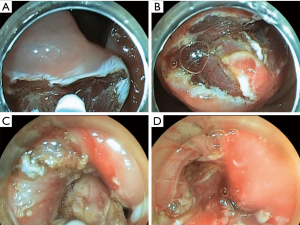
Type I: the tumor originates from the muscularis mucosa (second echo layer) (Figure 2). In this type, the tumor is characterized by intra-organ growth, is easily displaced relative to the organ wall during instrumental palpation. The most effective intervention for such tumors is the intraluminal removal of the tumor with the mucosa covering it by submucosal dissection or, for small tumors, endoscopic mucosal resection. During these techniques we always use lifting by submucosal injection of HES with indigo carmine dye. In cases of endoscopic removal of submucosal tumor (SMT) by means of endoscopic mucosal resection with tumor we never close the defect of mucosa. In all cases of tumor removal by means of endoscopic tunneling dissection we close the entry point to the tunnel routinely with endoscopic clips (Figure 3).
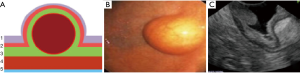
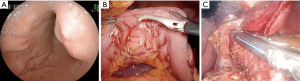
Type II: the tumor is localized in the submucosal layer, is predominantly intra-organ, the base of the tumor can reach its largest diameter (Figure 4). With such tumors, endoscopic intraluminal resection is also the most effective and safe. With such localization, endoscopic mucosal resection is usually not effective; the tumor is enucleated from the submucosal layer after resection of the mucosa over the tumor (Figure 5).
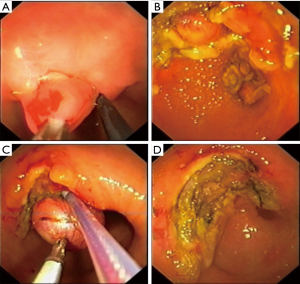
Type III: the tumor originates from the muscular layer of the organ wall. With such localization, intraluminal removal of the tumor sometimes leads to perforation of the wall, therefore, laparo-/thoracoscopic access is often used; however, endoscopic tunnel dissection is also possible. The choice of surgical access and the extent of resection is determined by the size of the tumor base in the muscular wall layer and the type of growth relative to the lumen—intra- or extra-organ. Based on these features, Type III is divided into four subtypes.
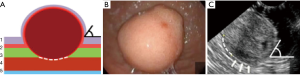
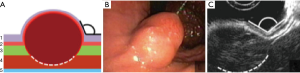
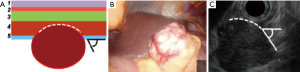
Type IIIa: the tumor is intra-organ and has a base that is less than half of the largest diameter of the tumor (Figure 6). In this type, the tumor originates from the inner circular muscular wall layer, and the angle between the inner surface of the organ wall and the tumor is acute. For organ preservation, the tunnel dissection with resection of the inner muscular wall layer several millimeters from the tumor base is the most optimal (Figure 7).
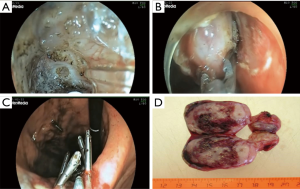
Type IIIb: the tumor is predominantly intra-organ; however, the diameter of its base is more than half of the largest tumor diameter (Figure 8). In this type, the tumor can originate from both the circular and longitudinal muscular layers of the stomach, and the angle between the inner surface of the organ wall and the tumor, as determined by EUS, is obtuse. Both endoscopic tunnel dissection and laparoscopic gastric resection can be used. The endoscopic approach is justified when the tumor size is less than 3 cm and the surgeon has extensive experience of such operations. With the laparoscopic access, the tumor removal is most effective when it is excised after a gastro- or duodenotomy and with subsequent suturing for defect closure (Figure 9). This approach involves the least extensive resection of the organ wall with the tumor base, which is especially relevant in anatomically narrow areas.
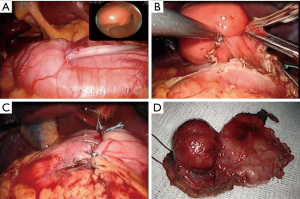
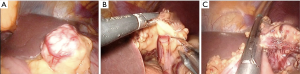
Type IIIc: the tumor is both intra-organ and extra-organ (Figure 10). It is impossible to determine from which inner or outer muscular layer the tumor originates in most such cases. When viewed from the abdominal cavity, the angle between the outer surface of the organ wall and the tumor is most often obtuse, but with an “hourglass” tumor growth it may be acute. In this type of tumor, laparoscopic atypical resection of the stomach is the most justified both using a endoscopic stapler technique or hand-sewing (Figure 11).
Type IIId: the tumor is extra-organ. In this situation, the tumor originates from the outer layers of the muscular organ wall (Figure 12). The angle between the outer surface of the wall and the tumor is acute. With this type of tumor, laparoscopic tumor enucleation can be performed with resection of the seromuscular layer 2–4 mm away from the tumor base and preservation of the submucosal and mucosal layers of the stomach wall if the tumor located close to the anatomical sphincters or narrow spaces, otherwise using endoscopic stapler is the most fastest and easiest way to perform a resection (Figure 13).
Discussion
Non-epithelial tumors of the gastrointestinal tract are a heterogeneous group with different histological structure, growth pattern and prognosis. With the development of endoscopic methods of diagnosis and treatment, the approach to the tactics of treating patients with this pathology has changed. As the majority of upper gastrointestinal non-epithelial tumors are benign, small tumors with diameter <2 cm were primarily subjected to follow-up observation in the past. If a tumor had a larger diameter or clinical symptoms have developed, the tumor was resected. With advances in endoscopic ultrasonography technology, this technique has become the primary screening method for the diagnosis of upper gastrointestinal non-epithelial tumors and is capable of determining, in general, the nature of a lesion based on the originating layer, size, and internal echoes of the lesion, therefore, EUS may assist in both diagnosis and guiding treatment (1).
Most non-epithelial tumors have a favorable prognosis and require surgical intervention only in rapid tumor growth or impaired patency of the hollow organ. Only the gastrointestinal stromal tumor (GIST) has the ability to progress, which necessitates a more active surgical tactics. A feature of GISTs is their non-infiltrative growth, the formation of a pseudocapsule, while lymphogenous metastasis is uncommon; this is reflected in the approaches to surgical removal of these tumors. According to the NCCN recommendations, the standard of treatment for localized forms of GIST is complete surgical excision; removing a tumor without rupturing the pseudocapsule is sufficient, and a routine lymphadenectomy is not needed (2). Therefore, laparoscopic, thoracoscopic and flexible intraluminal endoscopic approaches are common in the treatment of non-epithelial upper gastrointestinal tumors (3-6).
The general principle of the treatment for SMTs is local resection with negative resection margin. There is no exception to this principle, even with laparoscopic surgery; however, different surgical techniques are required depending on the location and configuration (i.e., endophytic or exophytic) of the tumor (7).
Although laparoscopic resection has been the main stream of minimally invasive surgery for gastrointestinal SMTs, recent advances in endoluminal endoscopic procedures now provide various treatment modalities for gastric SMTs (8).
The main reason for using endoluminal endoscopy is the difficulties of localizing small endophytic tumors by laparoscopy. In that sense, the endoscopic approach has advantages such as easy localization of the tumors and less invasiveness. However, it also carries a risk of bleeding, perforation, and less radicality. Therefore, to overcome these problems, investigators have developed several hybrid techniques combined the laparoscopic method with endoscopic procedures that include the advantages of both laparoscopic and endoscopic procedure (8-14).
Another limitation of laparoscopic and thoracoscopic surgery is the localization of tumors in the difficult places such as esophagus, esophagogastric junction and pylorus. Non-epithelial tumors located very close to the functional sphincters remain a challenge for surgeons because of the narrow space, the absence of any redundant gastric wall for the laparoscopic linear stapler, and what is more important the possibility of postoperative deformity, stenosis, and leakage.
Some have reported that anatomical partial gastrectomy unavoidable when the tumor is located near the esophagogastric junction or pylorus, but major gastric resection might be too invasive for a gastric SMTs most of which are benign tumors. Therefore, various stomach preserving techniques including endoluminal endoscopy, hybrid laparoendoscopic approach or transgastric approach have already been introduced with the main aim to prevent deformity, stenosis or sphincters dysfunction (15-17).
Moreover, with the development and technological advances in flexible endoscopy recently, endoscopic therapy has become the concurrent approach for the treatment of upper gastrointestinal non-epithelial tumors (18). In particular, the development and maturation of endoscopic submucosal dissection (ESD) and peroral submucosal tunneling endoscopic resection (STER) allow for the endoscopic resection of SMTs with larger diameters (>2 cm) and SMTs originating from the deeper layer of the gastrointestinal tract—muscularis propria (1). ESD, including endoscopic muscularis dissection, is a technically feasible procedure for the treatment of SETs. However, selection bias is suspected from the enrolled studies. For the development of a proper indication of ESD for SETs, further studies are needed (19). Publications on STER for SMTs conclude that STER is a safe and efficient technique for treating SMTs originating from muscularis propria layer, which can avoid patients suffering repeated resections (20). Furthermore, studies comparing endoluminal endoscopic techniques confirmed that both endoscopic nontunneling and tunneling resection seem to be effective and safe methods for removing relatively small gastric SMTs. Compared with endoscopic nontunneling, tunneling resection does not seem to have distinct advantages for gastric SMTs, but has a longer mean operative time (21).
Conclusions
There is a diversity of surgical approaches for non-epithelial tumors treatment. From our point of view, individualization is necessary in each specific case. The relevance of this area of endoscopic surgery is confirmed by many presentations at world congresses and publications. In clinical practice, surgeons and advanced surgical endoscopists most often offer the techniques that they are skilled at (22-26). Only a few departments and clinics that have laparoscopic and flexible endoscopic technologies in their hands can offer the patient the optimal surgical treatment method.
Our classification considers the originating GI wall layer, the size of the tumor base and the growth type (intra- or extra-organ). Based on this classification, the surgical approach, the nature and extent of the surgical procedure for R0 resection in compliance with the organ preservation principles can be selected individually for each patient at the preoperative stage (Figure 14).
In all cases, the classification of the non-epithelial tumor types proposed in our department led to effective hollow organ sparing resection in compliance with the oncological principles for the removal of particular tumor type, including GIST, performed radically without mortality or serious morbidity.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Muhammed Ashraf Memon and Abe Fingerhut) for the series “Laparoendoscopic Surgery for Benign Oesophagogastric Conditions” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2019.06.08). The series “Laparoendoscopic Surgery for Benign Oesophagogastric Conditions” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Institutional ethical approval and individual informed consent were waived due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zhang J, Huang K, Ding S, et al. Clinical Applicability of Various Treatment Approaches for Upper Gastrointestinal Submucosal Tumors. Gastroenterol Res Pract 2016;2016:9430652 [Crossref] [PubMed]
- Demetri GD, Benjamin R, Blanke CD, et al. NCCN Task Force report: optimal management of patients with gastrointestinal stromal tumor (GIST)--expansion and update of NCCN clinical practice guidelines. J Natl Compr Canc Netw 2004;2 Suppl 1:S-1-26; quiz 27-30.
- Chen QF, Huang CM, Lin M, et al. Short- and Long-Term Outcomes of Laparoscopic Versus Open Resection for Gastric Gastrointestinal Stromal Tumors: A Propensity Score-Matching Analysis. Medicine (Baltimore) 2016;95:e3135 [Crossref] [PubMed]
- Huang CM, Chen QF, Lin JX, et al. Can laparoscopic surgery be applied in gastric gastrointestinal stromal tumors located in unfavorable sites?: A study based on the NCCN guidelines. Medicine (Baltimore) 2017;96:e6535 [Crossref] [PubMed]
- Judson I, Bulusu R, Seddon B, et al. UK clinical practice guidelines for the management of gastrointestinal stromal tumours (GIST). Clin Sarcoma Res 2017;7:6. [Crossref] [PubMed]
- Severino BU, Fuks D, Lainas P, et al. Large gastrointestinal stromal tumours of the stomach: Is laparoscopy reasonable? J Minim Access Surg 2016;12:148-53. [Crossref] [PubMed]
- Hwang SH, Park DJ, Kim YH, et al. Laparoscopic surgery for submucosal tumors located at the esophagogastric junction and the prepylorus. Surg Endosc 2009;23:1980-7. [Crossref] [PubMed]
- Lee CM, Kim HH. Minimally invasive surgery for submucosal (subepithelial) tumors of the stomach. World J Gastroenterol 2014;20:13035-43. [Crossref] [PubMed]
- Aisu Y, Yasukawa D, Kimura Y, et al. Laparoscopic and endoscopic cooperative surgery for gastric tumors: Perspective for actual practice and oncological benefits. World J Gastrointest Oncol 2018;10:381-97. [Crossref] [PubMed]
- Barajas-Gamboa JS, Acosta G, Savides TJ, et al. Laparo-endoscopic transgastric resection of gastric submucosal tumors. Surg Endosc 2015;29:2149-57. [Crossref] [PubMed]
- Caron PH, Martins MI, Bertevello PL. Preliminary analysis of hybrid laparoscopic procedure for resection of gastric submucosal tumors. Rev Col Bras Cir 2016;43:129-35. [Crossref] [PubMed]
- Daiko H, Fujita T, Ohgara T, et al. Minimally invasive hybrid surgery combined with endoscopic and thoracoscopic approaches for submucosal tumor originating from thoracic esophagus. World J Surg Oncol 2015;13:40. [Crossref] [PubMed]
- Matsuda T, Nunobe S, Ohashi M, et al. Laparoscopic endoscopic cooperative surgery (LECS) for the upper gastrointestinal tract. Transl Gastroenterol Hepatol 2017;2:40. [Crossref] [PubMed]
- Ntourakis D, Mavrogenis G. Cooperative laparoscopic endoscopic and hybrid laparoscopic surgery for upper gastrointestinal tumors: current status. World J Gastroenterol 2015;21:12482-97. [Crossref] [PubMed]
- Kanehira E, Kanehira AK, Tanida T, et al. Percutaneous endoscopic intragastric surgery: an organ preserving approach to submucosal tumors at esophagogastric junction. Transl Gastroenterol Hepatol 2017;2:48. [Crossref] [PubMed]
- Kwon OK, Yu W. Endoscopic and Laparoscopic Full-Thickness Resection of Endophytic Gastric Submucosal Tumors Very Close to the Esophagogastric Junction. J Gastric Cancer 2015;15:278-85. [Crossref] [PubMed]
- Zhou DJ, Dai ZB, Wells MM, et al. Submucosal tunneling and endoscopic resection of submucosal tumors at the esophagogastric junction. World J Gastroenterol 2015;21:578-83. [Crossref] [PubMed]
- Kim SY, Kim KO. Management of gastric subepithelial tumors: The role of endoscopy. World J Gastrointest Endosc 2016;8:418-24. [Crossref] [PubMed]
- Bang CS, Baik GH, Shin IS, et al. Endoscopic submucosal dissection of gastric subepithelial tumors: a systematic review and meta-analysis. Korean J Intern Med 2016;31:860-71. [Crossref] [PubMed]
- Zhang C, Hu JW, Chen T, et al. Submucosal tunneling endoscopic resection for upper gastrointestinal multiple submucosal tumors originating from the muscular propria layer: A feasibility study. Indian J Cancer 2015;51:e52-5. [PubMed]
- Zhang Q, Wang F, Wei G, et al. Endoscopic Resection of Gastric Submucosal Tumors: A Comparison of Endoscopic Nontunneling with Tunneling Resection and a Systematic Review. Saudi J Gastroenterol 2017;23:52-9. [Crossref] [PubMed]
- Jain D, Desai A, Mahmood E, et al. Submucosal tunneling endoscopic resection of upper gastrointestinal tract tumors arising from muscularis propria. Ann Gastroenterol 2017;30:262-72. [PubMed]
- Loureiro MP, Almeida RA, Claus CM, et al. Laparoscopic resection of gastrointestinal stromaltumors (GIST). Arq Bras Cir Dig 2016;29:1-4. [Crossref] [PubMed]
- Nishida T, Blay JY, Hirota S, et al. The standard diagnosis, treatment, and follow-up of gastrointestinal stromal tumors based on guidelines. Gastric Cancer 2016;19:3-14. [Crossref] [PubMed]
- Tang X, Ren Y, Huang S, et al. Endoscopic Submucosal Tunnel Dissection for Upper Gastrointestinal Submucosal Tumors Originating from the Muscularis Propria Layer: A Single-Center Study. Gut Liver 2017;11:620-7. [Crossref] [PubMed]
- Zhou JQ, Tang XW, Ren YT, et al. Endoscopic submucosal tunnel dissection of upper gastrointestinal submucosal tumors: A comparative study of hook knife vs hybrid knife. World J Gastroenterol 2017;23:1843-50. [Crossref] [PubMed]
Cite this article as: Dzhantukhanova S, Starkov Y, Solodinina E, Zamolodchikov R. The smart approach to surgical treatment of submucosal tumors based on preoperative EUS-typing. Ann Laparosc Endosc Surg 2019;4:81.


