Update in parastomal hernia
Introductions
Parastomal hernias (PSHs) are a common complication whereby the contents of the abdomen are able to penetrate the abdominal wall via the opening of the stoma (1). It is estimated that this problem arises in 30–60% of created stomata, but the diagnosis approaches vary along with the definitions, and hence the incidence data are not necessarily consistent; where CT scans are employed, the recorded incidence rate can reach 70% (2). When the intra-abdominal pressure increases while the abdominal wall is weakened as a result of stoma creation, the outcome can often be fascial discontinuity. However, the risks vary along with the duration of stoma creation, the type of stoma involved, and factors related to the individual patient. It is also possible that the true rate of incidence may be higher than suggested here, since many cases may not be recognized, or could be misdiagnosed. From the patient’s perspective, PSHs can cause a deterioration in quality of life, while also leading to financial problems since the treatment is expensive (3). Treatments which do exist pose technical challenges in surgery, and lead to a high rate of recurrence. The PSH is thus a problem which will be commonly encountered be medical professionals as its incidence increases.
Incidence and risk factors
PSHs usually develop in the year immediately following the creation of the stoma, but in some cases have occurred twenty years later. In fact, the risk increases as the stoma is in existence for longer periods. It has been observed that the incidence rate one year after colostomy implementation stands at 30%, rising to 40% in the second year, and 50% by the third year. Eventually, at the twenty-year, the rate can reach as high as 76% (4-6). It is thus believed that the hernia risk may be significantly influenced by the ageing process in the form of musculoskeletal degeneration; as patients age, each year increases the risk of hernia by 4% [odds ratio (OR) −1.04; P=0.04] (7).
Examination of the literature also indicates that PSHs have the highest incidence rate in the case of end colostomy, with an incidence rate rising to 48.1% with a mean of 15.3%. In contrast, end ileostomy gives a rate up to 28.3% but a mean of 4%. Similarly, PSHs are more prevalent with loop colostomy than with loop ileostomy; the means are 4% and 1.3%, respectively. It is also assumed that the incidence of loop fashion ostoma would generally be lower than that of end fashion ostoma, while colostomy is more problematic than ileostomy (8-11). To determine this suspicion definitively would, however, require further studies with substantially larger sample sizes, so that the rates for different stoma types could be compared (12).
When considering risk factors, the age of the patient (over 60 years) is the only factor which has been deemed to be statistically significant. Many other factors have been proposed and studied in order to determine the nature of their influence, including obesity (BMI in excess of 30 kg/m2), waist size (in excess of 100 cm), ASA classification (greater than II), smoking, diabetes, the effects of physical labour, chronic cough, systemic infections, poor tensile strength of the abdominal wall, COPD (chronic obstructive pulmonary disease), immune disorders, steroid therapy, cancer, ischemia, Crohn’s disease, disorders involving collagen metabolism, or previous history of hernias. However, none of these have proved to be statistically significantly linked to PSHs, and in general there have been few studies capable of providing reliable data (4,7,13-16).
It is also the case that aspects of the surgical procedure can influence the risk of PSHs. It has been difficult to provide direct proof through statistical significance which would indicate that such factors affect the incidence of PSH, but it can be argued that the urgency of the surgery, the experience of the surgeon, the pre-operative marking of the stoma site, and the occurrence of prior PSH surgery might all be influential (15-17). The method used to create the stoma can also affect subsequent hernia development; the extra-peritoneal and transperitoneal stoma creation techniques have been compared, while the lateral para-rectus location has been compared to a transrectus location. Reports stemming from a meta-analysis of randomized and non-randomized controlled trials suggest that the incidence of PSH is lower with the extra-peritoneal technique, with a relative risk factor of 0.36 (95% CI: 0.21–0.62), thus confirming earlier meta-analysis of observational studies but not advocated by Cochrane review (18-20). It is still, however, necessary to conduct further studies using larger sample sizes and with longer-term follow-up in order to establish whether or not the extra-peritoneal approach may offer an effective means of preventing the incidence of PSHs. Sjodahl et al. (21) advised that the positioning of the stoma through the rectus sheath may have the effect of lowering the risk of PSH, whereas according to Stephenson et al., the lateral rectus abdominis positioned stoma (LRAPS) might also prove effective in risk reduction (22). This particular stoma technique offers minimal disruption of the anterior abdominal wall, and resulted in no cases of PSH at the 14-month check-up using CT scan evidence in a small sample group of patients. Meanwhile, in the PATRASTOM trial, a pilot single-centre randomized trial was conducted involving 56 patients undergoing loop ileostomy creation; in this study there was no significant difference found between the LRAPS and transrectal methods in terms of PSH development (23). Furthermore, later work in 2013, which included both systematic reviews and the Cochrane review, also found no satisfactory evidence linking PSHs to the position of the stoma with regard to the rectus sheath (1,24).
In the case of alternative surgical approaches, including attachment of the fixing sutures to the fascia, or closing the lateral extra-intestinal space of the stoma, little evidence was found to link the approach to the development of PSHs. A number of case studies have suggested, however, that where the stoma orifice size exceeds 3 cm, the PSH risk may be increased (1,17,25). Multi-factor analysis studies have also indicated this notion, reporting that for every extra millimeter, the hernia risk is increased by 10% (OR −1.1; P=0.005) (16).
Furthermore, it has also been suggested that laparoscopic surgeries pose a higher risk of hernia development than open surgery, although the large number of variables involved make it difficult to attribute any particular outcome to a single specific factor. One study did compare open surgery with laparoscopic surgery over a ten-year period involving 148 low rectal cancer patients. Analysis of the subgroups reveled that although no statistically significant differences were found between the demographic and clinical factors of the open surgery and laparoscopic groups, the laparoscopic group showed greater incidence of PSH during the first and second years after the operation (26). Higher incidence of PSH should be considered as potential disadvantage of minimally invasive approach to patients with low rectal cancer or lead to consider method to prophylaxis.
Presentations
It is common for PSH patients to experience bulging when straining or coughing. When bulging occurs, the impact upon quality of life may vary between individuals. Usually, however, the principal complaint is of discomfort, while further issues may arise with stomal appliances, whereby the skin becomes irritated and leakage occurs. In cases where the bulge is very large, an unsightly lack of symmetry can be caused in the body of the patient, which will be more strongly perceived by the patient than even the original feeling of the stoma. Both the bulge and the stoma are likely to change shape and also size over time, forcing patients to adapt to the situation, which many choose to do instead of reporting the matter to their doctor. Psychosocial problems can, however, be experienced by some patients even when the bulge is rather small and shows few symptoms. It is still possible that the patient feels inadequate in terms of appearance or in having their activities restricted (27-31). In general, only around 30–40% of PSH patients report their symptoms, and hence only around one third receive treatment in the form of surgery. The technical challenges are usually in the form of non-virgin abdomen surgery, although further complications stem from the attitudes of patients towards surgery, along with the disease status or physiology of the patient (32).
Complications such as PSHs with incarceration or strangulation generally occur late if they occur at all, and are quite rare. Obstruction is an early problem, it can then cause strangulation and lead to an urgent need for emergency care. Diagnostic need high index of suspicious, investigation such as CT, or dynamic ultrasonography should be performed in case of unexplained abdominal pain associated with existing PSH (33,34). Problems with the stomach, leading to gastric outlet obstructions, are much rarer, although the in literature it was reported that elderly female colostomy patients may experience gastric herniation (35). One very rare complication is parastomal evisceration. Reports on very few cases have been published to date, with the majority focusing on ileostomy and the evisceration of the small bowel. This resulted in parastomal evisceration developing of its own accord at the edge of the end stoma, and this can be linked directly to a sharp rise in intra-abdominal pressure as well as coughing. This condition requires urgent surgical intervention (36,37).
Diagnosis and examinations
It is possible to make the clinical diagnosis if the patient presents visible symptoms of bulging, or signs of bulging which become apparent upon palpation of the ostomy site if the patient performs a Valsalva manoeuvre. In the case of PSHs, however, it is not always simple to provide the diagnosis due to the subjective nature of the symptoms; it can be hard to observe or palpate the bulge, especially in obese patients, and there is also the fact that many patients also have other signs to complicate the issue, such as laparotomy incisions, co-existing hernias, scar contraction, pain, or relaxed abdominal walls resulting from degenerative processes (38). Physical examinations provide sensitivity rates of 66–94% in the detection of PSHs, while rates for specificity can reach 100%. Incidentally, the negative predictive values for negative physical examinations are relatively high, at 63–96% (5,13).
Several studies have concluded that there are benefits to be drawn from computed tomography scans of the abdomen, while CT scans are also helpful due to their ability to identify occult hernias which cannot be readily discovered by physical examination. Furthermore, radiographic testing methods can also identify simultaneous incisional hernias as well as clarifying the anatomy of the abdomen. An increasing number of incisional hernias and occult hernias are now being discovered by CT scans having been missed by earlier clinical examinations. The rate stands at 35% but could be as high as 53–78% according to some studies (1). Estimates of these rates are made on the supine position of abdominal computed tomography scans, which may fail to detect genuine cases in around 7% of patients, so clinical correlation may be unreliable, especially in cases where the CT scan was performed without having first obtained clinical diagnosis of the hernia. Another reason for the lack of accuracy is the absence of any standardized protocol; indeed, studies of re-reported CT have shown that as many as half of all hernias reported in CT scans had not been reported in the original examination (39,40).
Detection rates can possibly be enhanced by using the Valsalva manoeuvre or placing the patient in a semi-prone position, but the evidence has not yet been gathered to determine definitively whether this is the case. Differences in reported observations from radiologists and surgeons show high correlation in the prone position with a Kappa value of 0.80. For CT scans in the prone position, the sensitivity and specificity are not yet known, however (41).
Alternatively, there are reports which argue that dynamic intrastromal 3D ultrasonography carried out without radiation exposed and in the supine position would be an effective an appropriate method. Various research studies have made the claim that 3D intrastromal ultrasonography offers a viable alternative to a CT scan since it can provide a superior positive likelihood ratio when compared to the CT scan, while the predictive values for the detection of PSHs are also favourable. These studies are, however, few in number. One particular benefit of performing ultrasonography is that it can be carried out at simultaneously with the clinical examination and hence it is possible to immediately match the outcomes with the symptoms which are presented. The procedure is not easy to perform, required operator’s skill set and may be uncomfortable for the patient. Moreover the study can be performed only when the opening of the stoma exceeds 17 mm (42-44).
To conclude, it can be hard to provide a conclusive diagnosis of PSH for some patients, since physical examinations can lack reliability, and CT scans may also be inconclusive, providing unknown specificity. In cases where symptoms are presented, however, CT scan may prove helpful in enhancing rates of detection, providing evaluation for concurrent incisional hernia, and assessing abdominal wall details while simultaneously excluding issues related to primary disease recurrence. This would permit the best treatment plan to be developed to repair the hernia (45).
Classifications
Five different categories have been determined for PSHs, which are listed as follows.
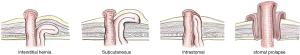
Rubin & Devlin [1973]: Rubin et al. classified as type I—true PSH (Ia: interstitial, Ib: subcutaneous); II—intra-stomal; III—subcutaneous prolapse; IV—pseudo hernia. Devlin et al. classified as type I—interstitial hernia; II—subcutaneous; III—intra-stomal; IV—peristomal hernia (stomal prolapse) (46). Samples of this classification demonstrate in Figure 1. Therefore, those classification were included both true and pseudo hernias.
According to Moreno-Matias [2009] and Seo [2011], on the basis of the radiological results, the following categories can be identified:
0—CT image normal, in this case the peritoneum follows the bowel wall to form the stoma without a sac;
Ia—bowel forming the colostomy with a sac smaller than 5 cm;
Ib—bowel forming the colostomy with a sac larger than 5 cm;
II—sac containing omentum;
III—sac containing an intestinal loop which is not the bowel which forms the stoma (47,48).
According to Gil [2011], on the basis of a physical examination of the patient, the following categories can be identified:
I—small and isolated PSH;
II—small PSH with coexisting midline incisional hernia with no deformation of the front wall of the abdomen;
III—isolated, large PSH with deformation of the front wall of the abdomen;
IV—large PSH with coexisting midline incisional hernia, with deformation of the front wall of the abdomen (49).
The European Hernia Society (EHS) classification of PSH [2014]: derived from radiological results and the clinical examination of the patient.
I—less than 5 cm in diameter with no coexisting incisional hernia;
II—less than 5 cm in diameter and including coexisting incisional hernia;
III—greater than 5 cm in diameter with no coexisting incisional hernia;
IV—greater than 5 cm in diameter and including coexisting incisional hernia.
Additionally, it should be explained in each scenario whether the hernia is recurrent or primary (50).
At present, there has been no validation of any of these various classifications and the evidence available is insufficient to support any particular one of those listed. The classification provided by Rubin and Devlin [1973] is not especially helpful in the clinical context. The classification of Moreno-Matias finds applications solely for PSHs in colostomy cases following radiological diagnosis (47). Meanwhile, the classification of Gil appears to be the preferred version for the practical context since it combines incisional hernias and PSH size with a physical patient examination. Therefore, the EHS has adapted this classification as their preferred version. The drawback is that none of the options has been widely used due to the lack of influence upon clinical practice. It has, however, been suggested that this version accepted by the EHS should be used to standardize research reporting on PSHs in the future, thus clarifying definitions and making it easier to validate future studies with consistent data (45).
Managements
Watch and wait
The widely used approach today is to watch and wait, given that only around one third of all PSH patients undergo surgery (8). There are several reasons why the remainders are not treated: there is often no clinical diagnosis, or in some cases the problem shows no symptoms. Some patients fail to report their symptoms, while others are already suffering from other conditions. The high recurrence rate deters some patients, while inexperience among surgeons and reluctance among patients can lead to surgery not being performed. Few studies have examined the outcomes and safety record of non-operative methods of managing the condition. Furthermore it has also been difficult to accurately track the progression of the condition over time along with sizes and symptoms, while the increasing risk of strangulation or increased risk of complications prior to surgical treatment have also not been determined and hence little exists in terms of statistical analysis.
In cases where patients exhibit symptoms of non-resolving bowel obstruction or indications of intestinal ischemia, the physician should recommend emergency surgery. A number of research studies have revealed that the risks of recurrence, morbidity, and mortality are higher following the operation in cases where emergency repair was carried out: among the risk factors for recurrence or death, multivariate analyses identified emergency repair as the most significant (OR, 7.6; 95% CI: 2.7–21.5) (51,52). Where patients were aged over 70, this also correlated with increased morbidity following emergency repair interventions (51). In the case of elective surgery, it has been difficult to find evidence that repairing a PSH can improve the patient’s quality of life or relieve the symptoms, especially when compared to the alternative of professional stomal care by a trained nurse. However, one recent study carried out in 2017 reported that in 93% of cases, the overall symptoms were relieved during the six months following surgery, although little benefit was observed in terms of leakage or skin irritation (31). At present it is difficult to determine the point at which surgery becomes necessary because it is hard to find adequate data to describe the extent to which quality of life is affected and to determine the level of the symptom burden. In these circumstances, it is necessary to consider the costs or risks of surgery and weigh these against the benefits of quality of life improvements when discussing the possibility of surgery with patients.
The options for care which do not involve surgery when addressing PSHs are listed by the Association of Stoma Care Nurses. This organization also offers guidance on the use of skin sealants, other stoma appliances, adhesive adjuncts, and also support garments, all of which can lead to an improved quality of life (53,54). It is thought that support garments might relieve the symptoms while simultaneously lowering the possibility of hernia enlargement and strangulation, but clinical evidence to support this notion is not readily available. One study performed in 2018 using a retrospective cohort across multiple centres revealed that this approach might be most suitable for patients suffering only mild symptoms or those patients with comorbidities. Among the group undergoing non-operative treatments, only 2.6% subsequently required emergency surgery as a consequence of hernia problems arising within 48 months during follow-up checks (55).
The EHS guidelines published in 2018 took the position that when no evidence is available to aid the decision, regarding the treatment of PSHs, then the policy of watching and waiting was one which could not invite any specific recommendation (45). In an asymptomatic patient with no risk for strangulation, conservative management with regular monitoring should be strongly considered.
Indications for surgery
In the case of incarceration incisional hernia, one risk factor is the size of the hernia opening, but for PSHs this relationship has not yet been studied in depth (56). Indications for surgery are instead focused predominantly on the prevailing symptoms and the quality of life of the patient. The different indications for emergency surgery and elective surgery are thus presented in Table 1 (1,10,15).
Table 1
| Indication |
| Indication in emergency condition |
| Strangulation |
| Obstruction |
| Perforation |
| Incarceration which fails to conservative |
| Somal ischaemia |
| Indication in elective condition |
| History of incarceration or obstruction |
| Present of parastomal fistulae |
| Difficulty in maintaining the collection device |
| Difficulty in visually control and treat the stoma |
| Problems with irrigation |
| Hernia-related pain with fail medication treatment |
| Erosion of the surrounding skin |
| Prolapse with difficult to reduce |
| Inability to accept the stoma aesthetically |
| Contraindications |
| Absolute contraindications for elective surgery |
| Terminal malignant disease |
| Relative contraindications for elective surgery |
| Unresectable or metastatic cancer |
| Serious comorbidity |
| Scheduled temporary stoma closure |
| Patient disagree with procedure with clear understanding of prognosis of disease |
It is important to balance the risks and benefits of the procedure, since there is a chance of morbidity after the operation, the symptoms may not all be relieved, and there exists a high rate of recurrence. These facts must be discussed with patients in order to manage expectations.
Preparation for surgery
First of all, it is compulsory to conduct the pre-operative assessment and to check all of the comorbidities of the patient, but in the case of PSH repair, this is by definition invariably a second or third procedure, and hence for some patients the complications and process itself can be unpredictable. For this reason, it is necessary to prepare the patient for the possibility that the surgery takes much longer than anticipated. Even laparoscopic procedures can overrun due to this unpredictability. The previous operative history of the patient should be examined carefully, especially where cancer is involved because of the frequency with which recurrence takes place. The stoma creation etiology and purpose must also be taken into account. For instance it can sometimes be difficult to relocate urostomies, while in the case of temporary ostomy, it is preferable to reverse the stoma if this proves possible, rather than to choose the option of repairing the PSH (15,45,57). Such decisions can be guided by the use of preoperative tomography scans. Where elective repairs are carried out, it is advised that at least three months should have passed since the previous intra-abdominal surgery (45,57). Patients who smoke must stop at least 4 weeks before their operation, and where patients are obese, defined as BMI >30, they should be instructed to lose weight. Cardiopulmonary exercise testing (CPET) is useful prior to surgery in order to assess the patient’s level of cardiorespiratory fitness. All of these described points have been widely used in the case of ventral hernia repairs with the objective of producing superior outcomes where abdominal wall hernia surgery is performed; however, no specific guidelines have focused directly upon PSHs (58,59). Antibiotics should be administered prior to surgery to address the problem of enteric bacteria, and medication should also be provided to counter the threat of deep vein thrombosis. Under general anaesthetic, the patient should be positioned supine with the arms beside the body. To monitor urine levels, a Foley catheter can be employed, and it would be sound practice to place a sterilized catheter within the stoma, since this can support the identification of the bowel loop during the procedure. The stoma should then be covered using an adhesive sterile drape in addition to the drapes which are normally used to cover all other skin surfaces.
Surgical techniques and outcomes
The literature describes a number of approaches for the surgical repair of PSHs, each with its own advantages and disadvantages.
Suture repair
A repair technique involving suturing was first described in 1965 by Thorlakson (60). In this procedure, an incision is made around the stoma at a distance of around 5 cm. The sac is then identified, and suturing performed to approximate both fascial edges is carried out under tension, with the aim of avoiding hernia recurrence. However, the recurrence rates in different studies varied from 46% up to 100%. In a systematic review it was shown that this technique leads to a greater risk of PSH recurrence than mesh repair (OR 8.9, 95% CI: 5.2–15.1, P<0.0001). In the review, the recurrence rates for suture patients recorded in the review stood at 96.4% (61). Since the failure rate is so high, the approach is not suitable for elective surgery, but in emergency cases where the circumstances preclude other option of repairing or patient condition are not appropriate to complex procedure, suturing can be considered, especially if it is in the context of a temporary measure before a definitive repair can be carried out if the patient’s general condition improves.
Relocation
In this procedure, an incision is made at the midline of the abdomen before the creation of a new stoma to allow an open approach. This can cause an incisional hernia at the midline, and can also heighten the risk of developing a hernia in the location of the old stoma, or a PSH at the location of the new stoma. It has been shown that when relocation is performed, the incidence of recurrence at the new stomal site was 76%, while the rate of recurrence at the old stoma site was reported to be around 52% (10,62). It would be advisable to select relocation for patients whose original stoma was not correctly created and whose stoma location was not suitable. Furthermore, the area for the new stoma should be selected with great care to limit the risk of recurrence; this should be discussed with patients prior to surgery.
In studies of relocation, if the relocations were simply made to the opposite sides, and placed through the rectus muscle with the use of prosthetic devices, the recurrence rate would be significantly lowered (21,63,64). The authors of this paper suggest that synthetic non-absorbent materials be used for covering the new stoma site, the old stoma site, and the midline incision in order to prevent hernias.
Nowadays laparoscopy is now a commonly approach in experience laparoscopic surgeons. With the use of laparoscopic approach together with mesh reinforcement can reduce postoperative pain, lower perioperative morbidity, intra-abdominal adhesion, and the development of midline incisional hernias (65,66).
Mesh repair
The mesh repair technique for PSH repair leads to a statistically significantly lower incidence rate for recurrence than would be the case without the mesh. Estimably, without mesh, the recurrence rate has been shown to be almost nine times higher (51,67). Hopkins and Trento (67) were the first to publish findings on the use of mesh for repairs since 1982, but in that case the procedure resulted in intestinal erosions and infections. Because of this, the technique found little use, and it was only when a modern mesh was developed that the approach became successful (67,68). However there are still controversial in the details of procedures as mentions in following.
The types of meshes
Different mesh types are available, such as the non-absorbable meshes made from polypropylene, polyester, or synthetic expanded polytetrafluoroethylene (ePTFE) which is the most commonly employed type. Few studies have sought to make comparisons between the various types and hence little guidance is available to inform mesh selection, although it is clear that different meshes offer different properties and characteristics. Despite using of non-absorbable mesh intra-abdomen, there is a heightened risk of postoperative mesh infection, and intestines frequently suffer erosion. With the widespread use of ePTFE, the shrinkage percentage has been reported be as high as 50% (69,70). To reduce the possibility of intestinal erosion and for better results in preventing contamination, biological mesh may be another option, but the cost is much higher. Studies have indicated that the recurrence rate is around 31% when using biological mesh, but concerns over bacterial contamination suggest this problem can adversely affect the tensile strength of the mesh, leading to its ultimate mechanical failure (71,72). Biological mesh is thus rarely used, since it is not easy to obtain, it is expensive, and the results over the longer term fail to justify these costs because there is no significant improvement when compared to synthetic mesh products (73,74). Since it is understood that an uncoated synthetic mesh is wholly inappropriate for intraperitoneal use because of intestinal erosion and adhesion, composite meshes have been developed to place intraperitoneally which reduce these risks by soluble partially material, and hence these are suitable for PSH repairs in intraperitoneal onlay mesh (IPOM) style (75). There are several composite meshes which are commercially available today. The best known is Parietex™ Composite mesh which is a composite coated polyester mesh. A popular alternative is polyvinylidene fluoride mesh (PVDF, DynaMesh®-IPOM). In one prospective observational study involving 344 participants undergoing PSH repair using PVDF mesh, the recurrence rate was a satisfactory 2.1%, and in the 20 months following the procedure, no complications were reported which could be attributed to the mesh (76). In contrast, retrospective cohort research involving ventral hernia repair showed intestinal obstruction secondary to adhesions occurring more frequently with PVDF than when Parietex mesh was employed (77). It would thus be necessary to use the same surgical method, standardize the research approach while taking in long-term outcomes, and make comparisons between all the different composite mesh types in order to find the best option for PSH repair using composite mesh (45,70). More recent findings suggest that when infection occurs, the most readily removable mesh would be the uncoated macroporous polypropylene light or medium-weight mesh, and may be the “most salvageable mesh” in case of mesh infection and this type of mesh may resist to bacterial contamination (78). The latest ideas recommend the use of the natural structure of the abdominal layer to protect this non-absorbable mesh type from the intra-abdominal contents when repairing parastomal or ventral hernias. The technique would be mention in the following.
The abdominal wall layers for placing meshes
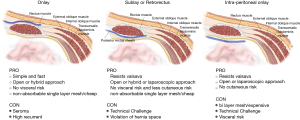
In the placement of mesh, the method used and the layers involved can also have an influence upon the rate of recurrence and the existence of complications. No studies have yet provided a suitable statistical analysis of the different techniques which are presented in Figure 2.
Open mesh repairs
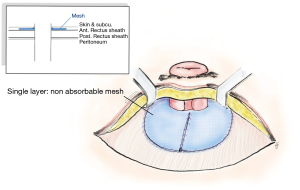
Onlay mesh repair, which requires the skin to be opened in L shape or semicircular incision along the lateral edge of stoma which no adhesiolysis needed. There are different ways to place the mesh, such as a keyhole technique, or the “stove pipe hat” technique, where the mesh sits over the fascial repair before the stoma is drawn through the middle of the mesh to achieve a 360-degree repair. A further piece of mesh is then attached to the circumference of the bowel and on to the onlay mesh (Figure 3). At the 48-month follow-up, the recurrence rate in one study was shown to be up to 25.9% (79-81). Meanwhile, meta-analysis revealed that figure to be 18.6% of recurrent after twelve months, while 1.9% of cases exhibited infections of the wound and 2.6% had mesh infections (61,82-84).
Retromuscular style, whereby the mesh is positioned beneath the rectus abdominis muscle. It is usually positioned using a keyhole or Sugarbaker fashion, and can also be performed using midline incision or parastomal incision. The concomitant midline hernias could be repair or stoma relocation may also be performed. Several techniques have been used, and the recurrence rate is around 28%, although the average pooled recurrence rate is not as high, at around 6.9%, while the incidence rate for wound infections was 4.8% in the absence of mesh infections (61,85-87). A modification of this approach is known as transverse abdominis release (TAR), whereby the retromuscular mesh is position using a modified Sugarbaker after conducting of PCS. In open approach showed good results in the reports but it was not possible to find any statistically significant difference in the recurrence rate when compared to keyhole techniques (88,89). By positioning the mesh in this particular layer, the intraperitoneal mesh placement can be avoided, which in turn permits the use of medium weight mesh polypropylene. This is cheaper, and also offers a lower infection rate. However, a rate of 8% was recorded for the erosion of the mesh. In order to perform, the retromuscular procedure demands detailed knowledge of the anatomy of the abdomen so as to avoid causing damage to the nerves and blood vessels in the abdomen wall. Muscle atrophy or bleeding is another potential danger if the surgeon lacks experience. Finally the risk of erosion by using polypropylene mesh still need long term result to confirm real benefit of this approach.
Intraperitoneal placement makes inflection less likely, as there is no need for dissection of muscle. Among the different mesh placement techniques, such as the Sugarbaker technique (1985, Sugarbaker), and the keyhole technique, the intraperitoneal approach is particularly interesting and will be discussed further. It involves an adhesiolysis if surgery was either laparoscopic or open, prior to placing the mesh in position. This leads to a greater probability of intestinal injury being caused (90,91).
A systematic review in 2014, concluded insufficient evidence to determine which mesh technique (onlay, sublay or intraperitoneal) is most successful in terms of recurrence rates and morbidity. The overall recurrent rate at 7.9–14.8% compare to the pooled rate of suture repair was 57.6% (92). Lately, in a systematic review evaluating the differences between the open approach with onlay, retromuscular, intraperitoneal Sugarbaker, and intraperitoneal keyhole methods, the findings confirmed that the lowest recurrence rate was for retromuscular recurrence, at 6.9%, while Sugarbaker (11.6%), and open onlay (17.2%) performed reasonably. The worst rate was for the intraperitoneal keyhole approach (34.6%) (61).
Selecting the right mesh, position and the right technique involves consideration of numerous factors. In the contaminated cases, biological mesh is still the popular choice, but it is very expensive and can lead to a high recurrence rate, so in such scenarios, medium-weight or light-weight, large-pore, polypropylene mesh placing in retro muscular fashion is effective and satisfactory regarding infection and recurrence for PSH repairs where there is a threat of contamination (86,87).
Laparoscopic repairs
For the treatment of PSH, various laparoscopic techniques have been proposed as an alternative approach to the traditional open repair. These techniques can involve either retromuscular mesh placement or intraperitoneal mesh placement (Lap IPOM), performed using any of the keyhole, Sugarbaker, or sandwich approaches. To date, however, very little research has been conducted to examine the merits of laparoscopic surgery in comparison to open repair for PSH. Upon examination of the data for 2,000 patients taken from the database of the American College of Surgeons’ National Quality Improvement Program, it was possible for the authors to present a comparison of the two approaches to treatment. In the laparoscopic treatment group, the chance of morbidity was reduced by around 60% (OR 0.42, 95% CI: 0.27–0.64) while the time taken for surgery was cut by around 13 minutes on average (mean difference −13.24, 95% CI: −24 to −3) (93). Hansson et al. carried out a systematic review of a series of cases which included a number of different PSH repair methods. Analysis was then conducted using logistic regression in order to make the comparisons between the laparoscopic and open techniques. It was found that the laparoscopic methods lead to a reduced level of recurrence and are as effective as open intraperitoneal and open retromuscular repair without significant difference in the incidence of wound infection at 3.3%, while the rate of mesh infection is 2.7% and morbidity 17.2% (61). Numerous small case series have been reported but they have selection bias and lack standardization in terms of the techniques and procedures followed. However, these studies do indicate the safety of the laparoscopic approach and confirm that complications associated with the wounds are avoided. Pain is reduced, as is morbidity, decrease hospital stay and can return to work much sooner. The greatest benefit of laparoscopic surgery, however, is that concurrent or hidden incisional hernias can also be repaired during the course of the operation (94,95). However, the latest 2018 EHS recommendations for PSH repair state that it is not possible to indicate a preference for laparoscopic or open repair with mesh for elective surgery (45). The laparoscopic intraperitoneal approach does bring with it concerns of the risk of intra-abdominal injury, with iatrogenic bowel injuries recorded in up to 4.1% of all cases, while the conversion rate is around 3.6%. While the incidence of iatrogenic injury causing peritonitis or the need for re-operation is very low without increasing of mortality (61,94,96,97).
Laparoscopic intraperitoneal only mesh: keyhole technique
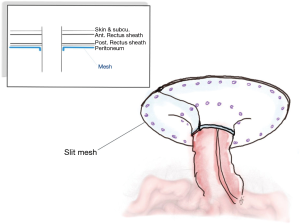
Hansson was the first to propose the keyhole approach (97). The general laparoscopic method commenced with entry to the abdomen via the contralateral side to the stoma, employing the technique corresponding to the personal preference of the surgeon. However, the workings ports should be away from the stoma. A careful adhesiolysis was performed to clarify the stoma and a clear area 5 cm away from all of the defects on the abdominal wall. If it is suspected that an injury to the bowel might have occurred, it is essential to perform the repair immediately before continuing with the surgical procedure. One piece of bilayer mesh is cut in the center to form a slit, whether with or without fascial closure, and this forms a hole of size up to 2 cm which can house the stoma in the center. The tails of the mesh are then wrapped around the stoma in order to create the keyhole shape, and are fixed in position using sutures or tacks (Figure 4). Finally, the release of the pneumoperitoneum and closure of the port sites completes the process (89-99). The approach is not suitable in the case of ileostomy where the small intestine can all too readily slide through the opening. The risk of recurrence increases if the mesh sinks and there is a wider opening.
Laparoscopic intraperitoneal only mesh: modified Sugarbaker
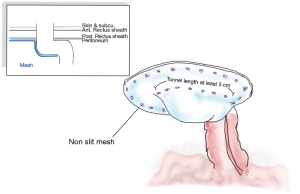
Voitk was the first to present a laparoscopic approach combined with a Sugarbaker technique, in which the non-slit bilayer or coated-non absorbable meshes were employed in covering the stoma and also the hernia (100).
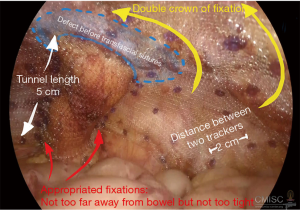
Following the general laparoscopic procedure, reduction of the hernia was carried out prior to covering with a mesh prosthetic over the fascial defect, while the mesh is centered over the site of the stoma. The mesh should then be extended no less than 5 cm. past the defect edge, and able to provide cover for the lateralized bowel up to the abdominal wall. It is possible to achieve sufficient length for the lateralized colon by forming a tunnel section with length of at least 5 cm. for the bowel prior to its entry to the enterocutaneous junction. It is possible to fix the mesh using only tacks, or by employing tacks along with the surgeon’s preferred style of extracorporal or intracorporal sutures. The main step in this process, however, is fixing the mesh where the bowel is entered between the mesh and the abdominal wall. This should be fixed using a suitable method to stop any possibility of hernia of the bowel into the mesh. Mesh fixation must be performed which duration between each tracker should be around 2 cm. away from each other and double crown fashion should be employed as shows in Figures 5 and 6. Proper fixation with great care to avoid injuries, prevent stenosis, and reduce the risk of bowel angulation (90,99,101-103).
Delays in the function of the bowels following surgery are reported more frequently with the Sugarbaker technique, although this issue can be controlled to some extent by diet management and medication. The flap which results when the non-slit mesh is implanted can stop PSH formation near the bowel, and achieves adequate hernia recurrence rates (101,103,104). One further shortcoming with this technique lies in the lateral defects of the hernia, where the rate of recurrence is notably increased, possibly as a consequence of the lateralization of the stoma loop, which is normally performed in any Sugarbaker repair. It can be inferred that the stoma loop which lies beneath the mesh covers the lateral defect, which is thus not stabilized and hence can grow laterally, causing the problem of recurrence.
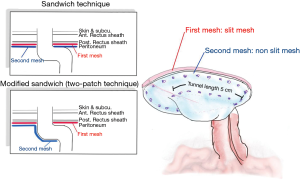
Laparoscopic intraperitoneal only mesh: sandwich or two-patch technique
Since the keyhole approach leads to a high rate of recurrence, LeBlanc and Bellanger reported on a Sandwich technique using a pair of patches of slit meshes to cover the opening to the stoma by placing the second mesh patch in the opposite direction to the first mesh patch. For this report, DualMesh Plus™ was employed, and the positioning of the slit was selected in order to cover the defect which had been created in the previous mesh. By combining two layers, the abdominal wall would be strengthened to a greater extent than if a single prosthesis were used. It was expected that using thicker material would improve the colostomy appliance fitting and would minimize any movement of the intestine on the sides of the repair site. Findings for a small case series report indicated 25% morbidity, 8% recurrence, and 8% mortality (105,106). Meanwhile, Berger et al. presented a modification of the sandwich technique or two-patch technique, combining the Sugarbaker and keyhole methods and employing a pair of mesh pieces. The first piece of mesh, measuring 15 cm × 15 cm is positioned as in the keyhole technique, with a hole at the center up to 1.5 cm in size. This first piece of mesh was then wrapped around the stoma loop in order to cover the fascial gap, while the parts of the mesh which were incised were then closed medially using spiral tacks and two transfascial sutures. These tacks were also used to fix the mesh in place. Another mesh which featured non-absorbable stay sutures at each corner was used to cover the first mesh as well as the whole of the abdominal wall. The stoma loop was then positioned between the two meshes in order to create the necessary lateralization of no less than 5 cm, since this tunnel length allowed the extraperitoneal parietal positioning of the bowel’s distal segment. Fixing is best carried out using both spiral tacks and transfascial sutures (Figure 7). During one study involving 66 patients, a 12% recurrence rate was reported, but notably with no recurrence at all in the 25 patients who used the PVDF mesh type instant of ePTFE (104). Subsequently, at the 20-month follow-up stage, a recurrence rate of 2% was reported along with 3 cases of wound infection in 47 patients with PVDF mesh employed (76). The use of two mesh layers ensured that any case of lateral hernia defect would be stabilized by the first mesh layer, prior to placement overlying the bowel loop where the mesh would again improve stabilization and the restoration of abdominal wall contours. However, in two cases obstruction of the stoma was reported which required surgery. In these cases, the stoma loop had a subcutaneous section which was very long and formed a lateral siphon-like curve. When the intra-abdominal section of the stoma loop was fixed between the meshes, this caused the bowel loop to be squeezed and resulted in a stenosis, which is a problem to be avoided wherever possible.
Very few meta-analyses of systematic reviews have examined the outcomes of laparoscopic PSH repair. The existing studies were not randomized, while a majority took the form of retrospective case series using a variety of materials for prosthetics, and a variety of surgical approaches (61,94). One report from 2015 involving 469 patients whose treatment was laparoscopic IPOM for PSH repair reported a morbidity rate after surgery of 1.8% (95% CI: 0.8–3.2), while no differences were reported between the different techniques used. Among the complications after surgery, the most frequent was infection of the operation site, at 3.8% of all cases (95% CI: 2.3–5.7). Infection of the mesh was recorded in 1.7% of all cases (95% CI: 0.7–3.1), while obstructions which required surgery were also seen in 1.7% of all cases (95% CI: 0.7–3.0). In 16.6% of cases, further complications including ileus, pneumonia, and infections of the urinary tract were found (95% CI: 11.9–22.1). After one year, the rate of recurrence was reported to be 17.4% (95% CI: 9.5–26.9). Taking all repairs into consideration, a conversion rate of 3.1%, predominantly due to bowel perforation or the existence of dense adhesions (94).
For the modified laparoscopic Sugarbaker method, the rate of recurrence was found to be 10.2% (95% CI: 3.9–19.0), while for the keyhole approach, the rate of recurrence rate was 27.9% (95% CI: 12.3–46.9). Meta-analysis showed that in the case of the pooled recurrence rates, the laparoscopic Sugarbaker technique revealed significantly lower values than was the case for the laparoscopic keyhole technique. However, in the case of the sandwich technique, since there was very low recurrent rate (2–8%) but there were only few case-series reported, the technique was not used as a part of this comparison (76,94,106). The findings generally confirm those of the earlier meta-analyses comparing the two different laparoscopic approaches. The Sugarbaker technique produced a significantly lower rate of recurrence when comparisons are made with the keyhole approach (OR 2.3, 95% CI: 1.2–4.6; P=0.016) (61).
Fascial closure (IPOM Plus)
During the last ten years, the IPOM procedure has been adjusted to include fascial closure of hernia defects. There were some evidence-base studies in ventral hernia repair with laparoscopic fascial defect closure is carried out with IPOM reinforcement (IPOM Plus) for ventral hernia repair, the surgical outcomes are enhanced, with dead space removed, can prevents the incidence of a seroma or mesh bulging. Through the mechanism which controls mesh bulging, the central section of the abdominal wall which is non-functioning can extend into the hernia sac as a consequence of intra-abdominal pressure. This is described by Laplace’s law. The bulging can be uncomfortable for the patient, and can easily be mistaken for recurrence. As the intra-abdominal pressure increases, the shear forces upon the mesh are no longer present, hence the sutures which fix the mesh will not be cut or broken. Such damage to the sutures would potentially lead to mesh migration and eventually recurrence, which can be defined as the presence of a gap between the edge of the hernia and the musculofascial tissue. Fascial closure can also assist in restoring the rigidity or functionality of the abdominal wall, leading to better functional outcomes. The IEHS guidelines recommend that in order to prevent seroma, the hernia sac should be included during fascial closure to remove dead space, since this might be effective when making repairs to a ventral or incisional hernia (45,107).
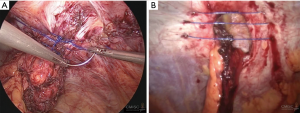
In the case of PSH repair, however, no similar recommendations were made, and while there have been detailed reports published to describe fascial defect closure and mesh handling, no standardization of the techniques involved has been established. Few case series have covered the possibility of providing suture reinforcement as a means of closing defects in PSH repair when using keyhole (108), Sugarbaker (109), or Sandwich-plus techniques (110). These authors also carried out a non-randomized trial in order to draw comparisons between defect closure and non-defect closure in performing laparoscopic modified Sugarbaker PSH repair. The findings indicate that defect closure would provide significantly lower incidence of seroma after surgery when compared to the non-closure sample group (12.7% vs. 37.5%, P value =0.002). No differences in pain levels were reported between the two groups after surgery.
While the data in the literature review have not been fully consistent, and number of unique suture methods have been described, but without any definitive conclusion as to which might be the most suitable. Accordingly, these authors advise that the defect be closed, and subsequently reinforced using mesh. It is recommended to use non-absorbable sutures, with the ideal approach involving intracorporeal suturing using V-Loc™ sutures 1/0 spacing the stitches at 1.5 cm, both between stitches and from stitches to the margin. Alternatively, an extracorporeal technique using Endoclose™ can be employed, involving small stab skin incisions and non-absorbable suture threads from 0–2. The spacing in this case should be 1 cm (Figure 8). When using these techniques, care must be taken to avoid stomal and mesentery injuries.
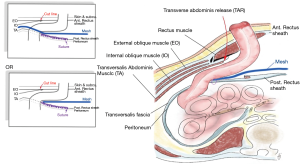
Laparoscopic transversus abdominis release (TAR) and modified retrorectus Sugarbaker
The separation of posterior components through the TAR method formed a large retrorectus space, which enabled the positioning of the mesh inside this space while not coming into contact with the intestine. Pauli et al. described open TAR combined with Sugarbaker repair (88), while more recently there have been reports using robotic approaches and laparoscopic techniques (111).
Electrocautery is used to start the process by making the first incision to the posterior rectus sheath around 0.5–1 cm lateral to the linea alba. The incision is then lengthened from the cephalad to caudal direction, covering the entire length of the rectus muscle. Blunt dissection is then used to continue the plane laterally towards the linea semilunaris at the lateral border of the rectus muscle, and the point where the anterior and posterior rectus sheaths are connected. It is vital to maintain the stoma during this process, and to find the deep inferior epigastric artery which runs through the posterior caudal section of the rectus abdominis muscle so as to ensure that this artery is not damaged. Upon reaching the level of the linea semilunaris, 1.5 cm medial to the linea semilunaris, the posterior lamina of the internal oblique fascia is found, which is a very thin layer which covers the transversalis muscle fibers. The posterior lamina of the internal oblique is then cut using hook electrocautery in order to expose the transversus abdominis muscle fibers which can then be divided. The neurovascular bundles which link the internal oblique and transversus muscles before perforating the rectus abdominis muscle can be identified laterally in this region, and must be maintained to ensure that rectus atrophy and loss of functionality will not occur. It is essential to remain medial to these neurovascular bundles. Dissection is then carried out through this layer close to the bowel before entering the pre-peritoneal/transversalis fascia plane, extending laterally past the linea semilunaris as far as the psoas muscle. In the next step, the defect in the posterior sheath where the stoma makes its passage can be laterally extended using scissors. The bowel which is next to the stoma is then delivered into the retromuscular plane, whereupon closure of the posterior pre-peritoneal/transversalis fascia plane is carried out at the medial side, by creating a lateralized bowel to the medial defect where the stoma was first formed. Retrorectus dissection is performed on the contralateral side, (making use of TAR, or not, as necessary). In this space there is room for a wide overlap of the mesh as it crosses the midline incision/midline hernia. Closure of the right and left posterior layers takes place at the midline, and thus the visceral sac is recreated.
In addition, the colon can then undergo lateralization with a 3-0 barbed suture attached to the bottom of the lateral wall of the abdomen. Another 3-0 barbed suture is then employed to affix the edges of the posterior layer to the conduit and to ensure continuity through the closure of any defects which can still be found in the posterior layer.
By using a transfascial suture passer, it is possible to place the mesh within the retrorectus space in a manner which is very similar to that used in modified Sugarbaker PSH repair. The mesh is positioned laterally in a sling around the bowel. Though not as effective, it is also possible to extend the mesh to cover the myopectineal orifice when this is considered necessary. The mesh is then medially extended as far as the contralateral linea semilunaris, or beyond in cases where contralateral TAR was used, so as to reinforce the midline in full. Once the mesh has been secured around the circumference, the primary closure of the parastomal defect is performed before recreating the linea alba through the suture of the anterior rectus sheaths to each other at the midline (Figure 9).
In the original posterior component separation with TAR described by Pauli et al., it was not necessary to take down the stoma, but the method did lower the rate of wound morbidity, skin necrosis and subcutaneous seroma compare to the operation resulting from anterior component separation, while simultaneously retaining the biomechanical properties of an effectively functioning abdominal wall and also making use of all the advantages of mesh reinforcement within a modified Sugarbaker configuration located in the retromuscular space, thus allowing a non-coated, non-absorbable mesh to be used. This approach also offers lower costs, while the positioning of the mesh permits the integration of both sides. One side integrates with the anterior abdominal wall while the other side integrates with the posterior fascial layer. This should lead to superior tensile strength in the repair. However, to perform this complex operation, it is necessary to have very good knowledge of the anatomy of the abdominal wall and the alignment of the stoma, as well as outstanding levels of laparoscopic skill. The need for complex dissection makes it difficult to perform the operation correctly. Furthermore, the lack of data concerning long-term results, allied to the concern about potential mesh-related complications and erosion of the bowel means further studies are necessary.
One further procedure related to TAR with modified retrorectus Sugarbaker repair is Stapled Transabdominal Ostomy Reinforcement with Retromuscular Mesh (STORRM) was presented by Majumder et al. (112) and has the goal of strengthening the aperture. The additional step is performed once the TAR and modified Retrorectus Sugarbaker section is complete, prior to taking down the stoma. The stem of the anvil of a circular end-to-end anastomosis (EEA) stapler must be passed through the mesh just behind the fenestration which is produced in the posterior sheath, and suitably aligned with the ultimate location of the stoma. The EEA stapler enters the stoma site through the skin and comes to rest against the anterior fascia. It is advisable to use a 25 EEA-stapler in the case of ileal conduits, a 25–28 mm stapler when performing ileostomies, and for colostomies, a 28–31 mm diameter stapler. Upon firing, the stapler connects the anterior fascia and the mesh. This stomal conduit is then externalized via the aperture. The aim is to produce a straight tunnel through the abdominal wall layers and the mesh, with standardized sizing, and then to fix the mesh, and substitute the traditional cruciate incisions by using mesh to produce a stapled reinforcement of the aperture. Initial findings indicate that the procedure is safe and repeatable, but the recurrence rate remains as high as 17% (112). It will be necessary to conduct follow-up observations over longer time periods to provide confirmation of the findings. The technique is, from our perspective, suitable for use while taking care to avoid contamination in cases where the enterocutaneous junction of stoma revision is planned or when the stoma must be relocated.
The same concept is employed in preventing PSH in the primary process when creating the stoma. The modified Stapled Mesh Stoma Reinforcement Technique (SMART) was described by Williams et al. (Permacol™ and 31 or 33 mm diameter circular stapler) (113). The findings indicated a significant reduction in the rate of recurrence using SMART to 19% from a high of 73%. A modified approach using polypropylene mesh has also been described, with a recurrence rate dropping from 39.5% to 13.8% using SMART, while there were no reports of mesh infection, stenosis, erosion, or fistulation within 24 months when using non-absorbable mesh (114,115).
Prophylaxis
When the stoma is created, the risk of PSH is increased because the abdominal wall, which was otherwise undamaged, now becomes defective. The underlying structure of the muscles of the abdomen provide a level of stability which, when applied in creating the stoma, can lower the potential for hernias to develop. The surgical technique can also be a factor leading to increased or reduced risk of hernia. Reduction of the risk can be achieved through careful preparation prior to surgery, choosing the optimal stoma location, providing patient education, and determining which patients might be at the greatest risk of PSH. Other factors which can be considered to lower the risk include participation in weight loss schemes, giving up smoking, taking physical exercise, and the identification and treatment of collagen metabolism disorders.
While potentially helpful, without evidence can be found to support the incorporation of sutures to stabilize the stoma to the rectus sheet, transrectus and lateral para-rectus location. Further studies are needed in the case of the extraperitoneal route for stoma placement (15,24). To date, it has been shown that the defect diameter at the abdominal wall is the only directly linked to the possibility of developing a hernia. Patients whose defects are enlarging, and those whose trephines exceed 3 cm may be at increased risk (16,116). However, the evidence cannot yet determine the optimal size or shape of the stomal aperture. Our suggestion would be to use the smallest trephine diameter possible on the condition that the passage of the afferent stomal limb is possible along with its mesentery, and ischaemia does not result. For standardization of the aperture size, it has been suggested that circular staplers should be used to more accurately control the size and shape of the trephine in the anterior rectus sheath. However, while these procedures showed low incidence of PSH development when compared to a non-prophylactic method at the follow-up after 24 months, but due to the limitations of the methodology and the absence of any standardization in terms of mesh size, staple size, and mesh reinforcement, it is necessary to remain cautious when interpreting any results thus far (52,113,114,116).
More recently, several studies have proposed the prophylactic use of a prosthetic mesh when the stoma is constructed. The evidence does support this approach in order to prevent PSH. To date there have been 14 randomized controlled trials from 12 researchers which make comparisons of prophylactic mess (4,5,117-127). These RCTs, however, have varied in quality and while the methodology has improved over time, the findings are still open to question. Most of the studies involved patients undergoing permanent end colostomy in elective surgery for bowel cancer, and the data do not take into consideration any other conditions, such as inflammatory bowel disease for instance. Few ileostomies have been reported, and there are very few reports on RCTs involving mesh prophylaxis in emergency cases. The primary end-point choice typically emphasizes the absence or presence of PSH in simple binary terms, without fully exploring the limits of clinical examination or CT imaging as a means of making diagnoses. Another problem is that studies to date have lacked follow-up over the longer term, with only two studies providing data covering as long as three years after surgery (4,123). Just two studies considered cost effectiveness, however these indicated that mesh prophylaxis could offer economic benefits (126,127). There have been few direct one to one comparisons of different mesh types and mesh positioning strategies. It has thus been the case that the studies examined have used a wide range of meshes and surgical approaches, placing different mesh types in different positions, using open and laparoscopic techniques. Thus it can be difficult to draw conclusions when comparing specific factors due to a lack of consistency in the other variables involved. One positive point is that the rates of erosion and mesh infection have to date been very low in the short term following surgery. Just two studies examined cross-linked porcine-derived collagen meshes for PSH prevention using both preperitoneal and intraperitoneal approaches (118,120). It is expected, however, that future trials will address these issues, and will help in developing new types of mesh and new surgical approaches which will deliver better outcomes over the longer term.
To identify benefit of prophylaxis mesh, the study comprised analysis of 12 systematic reviews and meta-analyses, including the Cochrane review, or a period covering 2010–2018. The analyzed studies included 451–844 participants in the randomized controlled trials which have been previously mentioned. The studies emphasized the overall incidence of PSH, and found that the rate was reduced in patients who were treated using a prophylactic mesh in comparison to patients receiving the standard ostomy formation. The Cochrane review for 2018 presented comparison findings involving the outcomes for prophylactic mesh and its protective capabilities, and the outcomes for non-mesh patients (RR 0.53, 95% CI: 0.43 to 0.66), whereby the results were similar to earlier meta-analyses which had shown reductions in the odds of RR at 0.44–0.53, and providing hernia rates of 10.8–22% for patients treated with prophylaxis mesh, and rates of 32.4–41% for those who did not receive mesh placements. There were no significant differences reported for wound infections or infections of the stoma when comparing the two groups, but the studies examined did not discuss such factors as cost, quality of life, or the rate of re-hospitalization (128-139). However, most of studies generally covered a wide range of mesh types, stoma types, and surgical methods, while the sample groups of patients were small, and the durations of the observation were limited. Only one research study it was revealed that no evidence could be presented to support the notion that type of mesh (regression coefficient −1.110, 95% CI: −3.208 to 0.983; P=0.298), or location of mesh (intraperitoneal vs. preperitoneal or intraperitoneal vs. retromuscular) have any influence upon outcomes (129).
As a result of the low cost, lack of adverse consequences, and comparatively beneficial outcomes, the ESH guidelines strongly recommend the use of a prophylactic mesh for PSH prevention. However, because of heterogeneity and poor-quality evidence in the existing studies, it is now suggested that while prophylactic mesh could be useful in preventing PSH in elective cancer surgery which plans end colostomy, it is vital to discuss the risks as well as the advantages with patients prior to surgery. At present, much of the evidence supports the use of synthetic un-coated meshes in the retrorectus space for both open and laparoscopic procedures, while coated-non absorbable mesh should also be considered for intraperitoneal treatment in laparoscopic procedures. In future it would also be beneficial to develop techniques which can be performed easily by inexperienced surgeons within a reasonable additional operative time and without excessive levels of complexity (45).
Conclusions
PSHs are a common problem, and their rate of incidence rises with time following surgery. The symptomatic cases should be treated while balances the risks and benefits of the procedure. Numerous approaches for treatment exist in the case of the hernia types with the highest rate of recurrence. These include the coated non-absorbable mesh repair, which is widely established, while the most popular today is the intraperitoneal onlay fashion using modified Sugarbaker due to its very promising outcomes. Moreover, techniques using two-patch mesh repairs are now gaining popularity as the recurrence rates are very low. Although retrorectus space placement of the mesh has been shown both to be possible and to bring positive outcomes. Accordingly, the best approach for PSH treatment is for the surgeon to apply the technique in which he is most experienced, which is most suitable for the condition of the patient. In elective cancer surgery, prophylaxis mesh are now recommended when end colostomy is the plan. Further studies are being undertaken to determine how to make improvements leading to long-lasting repairs offering enhanced durability to the benefit of patients suffering this type of hernia.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Davide Lomanto and Anil Sharma) for the series “Ventral Hernia” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2019.07.06). The series “Ventral Hernia” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Carne PW, Robertson GM, Frizelle FA. Parastomal hernia. Br J Surg 2003;90:784-93. [Crossref] [PubMed]
- Cingi A, Cakir T, Sever A, et al. Enterostomy site hernias: a clinical and computerized tomographic evaluation. Dis Colon Rectum 2006;49:1559-63. [Crossref] [PubMed]
- Londono-Schimmer EE, Leong AP, Phillips RK. Life table analysis of stomal complications following colostomy. Dis Colon Rectum 1994;37:916-20. [Crossref] [PubMed]
- Jänes A, Cengiz Y, Israelsson LA. Preventing parastomal hernia with a prosthetic mesh: a 5-year follow-up of a randomized study. World J Surg 2009;33:118-21. [Crossref] [PubMed]
- Serra-Aracil X, Bombardo-Junca J, Moreno-Matias J, et al. Randomized, controlled, prospective trial of the use of a mesh to prevent parastomal hernia. Ann Surg 2009;249:583-7. [Crossref] [PubMed]
- Sohn YJ, Moon SM, Shin US, et al. Incidence and risk factors of parastomal hernia. J Korean Soc Coloproctol 2012;28:241-6. [Crossref] [PubMed]
- Pilgrim CH, McIntyre R, Bailey M. Prospective audit of parastomal hernia: prevalence and associated comorbidities. Dis Colon Rectum 2010;53:71-6. [Crossref] [PubMed]
- Shah NR, Craft RO, Harold KL. Parastomal hernia repair. Surg Clin North Am 2013;93:1185-98. [Crossref] [PubMed]
- Cheung MT. Complications of an abdominal stoma: an analysis of 322 stomas. Aust N Z J Surg 1995;65:808-11. [Crossref] [PubMed]
- Aquina CT, Iannuzzi JC, Probst CP, et al. Parastomal hernia: a growing problem with new solutions. Dig Surg 2014;31:366-76. [Crossref] [PubMed]
- Leong AP, Londono-Schimmer EE, et al. Lifetable analysis of stomal complications following ileostomy. Br J Surg 1994;81:727-9. [Crossref] [PubMed]
- Narang SK, Alam NN, Campain NJ, et al. Parastomal hernia following cystectomy and ileal conduit urinary diversion: a systematic review. Hernia 2017;21:163-75. [Crossref] [PubMed]
- Andersen RM, Klausen TW, Danielsen AK, et al. Incidence and risk factors for parastomal bulging in patients with ileostomy or colostomy: a register-based study using data from the Danish Stoma Database Capital Region. Colorectal Dis 2018;20:331-40. [Crossref] [PubMed]
- Malik T, Lee MJ, Harikrishnan AB. The incidence of stoma related morbidity - a systematic review of randomised controlled trials. Ann R Coll Surg Engl 2018;100:501-8. [Crossref] [PubMed]
- Styliński R, Alzubedi A, Rudzki S. Parastomal hernia - current knowledge and treatment. Wideochir Inne Tech Maloinwazyjne 2018;13:1-8. [Crossref] [PubMed]
- Arumugam PJ, Bevan L, Macdonald L, et al. A prospective audit of stomas-analysis of risk factors and complications and their management. Colorectal Dis 2003;5:49-52. [Crossref] [PubMed]
- Shabbir J, Britton DC. Stoma complications: a literature overview. Colorectal Dis 2010;12:958-64. [Crossref] [PubMed]
- Kroese LF, de Smet GH, Jeekel J, et al. Systematic review and meta-analysis of extraperitoneal versus transperitoneal colostomy for preventing parastomal hernia. Dis Colon Rectum 2016;59:688-95. [Crossref] [PubMed]
- Lian L, Wu XR, He XS, et al. Extraperitoneal vs. intraperitoneal route for permanent colostomy: a meta-analysis of 1,071 patients. Int J Colorectal Dis 2012;27:59-64. [Crossref] [PubMed]
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Chichester: John Wiley & Sons, 2008:211-28.
- Sjödahl R, Anderberg B, Bolin T. Parastomal hernia in relation to site of the abdominal stoma. Br J Surg 1988;75:339-41. [Crossref] [PubMed]
- Stephenson BM, Evans MD, Hilton J, et al. Minimal anatomical disruption in stoma formation: the lateral rectus abdominis positioned stoma (LRAPS). Colorectal Dis 2010;12:1049-52. [Crossref] [PubMed]
- Hardt J, Seyfried S, Weiß C, et al. A pilot single-centre randomized trial assessing the safety and efficacy of lateral pararectus abdominis compared with transrectus abdominis muscle stoma placement in patients with temporary loop ileostomies: the PATRASTOM trial. Colorectal Dis 2016;18:O81-90. [Crossref] [PubMed]
- Hardt J, Meerpohl JJ, Metzendorf MI, et al. Lateral pararectal versus transrectal stoma placement for prevention of parastomal herniation. Cochrane Database Syst Rev 2013;CD009487 [PubMed]
- McGrath A, Porrett T, Heyman B. Parastomal hernia: an exploration of the risk factors and the implications. Br J Nurs 2006;15:317-21. [Crossref] [PubMed]
- Ihnát P, Tulinský L, Jonszta T, et al. Parastomal and incisional hernia following laparoscopic/open abdominoperineal resection: is there a real difference? Surg Endosc 2019; [Epub ahead of print]. [Crossref] [PubMed]
- Pearl RK. Parastomal hernias. World J Surg 1989;13:569-72. [Crossref] [PubMed]
- Carlsson E, Fingren J, Hallen AM, et al. The prevalence of ostomy-related complications 1 year after ostomy surgery: a prospective, descriptive, clinical study. Ostomy Wound Manage 2016;62:34-48. [PubMed]
- Krogsgaard M, Thomsen T, Vinther A, et al. Living with a parastomal bulge - patients' experiences of symptoms. J Clin Nurs 2017;26:5072-81. [Crossref] [PubMed]
- Kald A, Juul KN, Hjortsvang H, et al. Quality of life is impaired in patients with peristomal bulging of a sigmoid colostomy. Scand J Gastroenterol 2008;43:627-33. [Crossref] [PubMed]
- Krogsgaard M, Pilsgaard B, Borglit TB, et al. Symptom load and individual symptoms before and after repair of parastomal hernia: a prospective single centre study. Colorectal Dis 2017;19:200-7. [Crossref] [PubMed]
- Tadeo-Ruiz G, Picazo-Yeste JS, Moreno-Sanz C, et al. Parastomal hernias: background, current status and future prospects. Cir Esp 2010;87:339-49. [Crossref] [PubMed]
- Kwon OY, Lee KR, Kim SW. Massive parastomal hernia with strangulation. Am J Emerg Med 2008;26:109.e3-4. [Crossref] [PubMed]
- Markham DW, Ruppert M, Noel R, et al. A parastomal hernia causing small-bowel obstruction. J Clin Gastroenterol 1996;22:218-9. [Crossref] [PubMed]
- Bull N, Chan DL, Ravindran P, et al. Gastric outlet obstruction secondary to parastomal hernia: case report and literature review. ANZ J Surg 2019;89:E96-7. [Crossref] [PubMed]
- Yucel AF, Pergel A, Aydin I, et al. A rare stoma-related complication: parastomal evisceration. Indian J Surg 2014;76:154-5. [Crossref] [PubMed]
- Lolis ED, Savvidou P, Vardas K, et al. Parastomal evisceration as an extremely rare complication of a common procedure. Ann R Coll Surg Engl 2015;97:e103-4. [Crossref] [PubMed]
- Gurmu A, Matthiessen P, Nilsson S, et al. The inter-observer reliability is very low at clinical examination of parastomal hernia. Int J Colorectal Dis 2011;26:89-95. [Crossref] [PubMed]
- Naguib N, Rafique H, Dhruva Rao PK, et al. A review of the incidence of iatrogenic hernia in both laparoscopic and open colorectal surgery: Using CT as the gold standard of detection, cohort study. Int J Surg 2015;19:87-90. [Crossref] [PubMed]
- Williams JG, Etherington R, Hayward MW, et al. Paraileostomy hernia: a clinical and radiological study. Br J Surg 1990;77:1355-7. [Crossref] [PubMed]
- Jänes A, Weisby L, Israelsson LA. Parastomal hernia: clinical and radiological definitions. Hernia 2011;15:189-192. [Crossref] [PubMed]
- Sjödahl RI, Thorelius L, Hallböök OJ. Ultrasonographic findings in patients with peristomal bulging. Scand J Gastroenterol 2011;46:745-9. [Crossref] [PubMed]
- Strigård K, Gurmu A, Näsvall P, et al. Intrastomal 3D ultrasound; an inter- and intra-observer evaluation. Int J Colorectal Dis 2013;28:43-7. [Crossref] [PubMed]
- Näsvall P, Wikner F, Gunnarsson U, et al. A comparison between intrastomal 3D ultrasonography, CT scanning and findings at surgery in patients with stomal complaints. Int J Colorectal Dis 2014;29:1263-6. [Crossref] [PubMed]
- Antoniou SA, Agresta F, Garcia Alamino JM, et al. European Hernia Society guidelines on prevention and treatment of parastomal hernias. Hernia 2018;22:183-98. [Crossref] [PubMed]
- Devlin HB. Management of abdominal hernias. Oxford: Butterworth-Heinemann; 1988.
- Moreno-Matias J, Serra-Aracil X, Darnell-Martin A, et al. The prevalence of parastomal hernia after formation of an end colostomy. A new clinico-radiological classification. Colorectal Dis 2009;11:173-7. [Crossref] [PubMed]
- Seo SH, Kim HJ, Oh SY, et al. Computed tomography classification for parastomal hernia. J Korean Surg Soc 2011;81:111-4. [Crossref] [PubMed]
- Gil G, Szczepkowski M. A new classification of parastomal hernias: from the experience at Bielanski Hospital in Warsaw. Pol Przegl Chir 2011;83:430-7. [Crossref] [PubMed]
- Śmietański M, Szczepkowski M, Alexandre JA, et al. European Hernia Society classification of parastomal hernias. Hernia 2014;18:1-6. [Crossref] [PubMed]
- Gregg ZA, Dao HE, Schechter S, et al. Paracolostomy hernia repair: who and when? J Am Coll Surg 2014;218:1105-12. [Crossref] [PubMed]
- Helgstrand F, Rosenberg J, Kehlet H, et al. Risk of morbidity, mortality, and recurrence after parastomal hernia repair: a nationwide study. Dis Colon Rectum 2013;56:1265-72. [Crossref] [PubMed]
- Association of Stoma Care Nurses. ASCN Stoma Care National Clinical Guidelines. [internet] 2016 [cited 2019 Jan 15] Available online: http://ascnuk.com/wp-content/uploads/2016/03/ASCN-Clinical-Guidelines-Final-25-April-compressed-11-10-38.pdf
- Erwin-Toth P, Thompson SJ, Davis JS. Factors impacting the quality of life of people with an ostomy in North America: results from the Dialogue Study. J Wound Ostomy Continence Nurs 2012;39:417-22. [Crossref] [PubMed]
- Kroese LF, Lambrichts DPV, Jeekel J, et al. Non-operative treatment as a strategy for patients with parastomal hernia: a multicentre, retrospective cohort study. Colorectal Dis 2018;20:545-51. [Crossref] [PubMed]
- Dietz UA, Winkler MS, Härtel RW, et al. Importance of recurrence rating, morphology, hernial gap size, and risk factors in ventral and incisional hernia classification. Hernia 2014;18:19-30. [Crossref] [PubMed]
- ACPGBI Parastomal Hernia Group. Prevention and treatment of parastomal hernia: a position statement on behalf of the Association of Coloproctology of Great Britain and Ireland. Colorectal Dis 2018;20:5-19. [Crossref] [PubMed]
- Earle D, Roth JS, Saber A, et al. SAGES guidelines for laparoscopic ventral hernia repair. Surg Endosc 2016;30:3163-83. [Crossref] [PubMed]
- Silecchia G, Campanile FC, Sanchez L, et al. Laparoscopic ventral/incisional hernia repair: updated Consensus Development Conference based guidelines. Surg Endosc 2015;29:2463-84. [Crossref] [PubMed]
- Thorlakson RH. Technique of repair of herniations associated with colonic stomas. Surg Gynecol Obstet 1965;120:347-50. [PubMed]
- Hansson BM, Slater NJ, van der Velden AS, et al. Surgical techniques for parastomal hernia repair: a systematic review of the literature. Ann Surg 2012;255:685-95. [Crossref] [PubMed]
- Rubin MS, Schoetz DJ Jr, Matthews JB. Parastomal hernia. Is stoma relocation superior to fascial repair? Arch Surg 1994;129:413-8. [Crossref] [PubMed]
- Allen-Mersh TG, Thomson JP. Surgical treatment of colostomy complications. Br J Surg 1988;75:416-8. [Crossref] [PubMed]
- Riansuwan W, Hull TL, Millan MM, et al. Surgery of recurrent parastomal hernia: direct repair or relocation? Colorectal Dis 2010;12:681-6. [Crossref] [PubMed]
- Baig MK, Larach JA, Chang S, et al. Outcome of parastomal hernia repair with and without midline laparotomy. Tech Coloproctol 2006;10:282-6. [Crossref] [PubMed]
- García-Vallejo L, Concheiro P, Mena E, et al. Parastomal hernia repair: laparoscopic ventral hernia meshplasty with stoma relocation. The current state and a clinical case presentation. Hernia 2011;15:85-91. [Crossref] [PubMed]
- Hopkins TB, Trento A. Parastomal ileal loop hernia repair with marlex mesh. J Urol 1982;128:811-2. [Crossref] [PubMed]
- Hotouras A, Murphy J, Thaha M, et al. The persistent challenge of parastomal herniation: a review of the literature and future developments. Colorectal Dis 2013;15:e202-14. [Crossref] [PubMed]
- Ballas KD, Rafailidis SF, Marakis GN, et al. Intraperitoneal ePTFE mesh repair of parastomal hernias. Hernia 2006;10:350-3. [Crossref] [PubMed]
- Brown CN, Finch JG. Which mesh for hernia repair? Ann R Coll Surg Engl 2010;92:272-8. [Crossref] [PubMed]
- Rosen MJ, Krpata DM, Ermlich B, et al. A 5-year clinical experience with single- staged repairs of infected and contaminated abdominal wall defects utilizing biologic mesh. Ann Surg 2013;257:991-6. [Crossref] [PubMed]
- Majumder A, Winder JS, Wen Y, et al. Comparative analysis of biologic versus synthetic mesh outcomes in contaminated hernia repairs. Surgery 2016;160:828-38. [Crossref] [PubMed]
- Atema JJ, de Vries FE, Boermeester MA. Systematic review and meta-analysis of the repair of potentially contaminated and contaminated abdominal wall defects. Am J Surg 2016;212:982-95.e1. [Crossref] [PubMed]
- Kamarajah SK, Chapman SJ, Glasbey J, et al. Systematic review of the stage of innovation of biological mesh for complex or contaminated abdominal wall closure. BJS Open 2018;2:371-80. [Crossref] [PubMed]
- Morris-Stiff GJ, Hughes LE. The outcomes of nonabsorbable mesh placed within the abdominal cavity: literature review and clinical experience. J Am Coll Surg 1998;186:352-67. [Crossref] [PubMed]
- Berger D, Bientzle M. Polyvinylidene fluoride: a suitable mesh material for laparoscopic incisional and parastomal hernia repair! A prospective, observational study with 344 patients. Hernia 2009;13:167-72. [Crossref] [PubMed]
- Tandon A, Shahzad K, Pathak S, et al. ParietexTM Composite mesh versus DynaMesh®-IPOM for laparoscopic incisional and ventral hernia repair: a retrospective cohort study. Ann R Coll Surg Engl 2016;98:568-73. [Crossref] [PubMed]
- Kao AM, Arnold MR, Augenstein VA, et al. Prevention and Treatment Strategies for Mesh Infection in Abdominal Wall Reconstruction. Plast Reconstr Surg 2018;142:149S-55S. [Crossref] [PubMed]
- Rosin JD, Bonardi RA. Paracolostomy hernia repair with Marlex mesh: a new technique. Dis Colon Rectum 1977;20:299-302. [Crossref] [PubMed]
- Tekkis PP, Kocher HM, Payne JG. Parastomal hernia repair: modified Thorlakson technique, reinforced by polypropylene mesh. Dis Colon Rectum 1999;42:1505-8. [Crossref] [PubMed]
- Steele SR, Lee P, Martin MJ, et al. Is parastomal hernia repair with polypropylene mesh safe? Am J Surg 2003;185:436-40. [Crossref] [PubMed]
- Leslie D. The parastomal hernia. Surg Clin North Am 1984;64:407-15. [Crossref] [PubMed]
- Kald A, Landin S, Masreliez C, et al. Mesh repair of parastomal hernias: new aspects of the onlay technique. Tech Coloproctol 2001;5:169-71. [Crossref] [PubMed]
- Lüning TH, Spillenaar-Bilgen EJ. Parastomal hernia: complications of extra- peritoneal onlay mesh placement. Hernia 2009;13:487-90. [Crossref] [PubMed]
- Longman RJ, Thomson WH. Mesh repair of parastomal hernias-a safety modification. Colorectal Dis 2005;7:292-4. [Crossref] [PubMed]
- Guzmán-Valdivia G, Guerrero TS, Laurrabaquio HV. Parastomal hernia-repair using mesh and an open technique. World J Surg 2008;32:465-70. [Crossref] [PubMed]
- Colvin J, Rosenblatt S. Surgical Management of Parastomal Hernias. Surg Clin North Am 2018;98:577-92. [Crossref] [PubMed]
- Pauli EM, Juza RM, Winder JS. How I do it: novel parastomal herniorrhaphy utilizing transversus abdominis release. Hernia 2016;20:547-52. [Crossref] [PubMed]
- Tastaldi L, Haskins IN, Perez AJ, et al. Single center experience with the modified retromuscular Sugarbaker technique for parastomal hernia repair. Hernia 2017;21:941-9. [Crossref] [PubMed]
- Stelzner S, Hellmich G, Ludwig K. Repair of paracolostomy hernias with a prosthetic mesh in the intraperitoneal onlay position: modified Sugarbaker technique. Dis Colon Rectum 2004;47:185-91. [Crossref] [PubMed]
- Sugarbaker PH. Peritoneal approach to prosthetic mesh repair of paraostomy hernias. Ann Surg 1985;201:344-6. [Crossref] [PubMed]
- Al Shakarchi J, Williams JG. Systematic review of open techniques for parastomal hernia repair. Tech Coloproctol 2014;18:427-32. [Crossref] [PubMed]
- Halabi WJ, Jafari MD, Carmichael JC, et al. Laparoscopic versus open repair of parastomal hernias: an ACS-NSQIP analysis of short-term outcomes. Surg Endosc 2013;27:4067-72. [Crossref] [PubMed]
- DeAsis FJ, Lapin B, Gitelis ME, et al. Current state of laparoscopic parastomal hernia repair: a meta-analysis. World J Gastroenterol 2015;21:8670-7. [Crossref] [PubMed]
- Safadi B. Laparoscopic repair of parastomal hernias: early results. Surg Endosc 2004;18:676-80. [Crossref] [PubMed]
- Wara P, Andersen LM. Long-term follow-up of laparoscopic repair of parastomal hernia using a bilayer mesh with a slit. Surg Endosc 2011;25:526-30. [Crossref] [PubMed]
- Hansson BM, Bleichrodt RP, de Hingh IH. Laparoscopic parastomal hernia repair using a keyhole technique results in a high recurrence rate. Surg Endosc 2009;23:1456-9. [Crossref] [PubMed]
- Jani K. Laparoscopic paracolostomy hernia repair: a retrospective case series at a tertiary care center. Surg Laparosc Endosc Percutan Tech 2010;20:395-8. [Crossref] [PubMed]
- Yang X, He K, Hua R, et al. Laparoscopic repair of parastomal hernia. Ann Transl Med 2017;5:45. [Crossref] [PubMed]
- Voitk A. Simple technique for laparoscopic paracolostomy hernia repair. Dis Colon Rectum 2000;43:1451-3. [Crossref] [PubMed]
- Hansson BM, Morales-Conde S, Mussack T, et al. The laparoscopic modified Sugarbaker technique is safe and has a low recurrence rate: a multicenter cohort study. Surg Endosc 2013;27:494-500. [Crossref] [PubMed]
- Mancini GJ, McClusky DA, Khaitan L, et al. Laparoscopic parastomal hernia repair using a nonslit mesh technique. Surg Endosc 2007;21:1487-91. [Crossref] [PubMed]
- Muysoms F. Laparoscopic repair of parastomal hernias with a modified Sugarbaker technique. Acta Chir Belg 2007;107:476-80. [Crossref] [PubMed]
- Berger D, Bientzle M. Laparoscopic repair of parastomal hernias: a single surgeon’s experience in 66 patients. Dis Colon Rectum 2007;50:1668-73. [Crossref] [PubMed]
- LeBlanc KA, Bellanger DE. Laparoscopic repair of paraostomy hernias: early results. J Am Coll Surg 2002;194:232-9. [Crossref] [PubMed]
- LeBlanc KA, Bellanger DE, Whitaker JM, et al. Laparoscopic parastomal hernia repair. Hernia 2005;9:140-4. [Crossref] [PubMed]
- Suwa K, Okamoto T, Yanaga K. Is fascial defect closure with intraperitoneal onlay mesh superior to standard intraperitoneal onlay mesh for laparoscopic repair of large incisional hernia? Asian J Endosc Surg 2018;11:378-84. [Crossref] [PubMed]
- Hansson BM, van Nieuwenhoven EJ, Bleichrodt RP. Promising new technique in the repair of parastomal hernia. Surg Endosc 2003;17:1789-91. [Crossref] [PubMed]
- Jani K, Palanivelu C, Parthasarathi R, et al. Laparoscopic repair of a paracolostomy hernia: secure reinforced closure of the defect prevents recurrence. J Laparoendosc Adv Surg Tech A 2007;17:216-8. [Crossref] [PubMed]
- Wiessner R, Vorwerk T, Gehring A. Laparoscopic repair for parastomal hernia with ongoing barbed suture followed by sandwich-technique: ‘Sandwich-plus-technique’. J Minim Access Surg 2018; [Epub ahead of print]. [PubMed]
- Belyansky I, Zahiri HR, Park A. Laparoscopic Transversus Abdominis Release, a Novel Minimally Invasive Approach to Complex Abdominal Wall Reconstruction. Surg Innov 2016;23:134-41. [Crossref] [PubMed]
- Majumder A, Orenstein SB, Miller HJ, et al. Stapled transabdominal ostomy reinforcement with retromuscular mesh (STORRM): technical details and early outcomes of a novel approach for retromuscular repair of parastomal hernias. Am J Surg 2018;215:82-7. [Crossref] [PubMed]
- Williams NS, Hotouras A, Bhan C, et al. A case-controlled pilot study assessing the safety and efficacy of the Stapled Mesh stomA Reinforcement Technique (SMART) in reducing the incidence of parastomal herniation. Hernia 2015;19:949-54. [Crossref] [PubMed]
- Canda AE, Terzi C, Agalar C, et al. Preventing parastomal hernia with modified stapled mesh stoma reinforcement technique (SMART) in patients who underwent surgery for rectal cancer: a case-control study. Hernia 2018;22:379-84. [Crossref] [PubMed]
- Ng ZQ, Tan P, Theophilus M. Stapled Mesh stomA Reinforcement Technique (SMART) in the prevention of parastomal hernia: a single-centre experience. Hernia 2017;21:469-75. [Crossref] [PubMed]
- Hotouras A, Murphy J, Power N, et al. Radiological incidence of parastomal herniation in cancer patients with permanent colostomy: what is the ideal size of the surgical aperture? Int J Surg 2013;11:425-7. [Crossref] [PubMed]
- Jänes A, Cengiz Y, Israelsson LA. Randomized clinical trial of the use of a prosthetic mesh to prevent parastomal hernia. Br J Surg 2004;91:280-2. [Crossref] [PubMed]
- Hammond TM, Huang A, Prosser K, et al. Parastomal hernia prevention using a novel collagen implant: a randomised controlled phase 1 study. Hernia 2008;12:475-81. [Crossref] [PubMed]
- López-Cano M, Lozoya-Trujillo R, Quiroga S, et al. Use of a prosthetic mesh to prevent parastomal hernia during laparoscopic abdominoperineal resection: a randomized controlled trial. Hernia 2012;16:661-7. [Crossref] [PubMed]
- Fleshman JW, Beck DE, Hyman N, et al. A prospective, multicenter, randomized, controlled study of non-cross-linked porcine acellular dermal matrix fascial sublay for parastomal reinforcement in patients undergoing surgery for permanent abdominal wall ostomies. Dis Colon Rectum 2014;57:623-31. [Crossref] [PubMed]
- Târcoveanu E, Vasilescu E, Cotea E, et al. Parastomal hernias - clinical study of therapeutic strategies. Chirurgia (Bucur) 2014;109:179-84. [PubMed]
- Vierimaa M, Klintrup K, Biancari F, et al. Prospective, randomized study on the use of a prosthetic mesh for prevention of parastomal hernia of permanent colostomy. Dis Colon Rectum 2015;58:943-9. [Crossref] [PubMed]
- Lambrecht JR, Larsen SG, Reiertsen O, et al. Prophylactic mesh at end-colostomy construction reduces parastomal hernia rate: a randomized trial. Colorectal Dis 2015;17:O191-7. [Crossref] [PubMed]
- López-Cano M, Serra-Aracil X, Mora L, et al. Preventing parastomal hernia using a modified Sugarbaker technique with composite mesh during laparoscopic abdominoperineal resection: a randomized controlled trial. Ann Surg 2016;264:923-8. [Crossref] [PubMed]
- Brandsma HT, Hansson BM, Aufenacker TJ, et al. Prophylactic mesh placement to prevent parastomal hernia, early results of a prospective multicentre randomized trial. Hernia 2016;20:535-41. [Crossref] [PubMed]
- Brandsma HT, Hansson BM, Aufenacker TJ, et al. Prophylactic Mesh Placement During Formation of an End-colostomy Reduces the Rate of Parastomal Hernia: Short-term Results of the Dutch PREVENT-trial. Ann Surg 2017;265:663-9. [Crossref] [PubMed]
- Odensten C, Strigard K, Rutegard J, et al. Use of prophylactic mesh when creating a colostomy does not prevent parastomal hernia: a randomized controlled trial - STOMAMESH. Ann Surg 2019;269:427-31. [Crossref] [PubMed]
- Pianka F, Probst P, Keller AV, et al. Prophylactic mesh placement for the PREvention of paraSTOmal hernias: the PRESTO systematic review and meta-analysis. PLoS One 2017;12:e0171548 [Crossref] [PubMed]
- Cross AJ, Buchwald PL, Frizelle FA, et al. Meta analysis of prophylactic mesh to prevent parastomal hernia. Br J Surg 2017;104:179-86. [Crossref] [PubMed]
- López-Cano M, Brandsma HT, Bury K, et al. Prophylactic mesh to prevent parastomal hernia after end colostomy: a meta-analysis and trial sequential analysis. Hernia 2017;177-89. [Crossref] [PubMed]
- Patel SV, Zhang L, Chadi SA, et al. Prophylactic mesh to prevent parastomal hernia: a meta-analysis of randomized controlled studies. Tech Coloproctol 2017;21:5-13. [Crossref] [PubMed]
- Chapman SJ, Wood B, Drake TM, et al. Systematic review and meta-analysis of prophylactic mesh during primary stoma formation to prevent parastomal hernia. Dis Colon Rectum 2017;60:107-15. [Crossref] [PubMed]
- Cornille JB, Pathak S, Daniels IR, Smart NJ. Prophylactic mesh use during primary stoma formation to prevent parastomal hernia. Ann R Coll Surg Engl 2017;99:2-11. [Crossref] [PubMed]
- Wang S, Wang W, Zhu B, et al. Efficacy of prophylactic mesh in end-colostomy construction: a systematic review and meta-analysis of randomized controlled trials. World J Surg 2016;40:2528-36. [Crossref] [PubMed]
- Sajid MS, Kalra L, Hutson K, et al. Parastomal hernia as a consequence of colorectal cancer resections can prophylactically be controlled by mesh insertion at the time of primary surgery: a literature based systematic review of published trials. Minerva Chir 2012;67:289-96. [PubMed]
- Shabbir J, Chaudhary BN, Dawson R. A systematic review on the use of prophylactic mesh during primary stoma formation to prevent parastomal hernia formation. Colorectal Dis 2012;14:931-6. [Crossref] [PubMed]
- Wijeyekoon SP, Gurusamy K, El-Gendy K, et al. Prevention of parastomal herniation with biologic/composite prosthetic mesh: a systematic review and meta-analysis of randomized controlled trials. J Am Coll Surg 2010;211:637-45. [Crossref] [PubMed]
- Tam KW, Wei PL, Kuo LJ, et al. Systematic review of the use of a mesh to prevent parastomal hernia. World J Surg 2010;34:2723-9. [Crossref] [PubMed]
- Jones HG, Rees M, Aboumarzouk OM, Brown J, et al. Prosthetic mesh placement for the prevention of parastomal herniation. Cochrane Database Syst Rev 2018;7:CD008905 [PubMed]
Cite this article as: Techagumpuch A, Udomsawaengsup S. Update in parastomal hernia. Ann Laparosc Endosc Surg 2019;4:75.