The inaccuracy of the endoscopic anastomotic measurement techniques
Introduction
Obesity and its related complications pose a grave threat to national and worldwide public health (1,2). More than 30% of the people worldwide are struggling with obesity and its related complications, and nearly 5% of the global fatalities are attributed to obesity alone (1,3). According to a recent study, if left unattended, more than 50% of the world's population will undoubtedly be obese by the end of 2030 (4). Unfortunately, most of the treatments options for the obesity such as dietary adjustment, exercise, cognitive behavioral therapy, as well as pharmacotherapy are less reliable compared to the surgical interventions, which can attain more substantial and sustainable weight loss (1,2).
Over the past few decades, RYGB has emerged as the most effective and prominent approach for weight loss in morbidly obese individuals (5,6). Although this procedure is risk-free and reliable, weight gain can still occur in almost 20% of the patients undergoing RYGB for obesity (6,7). Amongst various factors connected with weight gain after the RYGB, diameter of GJ (due to failing to measure the anastomosis size at the GJ site precisely) is one of the most vital and also less documented factors (6,8). Currently, there seems to be no agreement on exactly how to best endoscopically determine anastomosis size at the GJ site (2,6).
As a result of the absence of standardized endoscopic measurement techniques of the GJ anastomosis size, the subsequent therapies may result in an inefficient intervention, therefore causing the weight gain after the RYGB (6). The current study aims to identify the most accurate method to endoscopically gauge the lumen diameter at the anastomosis to permit better management of the patients undergoing RYGB.
Methods
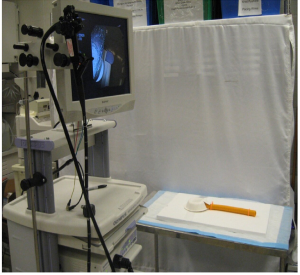
The current prospective study was conducted at Case Western Reserve University, Cleveland, Ohio. The primary objective of the study was to measure a ring of known diameter in a standardized plastic model of the esophagus and gastric pouch using four commonly used endoscopic measuring techniques and a double channel endoscope. A total of 10 participants, including 9 general surgeons and 1 gastrointestinal fellow, were asked to participate in the study voluntarily. There was no financial compensation to participate in the study, and no repercussions for not participating. The demographical data, years of endoscopic experience, and the number of endoscopies performed by the participants are summarized in (Table 1).
Table 1
| Participants initials | Gender | Specialty of participant | Endoscopic procedures performed (N) | Years of experience |
|---|---|---|---|---|
| Participant 1 | M | Surgery | >1,000 | N/A |
| Participant 2 | M | Surgery | <100 | 0 |
| Participant 3 | M | Surgery | 250–500 | 0 |
| Participant 4 | M | Surgery | 250–500 | 2 |
| Participant 5 | M | Surgery | 250–500 | 2 |
| Participant 6 | M | Surgery | >1,000 | N/A |
| Participant 7 | M | GI | >1,000 | 0 |
| Participant 8 | M | Surgery | <100 | 0 |
| Participant 9 | F | Surgery | >1,000 | 6 |
| Participant 10 | M | Surgery | <100 | 0 |
GI, gastrointestinal; N, number; N/A, not available.
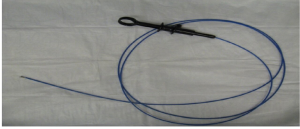
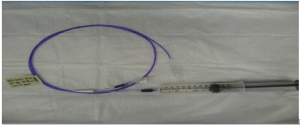
At the beginning of the study, five plastic models of the esophagus and gastric pouch were created (Figure 1). All the participants were asked to endoscopically measure the rings of a known diameters of 33, 27, 24, 18, and 13 mm, respectively (Figures 2,3). Participants used visual estimation (VE), instrument reference (IR) to a biopsy forceps, an 18 mm esophageal dilating balloon (EDB) as a reference, and a 30 mm endoscopic ruler (ER) made from an ERCP guide wire tip (Figures 4-6). The 5 models (33, 27, 24, 18, and 13 mm) were presented in random order.

Furthermore, the data was collected and transferred to the Microsoft Excel (Microsoft Corporation, Redmond, Washington) spreadsheet. The measurements obtained were analyzed for the average and the standard deviation using the basic statistical function of Microsoft Excel (Microsoft Corporation, Redmond, Washington). Moreover, the data is only shared with the participant and the individuals related to the study, and a copy of the data was also saved in the institutional database at the Case Western Reserve University, Cleveland, Ohio.
Results
A total of 10 participants consisting of 9 surgeons and also 1 gastroenterologist (Table 1) performed various measurements of GJ model, using VE, IR to a biopsy forceps, an 18 mm EDB as a reference, and a 30 mm endoscopic ruler (ER) made from an ERCP guide wire tip. Following observations were noticed for each category.
Visual estimation (VA) method
The VE was the least accurate method with an average diversion (AD) from the actual diameter of 6.25±4.95 mm (24.2%). Based upon the measurements obtained during the testing, the maximum difference from the least accurate single measurement was noted to be 22 mm. In the largest 33 mm ring diameter model, the average measurement was observed to be 19.3±6.4 mm, accounting for a 13.7 mm (42.0%) difference between actual sizes (33 mm) and the average measurements. Likewise, in the 27 mm ring diameter model, the average measurement was observed to be 19.35±6.5 mm, accounting for a 7.65 mm (28.0%) difference between actual sizes (27 mm) and the average measurements (Table 2).
Table 2
| Variable | 33 mm ring diameter model measurement in mm | 27 mm ring diameter model measurement in mm | 18 mm ring diameter model measurement in mm | 24 mm ring diameter model measurement in mm | 13 mm ring diameter model measurement in mm |
|---|---|---|---|---|---|
| Participants | |||||
| Participant 1 | 20 | 21 | 15 | 18 | 16 |
| Participant 2 | 30 | 30 | 25 | 25 | 20 |
| Participant 3 | 12 | 9 | 11 | 10 | 7 |
| Participant 4 | 20 | 20 | 15 | 18 | 11 |
| Participant 5 | 25 | 18 | 14 | 17 | 12 |
| Participant 6 | 25 | 20 | 15 | 20 | 14 |
| Participant 7 | 11 | 11 | 7 | 12 | 10 |
| Participant 8 | 22 | 27 | 18 | 25 | 12 |
| Participant 9 | 14 | 22 | 15 | 12 | 10 |
| Participant 10 | 14 | 15.5 | 13.5 | 15.5 | 11 |
| Average in mm (SD) | 19.3 (6.4) | 19.35 (6.5) | 14.85 (4.6) | 17.25 (5.1) | 12.3 (3.2) |
| Difference from the actual measurement in mm (%) | 13.7 (42.0) | 7.65 (28.0) | 3.15 (18.0) | 6.75 (28.0) | 0.7 (5.0) |
| Participant who performed >1,000 | 17.5 | 18.5 | 13 | 15.5 | 12.5 |
| Participant who performed 250–500 | 19 | 15.7 | 13.3 | 15 | 10 |
| Participant who performed <100 | 22 | 24.2 | 15.5 | 21.8 | 14.3 |
SD, standard deviation; mm, millimeter.
Additionally, in 18 mm ring diameter model, the average measurement was observed to be 14.85±4.6 mm, accounting for a 3.15 mm (18.0%) difference between actual size (18 mm) and the average measurements. Furthermore, in a 24 mm ring diameter model, the average measurement was observed to be 17.25±5.1 mm, accounting for a 6.75 mm (28.0%) between actual size (24 mm) and the average measurements. Finally, in the smallest 13 mm ring diameter model, the average measurement was observed to be 12.3±3.2 mm, accounting for a 0.7 mm (5.0%) difference between actual sizes (13 mm) and the average measurements. Furthermore, VA measurements variations on different models based on the endoscopic experience of the participants are explained in (Table 2). Not surprisingly, the underestimation was found in 82.5% (33/40) of each of the VE while the overestimation was lowest in VE 12.5% (5/40).
IR method
Using IR method, an AD from the actual diameter was 3.89±3.05 mm (14.8%), an overestimation of was noticed in IR 15% (6/40) measurements, while underestimation was seen in 82.5% (33/40) of the measurements. Additionally, based upon the measurements obtained during the testing, the maximum difference from the least accurate single measurement was noted to be 19 mm. In the largest 33 mm ring diameter model, the average measurement was observed to be 24.5 mm, accounting for an 8.5 mm (26.0%) difference between actual sizes (33 mm) and the average measurements. Also, in the 27 mm ring diameter model, the average measurement was observed to be 22.1 mm, accounting for a 4.9 mm (18.0%) difference between actual sizes (27 mm) and the average measurements (Table 3).
Table 3
| Variable | 33 mm ring diameter model measurement in mm | 27 mm ring diameter model measurement in mm | 18 mm ring diameter model measurement in mm | 24 mm ring diameter model measurement in mm | 13 mm ring diameter model measurement in mm |
|---|---|---|---|---|---|
| Participants | |||||
| Participant 1 | 23 | 26 | 15 | 24 | 16 |
| Participant 2 | 25 | 25 | 17 | 21 | 12 |
| Participant 3 | 20 | 22 | 15 | 15 | 9 |
| Participant 4 | 28 | 22 | 17 | 22 | 12 |
| Participant 5 | 29 | 22 | 16 | 19 | 14 |
| Participant 6 | 20 | 25 | 14 | 20 | 15 |
| Participant 7 | 14 | 16 | 14 | 17 | 12 |
| Participant 8 | 28 | 17 | 19 | 24 | 16 |
| Participant 9 | 30 | 25 | 18 | 20 | 15 |
| Participant 10 | 28 | 21 | 17.5 | 22 | 12 |
| Average in mm (SD) | 19.3 (5.2) | 22.1 (3.4) | 14.85 (1.7) | 17.25 (2.9) | 12.3 (2.3) |
| Difference from the actual measurement in mm (%) | 8.5 (26.0) | 4.9 (18.0) | 1.75 (10.0) | 3.6 (15.0) | −0.7 (5.0) |
| Participant who performed >1,000 | 21.8 | 23 | 15.3 | 20.3 | 14.5 |
| Participant who performed 250–500 | 25.67 | 22 | 16 | 18.67 | 11.67 |
| Participant who performed <100 | 27 | 21 | 17.83 | 22.33 | 13.33 |
SD, standard deviation; mm, millimeter.
Similarly, in 18 mm ring diameter model, the average measurement was observed to be 16.25 mm, accounting for a 1.75 mm (10.0%) difference between actual sizes (18 mm) and the average measurements. Furthermore, in a 24 mm ring diameter model, the average measurement was observed to be 20.4 mm, accounting for a 3.6 mm (15.0%) difference between actual size (24 mm) and the average measurements. Finally, in the smallest 13 mm ring diameter model, the average measurement was observed to be 13.3 mm, accounting for a −0.7 mm (5.0%) between actual sizes (13 mm) and the average measurements. Furthermore, VA measurements variations on different models based on the endoscopic experience of the participants are explained in (Table 3).
EDB method
Using the EDB method, an AD from the actual diameter was 1.46±0.9 mm (7.2%) was noticed, while an overestimation of 55% (22/40), and an underestimation of 40% (16/40) of the measurements was also documented. Additionally, the maximum difference from the least accurate single measurement was noted to be 10 mm on the 33 mm ring model. Additionally, in the largest 33 mm ring diameter model, the average measurement was observed to be 30.7 mm, accounting for a 2.7 mm (8.0%) difference between actual sizes (33 mm) and the average measurements. Also, in the 27 mm ring diameter model, the average measurement noted was 27.8 mm, accounting for a −0.8 mm (3.0%) difference between actual sizes (27 mm) and the average measurements (Table 4).
Table 4
| Variable | 33 mm ring diameter model measurement in mm | 27 mm ring diameter model measurement in mm | 18 mm ring diameter model measurement in mm | 24 mm ring diameter model measurement in mm | 13 mm ring diameter model measurement in mm |
|---|---|---|---|---|---|
| Participants | |||||
| Participant 1 | 31 | 29 | 21 | 23 | 16 |
| Participant 2 | 42 | 35 | 20 | 25 | 15 |
| Participant 3 | 26 | 35 | 15 | 22 | 13 |
| Participant 4 | 31 | 27 | 21 | 23 | 16 |
| Participant 5 | 30 | 24 | 15 | 20 | 12 |
| Participant 6 | 24 | 25 | 22 | 25 | 15 |
| Participant 7 | 23 | 22 | 20 | 22 | 16 |
| Participant 8 | 32 | 30 | 23 | 25 | 15 |
| Participant 9 | 30 | 23 | 20 | 20 | 15 |
| Participant 10 | 38 | 28 | 22 | 30 | 12 |
| Average in mm (SD) | 30.7 (5.9) | 27.8 (4.6) | 19.9 (2.8) | 23.5 (2.9) | 14.5 (1.6) |
| Difference from the actual measurement in mm (%) | 2.3 (8.0) | −0.8 (3.0) | −1.9 (11.0) | 0.5 (2.0) | −1.5 (12.0) |
| Participant who performed >1,000 | 27 | 24.75 | 20.75 | 22.5 | 15.5 |
| Participant who performed 250–500 | 29 | 28.67 | 17 | 21.67 | 13.67 |
| Participant who performed <100 | 37.33 | 31 | 21.67 | 26.67 | 14 |
SD, standard deviation; mm, millimeter.
Likewise, in 18 mm ring diameter model, the average measurement noted was 19.9 mm, accounting for a −1.9 mm (11.0%) difference between the actual sizes (18 mm) and the average measurements. Furthermore, in the 24 mm ring diameter model, the average documented was observed to be 23.5 mm, accounting for a 0.5 mm (2.0%) difference between actual size (24 mm) and the average measurements. Ultimately, in the smallest 13 mm ring model, the average measurement noticed was 14.5 mm, accounting for a −1.5 mm (12.0%) difference between actual sizes (13 mm) and the average measurements. Furthermore, VA measurements variations on different models based on the endoscopic experience of the participants are explained in (Table 4).
ER method
Using the ER method, an AD from the actual diameter was 2.4±1.9 mm (9.2%) was noticed, while an overestimation of 35% (14/40), and an underestimation of 60% (24/40) of the measurements was also documented. Additionally, the maximum difference from the least accurate single measurement was noted to be 23 mm on the 33 mm ring model. Additionally, in the largest 33 mm ring diameter model, the average measurement was observed to be 28 mm, accounting for a 5 mm (15.0%) difference between actual sizes (33 mm) and the average measurements. Also, in the 27 mm ring diameter model, the average measurement noted was 25.4 mm, accounting for a 1.6 mm (6%) difference between actual sizes (27 mm) and the average measurements (Table 5).
Table 5
| Variable | 33 mm ring diameter model measurement in mm | 27 mm ring diameter model measurement in mm | 18 mm ring diameter model measurement in mm | 24 mm ring diameter model measurement in mm | 13 mm ring diameter model measurement in mm |
|---|---|---|---|---|---|
| Participants | |||||
| Participant 1 | 29 | 28 | 19 | 22 | 15 |
| Participant 2 | 30 | 28 | 15 | 23 | 13 |
| Participant 3 | 32 | 22 | 16 | 20 | 12 |
| Participant 4 | 30 | 25 | 15 | 20 | 15 |
| Participant 5 | 32 | 28 | 18 | 18 | 12 |
| Participant 6 | 30 | 25 | 15 | 20 | 15 |
| Participant 7 | 10 | 9 | 8 | 11 | 8 |
| Participant 8 | 31 | 30 | 20 | 25 | 14 |
| Participant 9 | 25 | 30 | 15 | 24 | 14 |
| Participant 10 | 31 | 29 | 19 | 23 | 12 |
| Average in mm (SD) | 28 (6.6) | 25.4 (6.3) | 16 (3.4) | 20.6 (4.0) | 13 (2.2) |
| Difference from the actual measurement in mm (%) | 5 (15.0) | 1.6 (6.0) | 2 (11.0) | 3.4 (14.0) | 0 (0) |
| Participant who performed >1,000 | 23.5 | 23 | 14.25 | 19.25 | 13 |
| Participant who performed 250–500 | 31.33 | 25 | 16.33 | 19.33 | 13 |
| Participant who performed <100 | 3.67 | 29 | 18 | 23.67 | N/A |
SD, standard deviation; mm, millimeter; N/A, not available.
Likewise, in 18 mm ring diameter model, the average measurement noted was 16 mm, accounting for a 2 mm (11%) difference between the actual sizes (18 mm) and the average measurements. Furthermore, in the 24 mm ring diameter model, the average documented was observed to be 20.6 mm, accounting for a 3.4 mm (14%) difference between actual size (24 mm) and the average measurements. Ultimately, in the smallest 13 mm ring model, the average measurement noticed was 13 mm, accounting for a 0 mm (0%) difference between actual sizes (13 mm) and the average measurements. Furthermore, VA measurements variations on different models based on the endoscopic experience of the participants are explained in (Table 5).
Discussion
Over the past few decades, the RYGB has emerged the most popular form of bariatric surgery, making up 65% of all weight-loss procedures carried out worldwide (9,10). The success of this innovative treatment option can be assessed by a mean excess body weight loss of 62% and also the resolution of obesity-related diabetes in about 84% of the patients (9,11,12). This significant weight reduction after the RYGB is mostly set off by the restrictive impact of a gastric pouch created and also, to a lesser extent, by the malabsorptive effect of the surgical bypass of the jejunum (9,13). However, RYGB can potentially lead to some severe complications including anastomotic ulcers and strictures, jejunal ulcers and strictures, small bowel obstruction, and most significantly the weight gain after the procedure (9,14-17).
Weight regain after RYGB is multi-factorial, and one of the critical factors is the size of the gastrojejunal (GJ) anastomosis following the RYGB procedure (6,18,19). The exact size of the GJ anastomosis plays an essential function in the future management of the people treated with RYGB (6). Quigley et al. in a study related to the endoscopic findings and their clinical correlations in patients with symptoms after RYGB reported that a GJ anastomosis with a size <5 mm would prevent patients from digesting liquids, a size <10 mm would potentially pose problems in digesting the solid food, while sizes >14 mm are significantly related with post-operative weight regain (20). This highlights the significance of accurate measurement of GJ anastomosis which is supported by the similar finding in a review article by Levine et al., related to the normal anatomy and postoperative complications encountered by the bariatric surgery patients (21).
GJ anastomosis measurement is an essential step in delineating a post-operative therapeutic plan. The available data evaluating the reliability associated with different measurement options is very limited (6,22). The data available so far also emphasizes the use of alternatives other than VE for the precise measurement of the GJ site which is in agreement with the findings of the current study (6). de Quadros et al. in an observer agreement study validating the use of an endoscopic guidewire for the measurement of the GJ anastomosis following RYGB reported that compared to the VE; the new instrument offers a higher degree of reliability and accuracy for the GJ anastomosis measurement which significantly leads to the few post-operative complications (6). Additionally, the precise endoscopic measurement of the GJ anastomosis also tailors the post-operative course of the patient (23).
Conclusions
Endoscopic measurement of the GJ anastomosis is a critical determinant of the postoperative outcomes of the patient. Monitoring the size of GJ anastomosis will not only assist the physician in tailoring the prompt postoperative treatment plan for the patient but can also assist the physician in keeping track of the long-term difficulties leading to the red surgical procedures. Endoscopic visualization though highly practiced by the endoscopists all around the globe, is the least accurate method with the high AD from the actual diameter leading to higher underestimation of the actual size. On the contrary, the balloon method was the most accurate way of measuring the GJ anastomosis of various sizes and also demonstrated low AD from the actual size, and also demonstrated the least of the measurements. Based on the results from the current study and in the light of previously published data, we firmly believe that physicians ought to not depend on the VE for the GJ measurement. Depending on VE may lead to inaccurate judgment, and inappropriate planning of management. We believe that physicians should use a more accurate method to measure the GJ other than VE. Further large scale studies are needed in order to explore the most accurate and validated tool for the measurement of the GJ anastomosis.
Limitations
There are limitations to the current study as well. First, the study was conducted on plastic models mimicking the esophagus and GJ junction. Although the efforts were put in to provide an ideal environment to the endoscopists miming the real patient environment, yet there might be some differences in the measurements due to lack of testing on human subjects. Second, a limited number of measurements were calculated, which might have impacted the measurements overall.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2019.06.12). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Case Western Reserve University, Cleveland, OH, USA. Informed consent was taken from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Panteliou E, Miras AD. What is the role of bariatric surgery in the management of obesity? Climacteric 2017;20:97-102. [Crossref] [PubMed]
- Baig SJ, Priya P, Mahawar KKWeight Regain After Bariatric Surgery-A Multicentre Study of 9617 Patients from Indian Bariatric Surgery Outcome Reporting Group, et al. Obes Surg 2019;29:1583-92. [Crossref] [PubMed]
- Tremmel M, Gerdtham UG, Nilsson PM, et al. Economic Burden of Obesity: A Systematic Literature Review. Int J Environ Res Public Health 2017; [Crossref] [PubMed]
- Kelly T, Yang W, Chen CS, et al. Global burden of obesity in 2005 and projections to 2030. Int J Obes (Lond) 2008;32:1431-7. [Crossref] [PubMed]
- Costa RC, Yamaguchi N, Santo MA, et al. Outcomes on quality of life, weight loss, and comorbidities after Roux-en-Y gastric bypass. Arq Gastroenterol 2014;51:165-70. [Crossref] [PubMed]
- de Quadros LG, Galvao Neto MD, Campos JM, et al. Validation of a new method for the endoscopic measurement of post-bariatric gastric outlet using a standard guidewire: an observer agreement study. BMC Res Notes 2017;10:13. [Crossref] [PubMed]
- Monaco-Ferreira DV, Leandro-Merhi VA. Weight Regain 10 Years After Roux-en-Y Gastric Bypass. Obes Surg 2017;27:1137-44. [Crossref] [PubMed]
- le Roux CW, Heneghan HM. Bariatric Surgery for Obesity. Med Clin North Am 2018;102:165-82. [Crossref] [PubMed]
- Wang B, Levine MS, Rubesin SE, et al. Utility of barium studies for patients with recurrent weight gain after Roux-en-Y gastric bypass. Clin Radiol 2015;70:67-73. [Crossref] [PubMed]
- Buchwald H, Williams SE. Bariatric surgery worldwide 2003. Obes Surg 2004;14:1157-64. [Crossref] [PubMed]
- Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA 2004;292:1724-37. [Crossref] [PubMed]
- Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med 2009;122:248-56.e5. [Crossref] [PubMed]
- Cummings DE, Overduin J, Foster-Schubert KE. Gastric bypass for obesity: mechanisms of weight loss and diabetes resolution. J Clin Endocrinol Metab 2004;89:2608-15. [Crossref] [PubMed]
- Patel S, Szomstein S, Rosenthal RJ. Reasons and outcomes of reoperative bariatric surgery for failed and complicated procedures (excluding adjustable gastric banding). Obes Surg 2011;21:1209-19. [Crossref] [PubMed]
- Yu J, Turner MA, Cho SR, et al. Normal anatomy and complications after gastric bypass surgery: helical CT findings. Radiology 2004;231:753-60. [Crossref] [PubMed]
- Carucci LR, Turner MA, Yu J. Imaging evaluation following Roux-en-Y gastric bypass surgery for morbid obesity. Radiol Clin North Am 2007;45:247-60. [Crossref] [PubMed]
- Miranda da Rocha LC, Ayub Perez OA, Arantes V. Endoscopic management of bariatric surgery complications: what the gastroenterologist should know. Rev Gastroenterol Mex 2016;81:35-47. [Crossref] [PubMed]
- Owers CE, Abbas Y, Ackroyd R, et al. Perioperative Optimization of Patients Undergoing Bariatric Surgery. J Obes 2012;2012:781546 [Crossref] [PubMed]
- Abu Dayyeh BK, Lautz DB, Thompson CC. Gastrojejunal stoma diameter predicts weight regain after Roux-en-Y gastric bypass. Clin Gastroenterol Hepatol 2011;9:228-33. [Crossref] [PubMed]
- Quigley S, Colledge J, Mukherjee S, et al. Bariatric surgery: a review of normal postoperative anatomy and complications. Clin Radiol 2011;66:903-14. [Crossref] [PubMed]
- Levine MS, Carucci LR. Imaging of bariatric surgery: normal anatomy and postoperative complications. Radiology 2014;270:327-41. [Crossref] [PubMed]
- Evans JA, Muthusamy VR, Acosta RD, et al. The role of endoscopy in the bariatric surgery patient. Gastrointest Endosc 2015;81:1063-72. [Crossref] [PubMed]
- Borbely Y, Winkler C, Kroll D, et al. Pouch Reshaping for Significant Weight Regain after Roux-en-Y Gastric Bypass. Obes Surg 2017;27:439-44. [Crossref] [PubMed]
Cite this article as: Tuma F, Waheed A, Khorgami Z, Khaitan L. The inaccuracy of the endoscopic anastomotic measurement techniques. Ann Laparosc Endosc Surg 2019;4:73.