
Right sided colon cancer and peritoneal carcinomatosis
Introduction
Peritoneal carcinomatosis from colon cancer is associated with poor overall prognosis (1,2). Traditionally, patients with colon cancer who develop peritoneal carcinomatosis was thought to have late stage cancer with little to offer medically and rapid progression in their disease (1,2). Patients with peritoneal carcinomatosis often develop poor appetite, malnutrition, bowel obstruction, and infection prior to terminal disease (1,2). With respect to colon cancer, the peritoneal surface actually represents the second most common location for recurrence after the liver (1-3). Approximately a third of the colon cancer patients eventually develop peritoneal carcinomatosis and up to 15% of patients risk developing isolated peritoneal carcinomatosis.
Because of the location of metastases, this patient group face some unique challenges. Prognosis of patients with peritoneal carcinomatosis from colon cancer has traditionally been less than 1 year (1,2). Based on the EVOCAPE trials, the natural history of patients with peritoneal carcinomatosis was well documented with a median survival of 5.2 months (1). Development of systemic chemotherapy has not met the same type of success for treatment of peritoneal carcinomatosis as compared to other distant metastatic sites from colon cancer. The peritoneal surface is thought to be not as well vascularized compared with solid organ metastases, leading to poor penetration of chemotherapy systemically. Chemotherapeutic agents have had limited effect in controlling disease progression in patients with peritoneal surface malignancies. In fact, patients with isolated peritoneal carcinomatosis tend to fare worse compared to other patients with isolated systemic metastases (3). In side-by-side comparison between isolated peritoneal metastases and other single site metastases, Franko et al. reported that the prognosis of these patients was worse than others as a whole (3). In fact, even when multiple sites of metastases were included, those with presence of peritoneal metastases did worse than those without (3).
Development of cytoreductive surgery (CRS) was first attempted on stage IV ovarian cancers as reported by Meigs et al. (4,5) (Figure 1). The application of CRS in colorectal cancer has also become more widespread following the description of the procedure in the early 1990’s (6-8). Combining CRS with either postoperative normothermic chemotherapy (EPIC) or intraoperative hyperthermic chemotherapy (HIPEC), several phase II studies have reported 3-year survival rates approaching 25% to 47%, similar to results following metastasectomy for isolated colorectal liver metastases (8-11). A phase III study from The Netherlands by Verwaal et al. was the first to confirm and demonstrate survival benefits of the combined procedure in patients with known colorectal peritoneal carcinomatosis (12). The study has met several criticisms: patients with appendiceal origin tumors were included, the choice of adjuvant chemotherapy employed afterwards, the vast improvements of systemic chemotherapy since the original trials were done, and the difficulty in discerning the contributions of either the CRS or HIPEC components to survival (12).

As a result, despite the impact CRS and HIPEC can have on management of colorectal cancer, there is significant controversy in wide spread adoption of the surgical technique and its implementation (4,13,14). The procedure is known to be associated with significant morbidity and mortality, approaching 40% and 5% respectively (15). The variability of the approaches employed from center to center, including extent of cytoreduction, the duration of hyperthermic chemoperfusion, the type of chemoperfusate, and even the role of HIPEC have hindered the widespread adoption of this technique (13).
With respect to right and left sided colon cancers, the results of CRS and HIPEC raised several questions due to the differences between the two types of cancer (16,17). Right sided colon cancers have traditionally differed from left sided colon cancers based on genetics, presentations, biological behavior and histological types (18,19). There is widespread knowledge that right sided colon cancers have more mucinous histology (18-20). In addition, right-sided colon cancers were more often B-RAF mutated and had microsatellite instability, whereas the frequency of RAS mutation was similar between right- and left-sided colorectal cancers (16). Do the genetic differences impact the metastatic potential leading to peritoneal carcinomatosis? Is there a difference in survival benefit following CRS and HIPEC between left and right sided colon cancer with peritoneal carcinomatosis? Using the prospectively maintained clinical and biological digestive peritoneal metastasis database of the BIG-RENAPE network, the differences between right- and left-sided colon cancers was compared (17). Overall, right sided colon cancers, though frequently thought to have worse prognosis, seem to have similar overall survival or progression free survival after patients developed peritoneal metastasis (17). This review aims to evaluate the role of CRS and HIPEC on patients with peritoneal carcinomatosis following right sided colon cancers.
Development of CRS and HIPEC
Peritoneal surface reflects a unique area for metastasis in patients with gastrointestinal origin malignancies (13,21). The current models of peritoneal carcinomatosis is based on developments in understanding of ovarian epithelial carcinoma (22). Currently, two models of metastasis predominate. The seed-and-soil hypothesis implies that the tumor cell clones from the original cancer is heterogeneous in population and likely acquired different metastatic phenotype based on the types of tumor. Alternatively, the stochastic model implies that all tumor cells are clones of the original tumor and develop new mutations that allow the daughter clones to adapt to their new surroundings. Likely both types of models contribute, to a varying degree, in the current metastatic patterns seen with different types of cancers. The genetic expression profiles of tumors such as breast cancer and colon cancer argue for the stochastic model where new alterations in the gene is needed to allow the next step in tumor metastasis (22,23).
Peritoneal metastasis suggests a different mechanism for tumor cell spread compared with solid organs and has implications in its management and prognosis. The coelomic cavity is noted for a continuous circulation of ascitic fluid allowing the abdominal contents and proteins to be transported to other regions of the cavity. Ascitic fluid is guided by gravity and then flows toward the diaphragm along the paracolic gutters due to the negative pressure generated during respiration (22). This continuous flow of intra-abdominal contents allows tumor cells access to all parts of the abdomen with preferential deposit sites such as the pelvis and the diaphragmatic undersurfaces due to dependency, a fact that will have implications during surgical debulking. This ascitic fluid also transports contents introduced or shed by epithelial cells and ends up being reabsorbed in the lymphovascular system through drainage sites such as the lymphatic network near the diaphragm or the dependent aspects of the abdominal cavity located in the pelvic brim. Periodically, when tumors gain access to this surface either through shedding of tumor cells by direct extension, surgical manipulation during prior surgeries, or metastasis to this surface from the vascular system, they are provided with a surface conducive to permeative growth with little to confine their spread save for the poor vascular supply and poor oxygen tension within this surface. The exact process by which tumor cells are able to adapt to this surface and proliferate remains poorly understood (22). Adenocarcinomas such as colonic origin tumors can develop isolated peritoneal surface malignancies as a result of gaining access to the abdominal cavity.
CRS in treatment for peritoneal carcinomatosis from colorectal surgery was popularized by Sugarbaker (8,24). The addition of HIPEC evolved following design and implementation of the perfusion circuit initially trialed in canines (25-27). The development of CRS hinges on the concept that the peritoneum is an organ and improved prognosis can be achieved following complete surgical resection of metastatic disease, similar to solid organ metastasis, such as liver resection for colorectal hepatic metastases (24). In order to perform CRS, several guidelines were established to help quantify completeness of cytoreduction. Two types of severity of carcinomatosis grading system have been proposed including the Gilly proposal and the Sugarbaker Peritoneal Cancer Index (4,14). The Gilly classification is similar to staging of ovarian carcinomatosis where the severity of carcinomatosis is based on the largest tumor nodules (4). In the Peritoneal Cancer Index proposed by Sugarbaker, the abdominal cavity is divided into 12 separate zone with each zone harboring a separate quantity of disease ranging from 0 to 3. As a result, the degree of tumor burden could be quantified and expressed ranging from 1 to 39 (12) (Figure 2).

The completeness of cytoreduction has important implications in the prognosis of the patients. In order to allow comparison and define prognosis uniformly, the completeness of cytoreduction also needs to be quantified and is graded as CC-0 (no visible disease), CC-1 (<2.5 mm residual disease), CC-2 (2.5–25 mm), and CC-3 (>25 mm residual disease). Alternatively, others have used the R score which is a rough estimate of the completeness of cytoreduction. This scale ranges from R0 = complete resection, R1 = no gross disease with microscopic positive margins, or R2= macroscopic residual disease [R2a ≤5 mm, R2b =6–20 mm, and R2c ≥20 mm].
Patients undergoing CRS require extensive surgical resection to achieve an R0 or macroscopic complete resection. Peritonectomy is the surgical removal of the peritoneal surface in the abdominal cavity. This often involves an exploratory laparotomy incision to expose the abdominal cavity. Bilateral peritoneal stripping is initiated from midline to the paracolic gutters on either side of the abdomen. Peritoneal stripping to resect burdensome tumor off bilateral diaphragmatic surfaces is frequently both tedious but also important as the lower recesses of the diaphragm can harbor tumor nodules. Since the tumor burden is frequently high in the perineal reflection, a low anterior resection along with hysterectomy is frequently performed in case of tumor involvement in the pelvis.
Complete surgical resection in peritoneal carcinomatosis also involves resection of all visible tumor and associated organs when there are inseparable disease or dense adhesions precluding safe removal of tumor without en-bloc organ resection (8,13). As a result, due to the dense tumor frequently seen in the lesser curvature, the gallbladder and occasionally antrum of the stomach is resected during lesser omentectomy (6,8). In terms of the greater omentum, a splenectomy and occasionally transverse colectomy needs to be performed for adequate cytoreduction. In addition, when tumors involve organ surfaces, distal pancreatectomy, partial colectomy, liver resection and multiple small bowel resections are often concurrently performed in radical CRS (15).
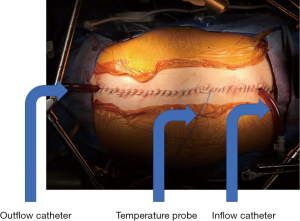
Once the CRS is completed, patients are planned for either EPIC or HIPEC in terms of instillation of chemotherapy (25-28). In cases of normothermic postoperative EPIC, patients have peritoneal access catheters placed prior to abdominal closure. In cases of HIPEC, both the coliseum technique and the closed technique have been used for heated chemotherapy perfusion (Figure 3). During the closed technique, temporary drainage catheters are placed in the abdomen with the incision closed. Perfusate heated to between 40 and 42 °C, with chemotherapy added, is used to perfuse the abdominal cavity for a set amount of time ranging typically from 60 to 120 minutes. The choice of chemotherapy can be a single agent, such as mitomycin C or oxaliplatin, or multiagent chemotherapy such as mitomycin C and cisplatin for their synergistic anti-tumor effects. The coliseum technique involves creating a silo where chemotherapy can be directly washed over the abdominal contents during the perfusion period. The closed technique utilizes a perfusion circuit to bath the abdomen for the duration of the treatment (27,29). Finally, the perfusate is removed and bowel anastomoses performed prior to definitive abdominal closure.
Considerations for laparoscopic CRS
In recent years, with the acceptance of CRS and HIPEC in the management of peritoneal carcinomatosis from colorectal cancer, the application of minimally invasive techniques to this demanding surgery has been reported in small case series (30,31). The application of laparoscopic techniques for advanced colorectal tumors including low rectal cancers, multi-visceral resections, and extended lymphadenectomies have all been described and shown to be associated with less postoperative pain and shorter hospital stays. The safety and feasibility of laparoscopic CRS was first explored in patients with heterogeneous characteristics and non-colorectal cancers. Small case control series comparing open CRS and laparoscopic CRS were published to date (31). Using laparoscopic techniques for CRS has inherent limitations when a larger midline incision is not utilized. At present peritoneal carcinomatosis with limited disease can be resected laparoscopically. In addition, a complete peritonectomy procedure is frequently not utilized in these scenarios because of the difficulty in completely removing all the peritoneal surfaces via laparoscopic approaches (30,31). Furthermore, diseases under the diaphragm are also difficult to access even with the use of angled scopes. Ha et al. have proposed that patients undergoing CRS laparoscopically have limited disease burden with a Peritoneal Carcinomatosis Index (PCI) score less than or equal to 10 (31). In addition, patients with subphrenic, subhepatic and porta hepatitis disease sites should also undergo traditional open surgical interventions (31).
Despite the limitations of laparoscopic approaches in CRS, it may still have a role in patients needing HIPEC. The indications for laparoscopic CRS and HIPEC are well suited for limited disease burdens and patients without gross disease in the presence of malignant ascites (32). Patients with unexpected peritoneal carcinomatosis detected intraoperatively may also be a suitable patient population (32). In patients with unexpected peritoneal carcinomatosis detected intraoperatively, surgical outcome is in line with low disease volume patients treated on a separate setting (32). Survival in this cohort based on available data shows good median survival overall. In addition, patients with limited disease burden with extensive ascites seem to be a good group of patients suitable for laparoscopic CRS with HIPEC to reduce malignant ascites.
Several trials are also underway for high-risk patients that are at risk for development of peritoneal carcinomatosis. Patients with T4 disease, perforated colon cancers, presence of positive peritoneal fluid cytology, or presence of Krukenberg tumors are at high-risk for peritoneal carcinomatosis. For patients undergoing second look procedures due to risk of recurrence, prophylactic HIPEC can be considered. These are patients ideally suited for laparoscopic CRS and HIPEC (33).
Primary colon cancer location and survival following CRS
The location of the primary tumor has an impact on the survival of patients. Patients with peritoneal carcinomatosis from right sided colon cancer has an overall worse prognosis compared with patients with other solid organ sites of systemic metastases (3). Based on the ARCAD database, the patients with peritoneal carcinomatosis overall have worse prognosis compared with patients having other types of metastatic disease (17). The impact of the primary CRC location on the survival outcome showed that patients with right sided colon cancer is worse in stage III disease. In the metastatic setting, the survival benefit for left sided lesions persisted in patients with solid organ disease. The difference in mutational variability between left and right sided colon cancer did not seem to impact the prognosis based on the tumor origins.
Since the location of the tumor might influence survival even after development of peritoneal metastasis, retrospective studies have evaluated whether there is a difference in survival in patients undergoing CRS based on the origin of their original tumor. The data was analyzed from the BIG-RENAPE clinical database on digestive peritoneal metastases, based on 16 different institutions with fourteen from France and two from Canada (17). For patients with isolated peritoneal carcinomatosis, the survival advantage for left sided CRC no longer persisted (17).
CRS in the era of PRODIGE 7
One of the earliest trials looking into benefits of CRS along with HIPEC was conducted in the The Netherlands by Verwaal et al. (12). Between 1998 and 2001, 105 patients were randomly assigned to receive either chemotherapy consisting of fluorouracil and leucovorin or aggressive cytoreduction with HIPEC followed by systemic chemotherapy regimen. Median survival improved from 12.6 to 22.3 months (12). The surgical treatment was associated with a mortality of 8% (12). Several interesting findings from the trial showed that patients with high PCI index had the worst survival. In addition, the patients with macroscopic complete (R1) cytoreduction had much more significant improvements in long-term outcome than incomplete CRS (12).
Based on the findings of survival benefit, the PRODIGE 7 trial was designed to evaluate the benefits of CRS and HIPEC (34). The study arms employed oxaliplatin as the perfusate of choice. Oxaliplatin was thought to be superior to mitomycin C in previous studies (35). Recently, the PRODIGE 7 trial was reported in abstract form in ASCO and raised several interesting findings. The addition of HIPEC to CRS did not increase survival (34). Furthermore, the use of HIPEC did not even improve recurrence free survival within the peritoneal cavity. The data based on the PRODIGE 7 trial seem to refute some of the benefits patients derive from HIPEC. At a median follow-up of 63.8 months, median overall survival was comparable at 41.7 and 41.2 months for patients randomized to CRS and HIPEC with oxaliplatin and CRS alone. There is no specific breakdown of the side of the primary lesion although there are more questions raised than answered. Patients who underwent HIPEC had significantly higher 60-day complication rates. However, the study patients with carcinomatosis, who all underwent CRS with or without HIPEC with oxaliplatin, performed much better than traditional cohort patients who did not undergo CRS and are treated with conventional chemotherapy only.
The addition of HIPEC was not beneficial in the PRODIGE 7 study and the choice of chemotherapeutic agent also raised new questions. In contrast to colorectal cancer with peritoneal metastases, recent developments in ovarian cancer showed significant benefit of HIPEC with over 13 months additional median survival (28). Therefore, rather than halting the use of CRS and HIPEC as a means to treat patients with colorectal cancer with peritoneal carcinomatosis, the current data seem to narrow the therapeutic bandwidth that should be employed on patients with this disease. It appears that the role of CRS in management of isolated peritoneal carcinomatosis is able to consistently offer prolonged median survival in comparison to chemotherapy alone. Optimal cytoreduction is the most important prognostic indicator, a finding long reached in earlier studies investigating the use of CRS (11). The question of the addition of HIPEC to the treatment fervently needs additional clarification.
Conclusions
CRS and hyperthermic intraperitoneal chemoperfusion for management of peritoneal carcinomatosis from colorectal cancer is a therapeutic modality that improves survival in a limited subset of patients. Right sided colon cancer, which has higher incidence of mucinous histology and overall worse prognosis, benefits equally from CRS and HIPEC. Consideration should be given to the patient cohort when they develop isolated peritoneal carcinomatosis.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Roberto Bergamaschi and Mahir Gachabayov) for the series “Right Colon Cancer Surgery: Current State” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2019.07.10). The series “Right Colon Cancer Surgery: Current State” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sadeghi B, Arvieux C, Glehen O, et al. Peritoneal carcinomatosis from non-gynecologic malignancies – Results of the EVOCAPE 1 multicentric prospective study. Cancer 2000;88:358-63. [Crossref] [PubMed]
- Jayne DG, Fook S, Loi C, et al. Peritoneal carcinomatosis from colorectal cancer. Br J Surg 2002;89:1545-50. [Crossref] [PubMed]
- Franko J, Shi Q, Meyers JP, et al. Prognosis of patients with peritoneal metastatic colorectal cancer given systemic therapy: an analysis of individual patient data from prospective randomized trials from the Analysis and Research in Cancers of the Digestive System (ARCAD) database. Lancet Oncol 2016;17:1709-19. [Crossref] [PubMed]
- Neuwirth MG, Alexander HR, Karakousis GC. Then and now: cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (HIPEC), a historical perspective. J Gastrointest Oncol 2016;7:18-28. [PubMed]
- Meigs JV. Tumors of the female pelvic organs. New York: The Macmillan Co, 1934.
- Sugarbaker PH, Kern K, Lack E. Malignant pseudomyxoma peritonei of colonic origin: natural history and presentation of a curative approach to treatment. Dis Colon rectum 1987;30:772-9. [Crossref] [PubMed]
- Sugarbaker PH, Gianola FJ, Speyer JC, et al. Prospective, randomized trial of intravenous versus intraperitoneal 5-fluorouracil in patients with advanced primary colon or rectal cancer. Surgery 1985;98:414-22. [PubMed]
- Sugarbaker PH. Peritonectomy procedures. Ann Surg 1995;221:29-42. [Crossref] [PubMed]
- Gilly FN, Sayag AC, Carry PY, et al. Intraperitoneal hyperthermic chemotherapy in the treatment of peritoneal carcinomatosis of digestive origin: a case report and physiopathology. J Chir (Paris) 1990;127:95-8. [PubMed]
- Gilly FN, Beaujard A, Glehen O, et al. Peritonectomy combined with intraperitoneal chemohyperthermia in abdominal cancer with peritoneal carcinomatosis: Phase I-II study. Anticancer Res 1999;19:2317-21. [PubMed]
- Glehen O, Kwiatkowski F, Sugarbaker PH, et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: A multi-institutional study. J Clin Oncol 2004;22:3284-92. [Crossref] [PubMed]
- Verwaal VJ, van Ruth S, de Bree E, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carciomatosis of colorectal cancer. J Clin Oncol 2003;21:3737-43. [Crossref] [PubMed]
- Bartlett DL. Current management and future opportunities for peritoneal metastases. Ann Surg Oncol 2018;25:2132-4. [Crossref] [PubMed]
- Rajeev R, Turaga KK. Hyperthermic intraperitoneal chemotherapy and cytoreductive surgery in the management of peritoneal carcinomatosis. Cancer Control 2016;23:36-46. [Crossref] [PubMed]
- Gusani NJ, Cho SW, Colovos C, et al. Aggressive surgical management of peritoneal carcinomatosis with low mortality in a high-volume tertiary cancer center. Ann Surg Oncol 2008;15:754-63. [Crossref] [PubMed]
- Petrelli F, Tomasello G, Borgonovo K, et al. Prognostic survival associated with left-sided vs right-sided colon cancer: A systematic review and eta-analysis. JAMA Oncol 2017;3:211-9. [Crossref] [PubMed]
- Péron J, Mercier F, Tuech JJ, et al. The location of the primary colon cancer has no impact on outcomes in patients undergoing cytoreductive surgery for peritoneal metastasis. Surgery 2019;165:476-84. [Crossref] [PubMed]
- Benedix F, Kube R, Meyer F, et al. Colon/Rectum carcinomas study G. Comparison of 17,641 patients with right- and left-sided colon cancer: differences in epidemiology, perioperative course, histology, and survival. Dis Colon Rectum 2010;53:57-64. [Crossref] [PubMed]
- Baran B, Ozupel NM, Tetik NY, et al. Difference between left-sided and right-sided colorectal cancer: A focused review of literature. Gastroenterology Res 2018;11:264-73. [Crossref] [PubMed]
- Hugen N, Brown G, Glynne-Jones R, et al. Advances in the care of patients with mucinous colorectal cancer. Nat Rev Clin Oncol 2016;13:361-9. [Crossref] [PubMed]
- Nagata H, Ishihara S, Hata K, et al. Survival and Prognostic Factors for Metachronous Peritoneal Metastasis in Patients with Colon Cancer. Ann Surg Oncol 2017;24:1269-80. [Crossref] [PubMed]
- Tan DS, Agarwal R, Kaye SB. Mechanisms of transcoelomic metastasis in ovarian cancer. Lancet Oncol 2006;7:925-34. [Crossref] [PubMed]
- Weigelt B, Glas AM, Wessels LF, et al. Gene expression profiles of primary breast tumors maintained in distant metastases. Proc Natl Acad Sci U S A 2003;100:15901-05. [Crossref] [PubMed]
- Sugarbaker PH, Jablonski KA. Prognostic features of 51 colorectal and 130 appendiceal cancer patients with peritoneal carcinomatosis treated by cytoreductive surgery and intraperitoneal chemotherapy. Ann Surg 1995;221:124-32. [Crossref] [PubMed]
- Spratt JS, Adcock RA, Sherrill W, et al. Hyperthermic peritoneal perfusion system in canines. Cancer Res 1980;40:253-5. [PubMed]
- Spratt JS, Adcock RA, Muskovin M, et al. Clinical delivery system for intraperitoneal hyperthemic chemotherapy. Cancer Res 1980;40:256-60. [PubMed]
- Alexander HR, Fraker DL. Treatment of peritoneal carcinomatosis by continuous hyperthemic peritoneal perfusion with cisplatin. Cancer Treat Res 1996;81:41-50. [Crossref] [PubMed]
- van Driel WJ, Koole SN, Sikorska K, et al. Hyperthermic intraperitoneal chemotherapy in ovarian cancer. N Engl J Med 2018;378:230-40. [Crossref] [PubMed]
- Dodson RM, Kuncewitch M, Votnopoulos KI, et al. Techniques for cytoreductive surgery with hyperthemic intraperitoneal chemotherapy. Ann Surg Oncol 2018;25:2152-8. [Crossref] [PubMed]
- Park SY, Choi GS, Park JS, et al. Laparoscopic cytoreductive surgery and early postoperative intraperitoneal chemotherapy for patients with colorectal cancer peritoneal carcinomatosis: initial results from a single center. Surg Endosc 2014;28:1555-62. [Crossref] [PubMed]
- Ha SH, Park SY, Park JS, et al. Short-term outcomes after laparoscopic cytoreductive surgery in patient with limited peritoneal metastases from colorectal cancer. Surgery 2019;165:775-81. [Crossref] [PubMed]
- Canda AE, Arslan C, Terzi C, et al. Treatment of intraoperatively detected peritoneal carcinomatosis of colorectal origin with cytoreductive surgery and intraoperative chemotherapy. World J Surg Oncol 2018;16:70. [Crossref] [PubMed]
- Paul BK, Ihemelandu C, Sugarbaker PH. Prior surgical score: An analysis of the prognostic significance of an initial nondefinitive surgical intervention in patients with peritoneal carcinomatosis of a colorectal origin undergoing cytoreductive surgery and perioperative intraperitoneal chemotherapy. Dis Colon Rectum 2018;61:347-54. [Crossref] [PubMed]
- Quenet F, Elias D, Roca L, et al. A UNICANCER phase III trial of hyperthemic intra-peritoneal chemotherapy (HIPEC) for colorectal peritoneal carcinomatosis (PC): PRODIGE 7. J Clin Oncol 2018;36:LBA3503 [Crossref]
- Leung V, Huo YR, Liau W, et al. Oxaliplatin versus Mitomycin C for HIPEC in colorectal cancer with peritoneal carcinomatosis. Eur J Surg Oncol 2017;43:144-9. [Crossref] [PubMed]
Cite this article as: Dong XD. Right sided colon cancer and peritoneal carcinomatosis. Ann Laparosc Endosc Surg 2019;4:72.


