Hand-assisted laparoscopic colon resection: review of literature and technique
Laparoscopic colon resections were first performed in the 1990s (1). However, as with any new technique and skill, there was an associated learning curve that initially limited its wide use and acceptance (2). Moreover, there were concerns that laparoscopy may be an inferior oncologic technique, compared to conventional open surgery (3). Hand-assisted laparoscopic surgery (HALS) was developed as a technique to mitigate these concerns. The concept of HALS for colorectal resection was first described in the mid-1990s as a way to bridge the gap between straight laparoscopy and traditional open surgery. In essence, HALS was proposed as a method to combine the best elements and overcome the difficulties of open and laparoscopic surgery in a hybrid technique (4). Ultimately, several studies demonstrated oncologic equivalency between laparoscopic and open colorectal surgery. Furthermore, laparoscopy appears to have several benefits over open surgery, including shorter length of stay and recovery as well as improved pain control and cosmesis (5). Over the last almost 30 years, there has been a steady increase in the use of laparoscopy in colorectal surgery and a concomitant decrease in the use of conventional open surgery (6,7). Because laparoscopy has become much more accepted and utilized, the modern colorectal surgeon is becoming increasingly facile with straight laparoscopic surgical (SLS) techniques. As colorectal surgeons become more proficient with laparoscopy, the role of HALS in colorectal surgery may be less well defined. Nevertheless, HALS can still provide an important tool in the repertoire of a colorectal surgeon. This article will discuss some of the advantages and disadvantages of HALS, as it compares to SLS, as well as describe some operative techniques in colorectal HALS.
HALS takes advantage of the fact that, regardless of laparoscopic technique, at some point in the operation a larger incision will need to be made to extract the specimen. Proponents of HALS therefore argue that this incision can be made early in the operation and be used throughout the surgery to enhance dexterity and efficiency of the surgeon. In HALS, the surgeon inserts one hand into the abdominal cavity through a hand assist device, typically to provide exposure, while the other hand works through a laparoscopic trocar, typically using an energy device. The goal of the hand-assist is to provide maximal exposure and assist with dissection while remaining as much out of camera view as possible. Often, the third, fourth and fifth fingers can provide retraction while the thumb and index finger are used to grasp, expose target anatomy or dissect. With the HALS approach, the surgeon can obtain further tactile feedback and obtain broader exposure that is less reliant on the assistant, compared with straight laparoscopy. A laparotomy pad can be placed within the abdomen prior to securing the hand assist device, which can help with retraction, cleaning the camera and keeping the operative field dry. Although, if this move is performed, it is crucial to have a reminder to remove it prior to final closure to avoid a retained foreign body. In general, HALS is best suited for patients who are candidates for laparoscopic surgery, but whose surgery may be difficult to performed using straight laparoscopy, such as those with a high body mass index (BMI), who have bulky disease, or if there is a high concern for conversion to open surgery (8).
Several hand assist devices are commercially available, and two of the most commonly used devices are GelPort (Applied Medical, USA) and Endopath Dextrus® (Ethicon, USA). Both devices feature a wound protector system that is inserted through the abdominal wall, as well as a cover placed externally which allows for maintenance of pneumoperitoneum and insertion of the surgeon’s hand. The cover is easily removed to allow for specimen extraction, direct “open” visualization or dissection (described below), bowel transection and anastomosis construction. In general, the size of the hand assist device incision will approximate the width of the surgeon’s hand, roughly 6–8 cm. Depending on the operation and on surgeon preference, the incision for the hand assist device can be placed as an upper midline, lower midline or low transverse (Pfannenstiel) incision.
Several studies have compared SLS and HALS in colorectal surgery. The HALS Study Group initially performed a prospective nonrandomized multicenter study designed to evaluate the feasibility and potential benefits of HALS. Although it was a small study (68 patients total) and did not exclusively examine colorectal procedures, it concluded that HALS appeared to be a safe and useful technique that compared favorably to historical studies of SLS (9). The HALS Study Group subsequently performed a prospective, randomized, multicenter study that included 40 patients with colorectal disease requiring elective resection. Patients were randomized to one of the two treatment arms (22 HALS, 18 SLS). The study found no difference in their main outcomes between the two groups, including operative time (152 vs. 141 minutes), blood loss, postoperative pain, time to oral intake, return of bowel function, length of stay, morbidity, and functional recovery. Additionally, the incision length for specimen extraction/bowel anastomosis was similar (HALS 7.4 vs. SLS 7.0 cm). These results led the authors to conclude that “hand-assisted laparoscopy retains the benefits of minimally invasive surgery and may allow the surgeon to perform complex operations more easily” (10).
A subsequent prospective randomized trial by Targarona et al. compared HALS to SLS in a single center. Operative times and post-operative outcomes were similar between the two groups. However, the study found that the conversion rate was higher in SLS (22% vs. 7%), although this difference was not statistically significant. Of the 6 SLS cases that were converted, 4 were completed using HALS, suggesting that the HALS technique can be used as an intermediary between SLS and open surgery, allowing the surgeon to perform the operation with improved access, while still preserving the benefits of minimally invasive surgery over open surgery. Interestingly, the study found that HALS induced a greater inflammatory response (presumably from more manipulation of the bowel) than SLS, as measured by interleukin (IL)-6 and C-reactive protein (CRP) over time. However, it appeared that this chemical difference did not result in a clinical difference, as the measured outcomes of morbidity and hospital stay were no different (11).
The Minimally Invasive Therapeutic Trial by Marcello et al. was a multicenter, prospective, randomized study whose primary outcome was to detect a difference in operating time between SLS and HALS for patients undergoing sigmoid/left colectomy and total colectomy. A total of 95 patients were examined (47 HALS and 48 SLS) and the results showed a significantly shorter operative time with HALS in both sigmoid colectomy (175±58 vs. 208±55; P=0.021) and total colectomy (127±31 vs. 184±72; P=0.015). Otherwise, there were no differences in post-operative outcomes, including return of bowel function, tolerance of diet, length of stay, postoperative pain scores, narcotic usage or complications between the two groups. The incision length was larger for the HALS sigmoid colectomy (8.2 vs. 6.1 cm; P<0.01) but not statistically different for the total colectomies (7.8 vs. 6.7 cm, P=0.09) (12).
As colorectal surgeons become more experienced with laparoscopic techniques and surgical residents increasingly train in an environment that includes laparoscopy, some argue that the utility of HALS may become less apparent compared to SLS. In a study by Hassan et al., a review of a single center prospectively maintained database was examined to compare the centers experience between SLS and HALS. The study found that the baseline characteristics between the two groups were similar, yet more patients in the HALS group underwent complex procedures and extensive resections. Outcomes including conversion, intraoperative complications, morbidity and reoperation were no different, but the HALS group had longer operative times (276 vs. 211 minutes, P<0.0001) and longer hospital stay (6 vs. 5 days, P=0.0009). The authors concluded that by using HALS selectively, they were able to increase number of extended and complex colorectal resections while maintaining surgical outcomes, allowing them to offer minimally invasive surgery to patients who may not have otherwise been candidates for laparoscopic surgery (13).
A study by Jadlowiec et al. found that HALS can still play an important role, even in a “mature colorectal practice” that favors SLS. The group performed a retrospective review to examine trends in the use of SLS and HALS as a single center. They found an overall decline in the use of HALS during the study period from 2005 to 2011, although HALS was not fully replaced by SLS. The greatest use of HALS was in diverticular disease, particularly in those with dense inflammatory adhesions, fistulas, and phlegmons, and in those with bulky colorectal tumors. They concluded that in a modern colorectal surgery practice, the decision to perform HALS can be made intraoperatively, and the surgeon’s ability to perform both SLS and HALS may improve ability to complete operations without a complete open conversion (14).
The cost of HALS appears to be comparable to that of SLS. Targarona et al. found no differences in the mean operative costs (11). Ozturk et al. performed a more in-depth analysis of the cost of HALS compared to SLS by performing case matched retrospective review of both operating room and total peri-operative care costs. In this study, 100 HALS colectomies were matched to 100 SLS colectomies performed concurrently from 2005–2008. Between the two groups, operating time, readmission and length of stay were no different. There was a higher rate of overall morbidity (32% vs. 16%, P=0.009). Operating room costs were higher for HALS ($3,476 versus $3,167), but total costs were similar ($8,521 versus $8,373) (15).
While several studies demonstrated similar short-term outcomes between HALS and SLS, because HALS can induce more inflammation (11) and may require a larger incision compared to SLS (12), some have suggested that HALS could lead to more long-term complications, such as incisional hernia or bowel obstruction. Sonoda et al. performed a retrospective review of 536 consecutive patients who underwent HALS or SLS colorectal resections, and compared rates of incisional hernia and bowel obstruction, with a median follow up of 27 months. They found that the rates of incisional hernia (6% with HALS vs. 4.8% with SLS, P=0.54) and the rate of bowel obstruction (4.5% with HALS vs. 7.4% with SLS, P=0.11) were no different (16). These results again suggested that HALS preserves the benefits of a minimally invasive approach.
Ultimately, the decision to use HALS is surgeon preference, but studies have consistently demonstrated that the outcomes between SLS and HALS appear to be similar. Depending on surgeon skill and comfort, HALS may shorten operative times and allow surgeons to perform a minimally invasive colorectal resection in a patient with high BMI, bulky disease, a hostile abdomen that may otherwise be too difficult to complete laparoscopically.
Right colectomy
Positioning
The patient should be positioned supine or in modified lithotomy position on the operating room table with both arms tucked at the sides to allow for maximum surgeon mobility. Lithotomy position allows the surgeon to stand between the legs if needed. As always, the patient should be well padded and strapped securely to the table to allow for bed tilt to facilitate operative exposure. The surgeon and assistant will stand on the patient’s left side and laparoscopic monitors and equipment towers are set up on the patient’s right side.
Port placement
The hand-assist device should be placed in the midline at the center of the abdomen, half way between the xyphoid and the pubis regardless of the location of the umbilicus. Placing the hand assist device in this location allows for optimal ergonomics as well as exposure when performing the bowel anastomosis. Pneumoperitoneum can be then be established by placing a trocar through the hand-assist device. Two additional 5 mm trocars are placed under direct visualization: a camera port in the epigastrium slightly to the left of the midline and a working port in the left upper abdomen in the midclavicular line approximately halfway between the costal margin and upper extension of the midline incision.
Operative technique
The hand assisted laparoscopic technique for the right colon is best approached from the top down, by initially mobilizing the transverse colon and hepatic flexure. The transverse colon, right colon and terminal ileum will be elevated from the retroperitoneum before dividing mesentery or intestine. The patient should first be placed in reverse Trendelenburg with the right side tilted up. The surgeon’s left hand in then placed through the hand-assist device and provides caudal traction on the transverse colon and greater omentum. Using a vessel sealing device, the lesser sac in opened by dividing the gastrocolic ligament. The dissection is carried to the right taking down the hepatic flexure and exposing the underlying duodenum and Gerota’s fascia. The back of the hand is used to elevate the colon off the retroperitoneum and the vessel sealer is used to divide the filmy retroperitoneal attachments. A generous mobilization of the right colon and ileal mesentery will facilitate specimen extraction and anastomosis. Once the mesentery is completely elevated, the lateral attachments of the right colon are divided. The right ureter and gonadal vessels should be visualized. The transverse colon is then grasped and elevated to expose the dorsal surface of the transverse mesocolon. A window in the transverse mesocolon is then developed to the left of the middle colic vessel. The vessel sealer can then be used to divide the middle colic and ileocolic vessels near their origins.
The specimen can then be extracted through the hand assist device. The remaining mesentery and marginal vessels at the transverse colon and ileum are divided at the transection points, and the bowel is divided. A stapled or hand-sewn ileocolic anastomosis is then performed, per surgeon preference, and then returned to the abdomen. The fascia of the hand port is closed and the remaining laparoscopic trocars are removed.
Left/sigmoid colectomy
Positioning
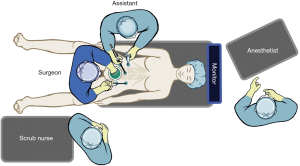
The patient should be placed in a modified lithotomy position or in a split leg position to allow the surgeon to stand between the legs for splenic flexure mobilization and to permit access to the anus for colonoscopy and insertion of the circular stapler. Adequate padding and securely strapping the patient to the operating room table is imperative to allow for bed tilt. Both arms are tucked at the sides and a bladder catheter is placed. To start, the surgeon will stand between the legs and the assistant first will stand on the patient’s right side. The laparoscopic monitors and equipment towers are set up on the patient’s left side (Figure 1).
Port placement
In most situations, the hand-assist device can be placed through a Pfannenstiel incision two fingerbreadths above the symphysis pubis. If the surgeon is concerned about a high risk of conversion to an open operation or if the patient had a previous lower midline incision, the hand-assist device can be placed through a lower midline incision. It is crucial to create generous flaps of the anterior rectus sheath and provide sufficient room for the surgeon’s hand. The surgeon’s hand is then used to protect the underlying viscera while placing a 5 mm trocar in the midline above the umbilicus. Pneumoperitoneum is established and two additional 5 mm trocars are placed under direct visualization: one in the right lower quadrant and one in the left lower quadrant.
Operative technique
Splenic flexure mobilization
Often, mobilization of the splenic flexure is essential to allow for a tension-free colorectal anastomosis. In the hand-assisted laparoscopic left/sigmoid colectomy, splenic flexure mobilization can be performed using a medial to lateral approach. With the patient placed in reverse Trendelenburg and tilted right side down, the surgeon stands between the patient’s legs. The assistant holds the camera and gently retracts the greater omentum and transverse colon cephalad using the right lower trocar. The surgeon uses a left hand in the hand assist port and a vessel sealing device in the left lower trocar. The inferior mesenteric vein (IMV) is identified lateral to the 4th portion of the duodenum and ligament of Treitz. The peritoneum between the IMV and duodenum is incised to enter the avascular plane between the colon mesentery and the retroperitoneum. This space can be developed using gentle blunt dissection with the left hand. It is critical to ensure that the tail of the pancreas is not elevated in this dissection plane. The dissection is carried laterally to the abdominal wall and superiorly over the anterior surface of the pancreas into the lesser sac. Once this dissection is complete, the IMV can be divided. Next, the assistant retracts the greater omentum cephalad while the surgeon retracts the transverse colon caudally such that the omentum and colon can be separated to enter the lesser sac, connecting to the previously developed medial to lateral dissection. The omentum should be fully released from the midline to the splenic flexure. The surgeon’s left hand can be placed posterior to the colon and mesentery to provide exposure, and the dissection can be carried laterally to release the colon from the abdominal side wall by incision the line of Toldt and again joining the medial to lateral dissection plane.
Inferior mesenteric artery (IMA) ligation
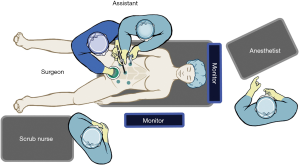
The surgeon then moves to stand at the patient’s right side with the right hand in the hand assist port and a vessel sealing device in the right lower port (Figure 2). The patient is placed in steep Trendelenburg still with the right side down. The right hand is used to grasp the IMA pedicle, elevating the colon mesentery. The peritoneum overlying the pedicle is incised and again the avascular plane between the colon mesentery and the retroperitoneum is entered. Using the hand, this plane is developed bluntly laterally and then cephalad to join the previous retromesenteric plane. Once this place is fully developed, the IMA can be ligated. The retromesenteric plane is then carried caudally to the sacral promontory and, the lateral attachments of the sigmoid colon are then divided. Depending on the location of the disease, the colon can either be divided at this location or the dissection can then be carried distally into the mesorectal plane.
Total mesorectal excision
The hand assisted laparoscopic technic offers a variety of approaches to performing the TME. Regardless of the approach, the principles of the TME are the same: to dissect an intact mesorectal envelope while preserving the surrounding structures, including hypogastric nerves, ureters, and blood vessels. To continue with a hand-assist approach, the surgeon remains on the patient’s right side with the right hand in the hand assist port and a vessel sealing device in the right lower port. Alternatively, the TME can be performed in an open fashion by removing the lid of the hand assist device. The small bowel can be packed in the upper abdomen using laparotomy pads. The sigmoid colon can be delivered through the incision and a proximal transection point is chosen where the colon is then divided. Lighted retractors are then used to provide retraction and the dissection is carried out in the usual open fashion. The distal transection point is chosen and the rectum is divided. The anastomosis can be performed in a hand-sewn or stapled end to end fashion.
Conclusions
HALS combines the advantages of both minimally invasive surgery and conventional open surgery. This technique offers similar short- and long-term benefits to that of straight laparoscopic procedures. Furthermore, it is easy to learn, easy to teach and can be especially helpful in complex procedures or in obese patients. HALS should be considered as an important tool in the surgeon’s armamentarium. It can be used as an initial approach or as an adjunct to straight laparoscopy depending on the surgeon’s preference and experience.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Michael J Stamos and Mehraneh Dorna Jafari) for the series “Laparoscopic Colon Surgery” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2019.03.01). The series “Laparoscopic Colon Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jacobs M, Verdeja JC, Goldstein HS. Minimally invasive colon resection (laparoscopic colectomy). Surg Laparosc Endosc 1991;1:144-50. [PubMed]
- Schlachta CM, Mamazza J, Seshadri PA, et al. Defining a learning curve for laparoscopic colorectal resections. Dis Colon Rectum 2001;44:217-22. [Crossref] [PubMed]
- Påhlman L. The problem of port-site metastases after laparoscopic cancer surgery. Ann Med 1997;29:477-81. [Crossref] [PubMed]
- Ou H. Laparoscopic-assisted mini laparatomy with colectomy. Dis Colon Rectum 1995;38:324-6. [Crossref] [PubMed]
- Aly EH. Laparoscopic colorectal surgery: summary of the current evidence. Ann R Coll Surg Engl 2009;91:541-4. [Crossref] [PubMed]
- Kang CY, Halabi WJ, Luo R, et al. Laparoscopic colorectal surgery: a better look into the latest trends. Arch Surg 2012;147:724-31. [Crossref] [PubMed]
- Davis CH, Shirkey BA, Moore LW, et al. Trends in laparoscopic colorectal surgery over time from 2005-2014 using the NSQIP database. J Surg Res 2018;223:16-21. [Crossref] [PubMed]
- Stein S, Whelan RL. The controversy regarding hand-assisted colorectal resection. Surg Endosc 2007;21:2123-6. [Crossref] [PubMed]
- Litwin DE, Darzi A, Jakimowicz J, et al. Hand-assisted laparoscopic surgery (HALS) with the HandPort system: initial experience with 68 patients. Ann Surg 2000;231:715-23. [Crossref] [PubMed]
- Hand-assisted laparoscopic surgery vs standard laparoscopic surgery for colorectal disease: a prospective randomized trial. HALS Study Group. Surg Endosc 2000;14:896-901. [Crossref] [PubMed]
- Targarona EM, Gracia E, Garriga J, et al. Prospective randomized trial comparing conventional laparoscopic colectomy with hand-assisted laparoscopic colectomy: applicability, immediate clinical outcome, inflammatory response, and cost. Surg Endosc 2002;16:234-9. [Crossref] [PubMed]
- Marcello PW, Fleshman JW, Milsom JW, et al. Hand-assisted laparoscopic vs. laparoscopic colorectal surgery: a multicenter, prospective, randomized trial. Dis Colon Rectum 2008;51:818-26; discussion 826-8. [Crossref] [PubMed]
- Hassan I, You YN, Cima RR, et al. Hand-assisted versus laparoscopic-assisted colorectal surgery: Practice patterns and clinical outcomes in a minimally-invasive colorectal practice. Surg Endosc 2008;22:739-43. [Crossref] [PubMed]
- Jadlowiec CC, Mannion EM, Thielman MJ, et al. Evolution of technique in performance of minimally invasive colectomies. Dis Colon Rectum 2014;57:1090-7. [Crossref] [PubMed]
- Ozturk E, Kiran RP, Geisler DP, et al. Hand-assisted laparoscopic colectomy: benefits of laparoscopic colectomy at no extra cost. J Am Coll Surg 2009;209:242-7. [Crossref] [PubMed]
- Sonoda T, Pandey S, Trencheva K, et al. Longterm complications of hand-assisted versus laparoscopic colectomy. J Am Coll Surg 2009;208:62-6. [Crossref] [PubMed]
Cite this article as: Gahagan JV, Garrett KA. Hand-assisted laparoscopic colon resection: review of literature and technique. Ann Laparosc Endosc Surg 2019;4:27.