
Which is safer in anastomosis, transanal or robotic total mesorectal excision?
The article “A multicenter matched comparison of transanal and robotic total mesorectal excision for mid and low-rectal adenocarcinoma” as published in the 2018 issue of Annals of Surgery, reports very informative data. The transanal total mesorectal excision (TaTME) is the most noteworthy item in recent years. Surgical robot enables precise dissection within narrow and deep pelvis. Therefore, it is a very useful tool to perform total mesorectal excision (robotic TME, RTME). This paper (1) is very interesting because it compares these two methods, which have great advantages in performing TME. In this paper, there were no differences in the incidence of poor quality resection including incomplete quality of TME and positive circumferential resection margin (CRM) in both groups. However, distal resection margin (DRM) involvement was higher in the TaTME group: the authors mentioned that this result may be related to a learning curve effect. They also stressed the importance of careful dissection to avoid DRM involvement for rectal cancers near to the anorectal ring. In this paper, there was no difference in anastomotic leakage rate in both groups (TaTME 11.1% vs. RTME 9.5%; P=0.612).
A simple comparison of TaTME and RTME seems to be unreasonable in the field. This is because it is uncommon for a surgeon who can perform RTME to require TaTME. TaTME may be considered when surgeons do not want to perform a robotic surgery or patients refuse to undergo a robotic surgery because of economic problems even if there is a surgical robot in the hospital. The rate of unsuccessful deep pelvic dissection is very low among surgeons performing robotic surgery. I personally have experience of 172 cases of robotic surgeries and only a few cases of unsuccessful deep pelvic dissection. In other words, surgeons who perform robotic surgery are more likely to successfully perform deep pelvic dissection, and TaTME is rarely needed. However, if for some reason the surgeon is unable to perform robotic surgery, it may be helpful to perform the TaTME.
Perez et al. (2) reported a comparison of 115 cases: 60 robotic low anterior resection (RLAR) and 55 TaTME. There were no differences in operating time, perioperative complications rate, oncologic outcomes except CRM (RLAR 19 mm vs. TaTME 12 mm; P<0.001) and DRM (RLAR 31 mm vs. TaTME 19 mm; P=0.007). There was no difference of the anastomotic leakage rate in both groups (RLAR 11.7% vs. TaTME 12.7%; P=0.862).
Law and Foo (3) reported a comparison of 40 RTME and 40 TaTME after propensity score matching. There were no significant differences between the groups in the incidences of anastomotic leakage (RTME 2 vs. TaTME 2; P=1.000), positive CRM (RTME 2 vs. TaTME 0; P=0.494) and DRM (RLAR 20 mm vs. TaTME 20 mm; P=0.116).
Lee et al. (4) reported a comparison of 24 RTME and 21 TaTME after case matching. There were no significant differences between the groups in the incidences of anastomotic leakage (RTME 3 vs. TaTME 1; P=0.363), positive CRM (RTME 2 vs. TaTME 1; P=0.738) and DRM (RLAR 19 mm vs. TaTME 22 mm; P=0.312).
Veltcamp Helbach et al. (5) reported the prevalence and localization of residual mesorectum by MRI after TaTME (32 patients) and laparoscopic TME (LapTME, 32 patients) in rectal cancer. MRI was performed at least 6 months after surgery. There were no differences in the sex, body mass index (BMI), neoadjuvant therapy, postoperative complications, TME specimen quality, and positive CRM in both groups. There were differences in the operative time (LapTME 164 min vs. TaTME 206 min; P<0.001), tumor height from anal verge (LapTME 8.7 cm vs. TaTME 7.4 cm; P=0.004), and anastomosis height (LapTME 7.3 cm vs. TaTME 4.7 cm; P<0.001). Residual mesorectum by MRI was detected in one patient (3.1%) after TaTME, and in 15 patients (46.9%) after LapTME (P<0.001). Multivariate analysis demonstrated only the type of surgery as a significant risk factor for leaving residual mesorectal tissue (P=0.005). They concluded that the complete mesorectal excision was significantly better with TaTME than with LapTME.
As seen in the above-mentioned papers, TaTME is evaluated as a satisfactory operation in terms of quality of surgical resection. However, is the incidence of anastomotic leakage after TaTME acceptable enough? Anastomotic leakage is a devastating complication to patients and surgeons after colorectal or coloanal anastomosis. The following two articles are large-scale studies of anastomotic leakage after TaTME.
Penna et al. (6) reported the results from the international TaTME registry about anastomotic failure in 1,594 patients who underwent TaTME. Anastomotic failure included leak (early or late), anastomotic fistula or stricture, chronic sinus, and pelvic abscess. The overall anastomotic failure rate was 15.7%: leak (early 7.8%, delay 2.0%); pelvic abscess (4.7%); anastomotic stricture (3.6%); chronic sinus (0.9%); anastomotic fistula (0.8%). Independent risk factors of early anastomotic leak included male sex, high BMI (≥30 kg/m2), smoking, diabetes, larger tumors (>25 mm), high tumor height (>4 cm) from anorectal junction on MRI, and excessive intraoperative blood loss (≥500 mL).
The 2017 European Society of Coloproctology (ESCP) collaborating group reported an international multicenter prospective audit (2,579 patients from 355 centers in 49 countries) of elective TME for rectal cancer (7). Type of surgical approach included open, non-transanal laparoscopic, transanal laparoscopic, non-transanal robotic, and transanal robotic TME. The primary end point was anastomotic leakage. The anastomotic leak rate was 6.7% for laparoscopic TME, 10.4% for TaTME, 5.5% for open TME, 6.5% for robotic TME, and 15.6% for robotic TaTME (P<0.001). In the univariate analysis both TaTME (P=0.038) and robotic TaTME (P=0.019) were associated with a higher risk of anastomotic leak than laparoscopic TME. However, in the multivariate analysis TaTME (P=0.38) and robotic TaTME (P=0.135) were no longer significantly associated with leak, but male gender (P<0.001) and low rectal anastomosis (P<0.001) remained strongly associated.
The two papers mentioned above re-consider whether anastomosis is safe after TaTME.
Which is safer in anastomosis, transanal or robotic total mesorectal excision?
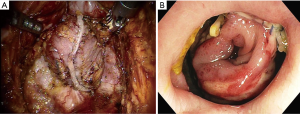
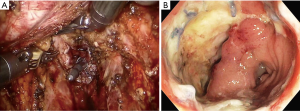
The answer to this question seems to be very difficult. However, I want to describe my personal opinion. Personally, I think the most serious complication after TaTME is anastomotic leakage. I recently experienced two patients with anastomotic leakage after RTME. One patient underwent double-stapling anastomosis after transection of the lower rectum (Figure 1A) using two robotic linear staplers. Anastomotic leakage occurred on postoperative day 6, and the disrupted anastomotic site (Figure 1B) was repaired on postoperative day 9, and ileostomy was created. Another patient underwent a RTME, which failed to complete the pelvic dissection due to easy touch bleeding of the tissue around rectal cancer. TME could be finished by adding dissection through the transanal approach. A single stapling anastomosis was performed after the purse string suture was made through the anus at the divided lower rectum (Figure 2A). Anastomotic leakage occurred at 4 days postoperatively and anastomotic repair was performed. In this patient, ileostomy was already made during the first operation. Two of these patients had the same complication of anastomotic leakage, but the results of wound healing of the two patients after the anastomotic repair were different. For these two patients, each anastomotic site was checked about 1 month after the first operation. In patient who underwent double stapling anastomosis, the anastomotic site was re-opened on rectal examination, but only a quarter of the circumference was separated, and the anastomotic site was considered to be highly naturally healed. However, in patient who underwent single stapling anastomosis, colonoscopy revealed that about three-quarters of the circumference was separated (Figure 2B) and the separation was too severe to be cured naturally and there was a possibility of permanent stoma. Why do these differences exist in the results? Overall 113 patients underwent RTME with colorectal or coloanal anastomosis for mid and low-rectal adenocarcinoma in my clinic. The anastomotic leak rate was 7% (8/113). My robotic surgical procedures to prevent anastomotic leakage include good blood supply to the two bowel ends for anastomosis, no using irradiated proximal colon for anastomosis, mesorectal clearing of the rectum involved in the anastomosis (Figure 1A), suturing of the crossing staple lines after double stapling anastomosis, tension free anastomosis, division of anococcygeal ligament and separation of the bilateral levator slings from the rectum for full lower rectal mobilization. However, in the TaTME, the mesorectal clearing and the surgical procedures for full lower rectal mobilization as described above is difficult when cutting the rectum (Figure 2A) and dissecting upwards. This is probably not a technical problem, but an inherent problem due to the limitations of visual field exposure and approach. These factors are probably the reason why patients undergoing TaTME may have higher anastomotic leak rate and worse outcome after anatomic repair than patients undergoing RTME. An insufficient mesorectal clearing may be associated with anastomotic bleeding and insufficient full lower rectal mobilization may be associated with anastomotic tension. Personally, I carefully evaluate that RTME is safer than TaTME in anastomosis.
In conclusion, the most important thing in implementing TME is to perform oncologically safe surgical procedure and to reduce anastomotic failure, whether it is TaTME or RTME. In order to achieve this, it is important that the surgeon is thoroughly trained in the surgical procedure, especially TaTME.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Laparoscopic and Endoscopic Surgery. The article did not undergo external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2019.01.12). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lee L, de Lacy B, Gomez Ruiz M, et al. A multicenter matched comparison of transanal and robotic total mesorectal excision for mid and low-rectal adenocarcinoma. Ann Surg 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Perez D, Melling N, Biebl M, et al. Robotic low anterior resection versus transanal total mesorectal excision in rectal cancer: a comparison of 115 cases. Eur J Surg Oncol 2018;44:237-42. [Crossref] [PubMed]
- Law WL, Foo DC. Comparison of early experience of robotic and transanal total mesorectal excision using propensity score matching. Surg Endosc 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Lee KY, Shin JK, Park YA, et al. Transanal endoscopic and transabdominal robotic total mesorectal excision for mid-to-low rectal cancer: comparison of short-term postoperative and oncologic outcomes by using a case-matched analysis. Ann Coloproctol 2018;34:29-35. [Crossref] [PubMed]
- Veltcamp Helbach M, Koedam TW, Knol JJ, et al. Residual mesorectum on postoperative magnetic resonance imaging following transanal total mesorectal excision (TaTME) and laparoscopic total mesorectal excision (LapTME) in rectal cancer. Surg Endosc 2019;33:94-102. [Crossref] [PubMed]
- Penna M, Hompes R, Arnold S, et al. Incidence and risk factors for anastomotic failure in 1594 patients treated by transanal total mesorectal excision: results from the International TaTME Registry. Ann Surg 2018; [Epub ahead of print]. [Crossref] [PubMed]
- 2017 European Society of Coloproctology (ESCP) collaborating group. An international multicentre prospective audit of elective rectal cancer surgery; operative approach versus outcome, including transanal total mesorectal excision (TaTME). Colorectal Dis 2018;20:33-46. [Crossref] [PubMed]
Cite this article as: Kim CN. Which is safer in anastomosis, transanal or robotic total mesorectal excision? Ann Laparosc Endosc Surg 2019;4:14.


