Managing complications in laparoscopic ventral hernia
Introduction
Advent of minimal invasive surgery has revolutionized surgical practice by obliterating the need for major abdominal incisions (1,2). Laparoscopy utilizes smaller incisions to access abdomen and should theoretically reduce the burden of incisional hernia. Till such time we will continue to encounter this morbidity of era of conventional open surgery and it will continue to remain a common problem in future.
For the purpose of this review we will be referring to ventral and incisional hernia as abdominal wall hernia (AWH). By definition abdominal wall hernia is a defect in the abdominal wall and may present at a variety of sites. A common condition encountered in clinical practice, surgical mesh repair of the hernia is accepted universally and considered a gold standard baring those with very small defects. Around 350,000 abdominal wall hernias are repaired in United States (US) annually (3). While in United Kingdom, annually around 600,000 laparotomies are performed for a variety of abdominal pathologies. Of these roughly 10–20% patients will develop an incisional hernia over the years. This itself will amount to 60,000–120,000 patients annually (4,5). The sheer numbers are fascinating, amounting to a significant strain on healthcare costs and resources. The morbidity associated with its management will further add to the burden. World over, surgeons and clinical researchers have worked relentlessly to improve our understanding of hernia and its management. Over the years the surgical technique has evolved, from approach to the plane of mesh placement in relation to abdominal wall, to newer meshes both in texture and design. With standardization of technique our attention has now focused on ways to reduce the morbidity and mortality associated with the procedure.
Except when defects are very small, most AWH today are repaired with mesh. It is now well established that use of mesh is associated with reduced recurrence rates when compared to primary suture repair (6). Based on the principle of Pascal’s law, placement of mesh in the retro-muscular plane i.e., sublay or underlay or pre-peritoneal plane has been shown to provide better mechanical stability (7,8). Almost two decades ago, laparoscopic ventral or incisional hernia repair (LVHR) was conceptualized and performed placing the mesh in an intra-peritoneal onlay (IPOM) manner, bridging the hernial defect. Multiple systematic and randomized control trials have showed benefit of LVHR in selected cases, with more surgeons readily accepting the procedure. These trials have shown the reliability of LVHR when compared to conventional open repair in term of clinical outcomes e.g., postoperative complications and recurrence (5,9). Due to heterogeneity of case mix, the technique used, length and accuracy of follow-up it is difficult to perform a definitive comparison. But LVHR has been proven to be as secure as open mesh repair if not better and this can be concluded from the similar prevalence of surgeries being performed for recurrent incisional before and after the inception of LVHR in a wider population (10). All the known benefits of minimal invasive approach namely faster recovery after surgery, reduced pulmonary events, reduced wound related complications especially in patients with raised body mass index (BMI), early recovery of bowel movements, lesser adhesions and better cosmesis is making practice of LVHR more popular.
Risk factors influencing complications and importance of perioperative evaluation in LVHR
No investigation or technological advancement can replace the importance of a detailed history taking and physical examination of the patient during surgical consultation. It helps us identify multiple patient related factors such as obesity, diabetes, smoking, pulmonary disorders, hypertension, coagulopathies etc. which will have a significant bearing on the perioperative morbidity and mortality. It is paramount to detect these factors and optimization before planning surgical intervention.
It is equally important to characterize the hernia in terms of location, defect size and number, type of hernia—primary or recurrent, previous intervention especially with prosthesis if any, other scars on the abdomen, contents of the hernia, reducibility, loss of domain. Most of these can be identified with a thorough clinical examination. Use of radiological imaging [computed tomography (CT) or magnetic resonance imaging (MRI)] is not routinely indicated for diagnosis or planning prior to hernia repair. Although in selected cases, especially in large or recurrent hernia, complex hernia, multiple hernias, defects located in challenging or uncommon sites and when physical examination is unrevealing e.g., obesity radiological examination can offer the surgeon valuable inputs with regards to planning access, trocar positioning, mesh size, technical modifications e.g., component separation.
Optimization and control of blood sugar levels in diabetics will help in minimizing wound infections. Smoking cessation has been proven to be vital and needs to be emphasized. In fact, some centres will routinely perform urinary cotinine levels to evaluate compliance with smoking cessation.
Similarly, in complex large hernia with significant contents, it is paramount to assess the respiratory functions properly in case we are planning to bridge the defect with mesh or defect closure.
Patient selection
Proper patient selection is paramount, as each and every patient may not be a suitable candidate for laparoscopic intervention, some patients may be well served with an open approach. Abdominal wall hernias manifest as wide spectrum of disease. Like any other surgery, the capacity to perform a successful LVHR, especially in difficult situations will be dependent on the surgeon experience and expertise e.g., during adhesiolysis in recurrent hernia following a previous mesh repair or in morbid obesity patients (11). These are the very patients who will benefit the most when a successful LVHR is performed (12). At the same time, limitations of laparoscopy need to be known, not all difficulties may be overcome by skill and expertise.
The most common laparoscopic technique offered is the IPOM, wherein the hernial defect is simply bridged, without an attempt to approximate or suture the fascial layers of the abdominal wall. In contrast, during the conventional open sublay repair technique, the fascia anterior to the mesh is re-approximated, if need be with lateral release incisions to offset the tension on the closure. The importance of this step is well emphasized by experts, especially in wider defects. The worry is that in the absence of an anterior support, in large defect the mesh may simple prolapse into the defect leading to recurrence. Another technical step when a mesh is bridged is to ensure a wide overlap all around the defect edge with good fixation so as to minimize the outward displacement pressure exerted on the bridged segment of mesh. In one large series of 850 patients evaluating LVHR, they reported that patients who developed recurrence had a mean defect size of 184 cm2 as compared to 124 cm2 in those without a recurrence (11).
So, anticipate technical difficulties in large hernias with wide defect, discuss the same with patient offering them the alternative of open mesh repair. Although, no guidelines define the exact selection criteria based on defect size, a prospective study by Moreno-Egea et al. performed data analysis on incisional hernias with defects larger than 5 cm, and co-related defect size with recurrence. They suggested that LVHR be restricted to defects ≤10 cm (13). Similarly, another study of 302 open AWH repair patients demonstrated that size of hernia to be a significant factor increasing risk of recurrence (14). These findings were also collaborated by the International Endo-Hernia Society guideline (IEHS) (15), and recommended that while LVHR is feasible in large defects, its use needs to be preferably preserved for defects smaller than 10 cm.
Obesity is another factor which influences post-operative outcomes following hernia repair, both in terms of recurrence as well complications. The IEHS guidelines state that a BMI >30 kg/m2 significantly increase recurrence risk. It also states that LVHR is associated with lesser wound infections in obese and should be preferred approach. In obese patients as surgeons we should attempt defect closure, more extensive mesh overlap and stronger fixation of mesh. The guidelines also advise us to anticipate larger defects than what are clinically apparent in obese. Recently, the American Society for Metabolic and Bariatric Surgery and the American Hernia Society consensus guideline on bariatric surgery and hernia surgery recommended that pre-operative weight loss to reduce BMI is desirable and will improve the perioperative outcomes in obese patients with hernia. Weight loss can be achieved by bariatric surgery, medically supervised weight loss diets, pharmacotherapy and endoscopic methods (16). Detailed patient counselling as to the most appropriate methods and timing of surgery is mandatory.
Post-operative complications and its management
Baring few, most complications of LVHR are not very different from those associated with open AWH repair. Overall complications following LVHR range from anywhere between 5–30%. The specific complications are:
Intra-operative hemorrhage
Though there is paucity of data with regards to the exact incidence of intra-operative hemorrhage, most surgeons will invariably encounter the same sometime during their surgical practice. One can encounter bleeding during trocar insertion or while manipulating or dissecting viscera. A high index of suspicion is necessary. Use of non-cutting trocars reduces risk of trocar site bleeding. Always make a point to visualize a trocar entry into the abdominal cavity, after trans-illuminating the abdominal wall. If we see a brisk and significant bleeding the management strategies range from changing to a larger trocar to tamponade the bleeding vessel. “Cantilevering” the trocar against the abdominal wall in four directions, helps us to identify the quadrant from where bleeding is coming to put pressure or an external trans-abdominal suture. Sometimes if a bleeder is seen direct cauterization can be done. Timely management will arrest the development of intra-muscular or extra-peritoneal hematoma.
Bleeding can also arise albeit more frequently during adhesiolysis. Most commonly it will be from an omental vessel and can be managed with electro-cautery or ultrasonic energy devices. Be careful while using an energy device as we can inadvertently damage a neighbouring intestine. Most of energy device injuries to the bowel are occult and will manifest at a later date when perforation sets in leading to significant morbidity and mortality. Sometimes we can use surgical clips, endo-loops or an intra- or extra-corporeal suturing of the bleeding point.
Not uncommonly one encounters bleeding while fixing the mesh either through trans-fascial sutures or tackers. Most of the times one will immediately see a brisk bleeding or hematoma formation at the site of insertion. Bleeding from laceration of a small vessel is self-limiting, but when one of the epigastric vessels is involved, we need to tackle it. Bleeding encountered during trans-fascial suture fixation will invariably stop once we tighten the suture and knot. If need be, we can add additional trans-fascial or intra-corporeal sutures.
Hematoma
Can occur due to trocar entry, especially the cutting trocars. Usually it will be due to injury to a small vessel in the abdominal wall, muscles or the epigastric vessels. Hematomas can also be seen within the hernial sac, usually due to adhesiolysis of a chronic attachment mainly the omentum. In most cases it is self-limiting. Warm compresses may help. A hematoma in the sac may benefit by compression with an abdominal binder. Attempts at aspiration may insert infection. Most cases can be managed by watchful observation. Very rarely these may get infected and may warrant a drainage or debridement.
Bowel injury
Bowel injury during LVHR can occur during trocar insertion or during the actual surgical dissection. Adhesions are not an uncommon finding during LVHR, and will invariably be found in most hernia repair particularly to the abdominal wall. While performing adhesiolysis, dissect only what is necessary. The FINHYST trial has demonstrated that adhesiolysis is the single most important risk factor for major complications as a whole (17). Increased extent of adhesiolysis is associated with increased frequency of life-threatening complications and offers no additional benefit e.g. reduced chronic abdominal pain (15). Dissect only as much is needed for adequate mesh overlap. Use of plain scissors while dissecting close to the bowel wall, dissecting on the abdominal wall away from the bowel adhesion are some of the strategies employed. Similarly, Ultrasonic shears have been shown to be safer as compared to conventional monopolar cautery (18,19).
Bowel injury during hernia repair is challenging. In a study by LeBlanc et al., the incidence of bowel injury during LVHR was 1.78% with an overall mortality of 2.8% (20). Small bowel is the most commonly injured. A Cochrane review, reported an iatrogenic enterotomy rate of 1.55% in LVHR as compared to 0.63% in open repair (21). Injuries can be detected immediately, within first 24 hours or later. Avoidance is more important, but when detected management will depend upon the extent of injury, the contamination, time when detected and the surgeon skill and experience.
If a surgeon is adept in laparoscopy skills an attempt can be made to repair the injury laparoscopically, otherwise it is advisable to convert to a laparotomy. When identified intra-operatively, most intestinal injuries can be repaired with interrupted tension free sutures to include both serosa and submucosa if the edges are healthy. In the event of an extensive damage, primary resection anastomosis is preferred. Keep a high index of suspicion for potential complications will aid in timely identification of injury. Take help of a specialist if need be, as it is paramount to restrict the damage and its associated morbidity and mortality.
Once the bowel injury is tackled, the hernial defect is managed based on the degree of contamination. How do we proceed?
- If there has been no enteric spillage, we can proceed with laparoscopic repair of the bowel injury and proceed with IPOM. Alternatively, we can perform a mini-laparotomy away from the hernia under direct laparoscopic vision, exteriorize and repair the injured segment of bowel extra-corporeally. After closing the incision, we can proceed with IPOM (11,22). Another option is to place the mesh in a different plane, i.e., a pre-peritoneal onlay mesh (PPOM) repair. Thorough peritoneal lavage should be done and post-operatively intra-venous antibiotics given.
- Staged repair: When in doubt about contamination following injury, it is advisable to defer the hernia repair. You can proceed to continue the adhesiolysis laparoscopically, repair the injury. Post-operatively after optimizing bacterial clearance, in a fairly short interval usually few days to week we can perform the hernia repair with a mesh as a second stage procedure (23,24). When you suspect an injury especially when adhesiolysis was extensive and difficult, again defer mesh insertion, plan a re-laparoscopy 24–48 hours later to rule out a missed injury and proceed with mesh repair.
- When there is gross spillage, it is advisable to primarily repair the hernia without a mesh (11,22). A biological mesh can be used safely in presence of contamination and in presence of infection. Franklin et al. used porcine derived biological mesh for LVHR in 43 patients with contaminated fields. They reported only one wound infection and fistula (25). Although as compared to biological mesh a synthetic mesh is preferred in term of recurrence reduction. Colonic injuries present a bigger dilemma as risk of contamination is much higher. Mesh infection at times are known to present in a delayed fashion, weeks to months later. In the Author own experience, in the absence of any evidence-based guide to help the decision-making process, a case-by-case management plan based on the extent of injury, segment of bowel involved, level of contamination, patient general condition and surgeon expertise should dictate the strategy.
For young surgeons and those working in set-up with poor post-operative back-up e.g., intensive care unit or patient monitoring facilities, a safe option is to perform a laparotomy if need be, repair the bowel injury, primary suture repair of the hernia and counsel the patient that the risk of causing a prosthesis infection has been traded for an increased risk of recurrence.
Surgical site infection (SSI)
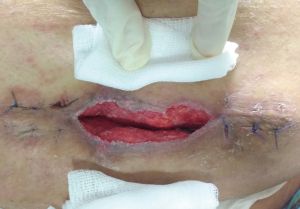
The incidence of SSI is less in laparoscopy (1.1%) as compared to open procedures (10%) (26). The reduced risk is the result of smaller incisions, probability of bacteria lodging in the subcutaneous space, reduced hospital stay and operative time (27-30). Wound infections are more commonly seen following open mesh repair particularly in obese (Figure 1). Patient related risk factors are age e.g., elderly, comorbid conditions e.g., COPD, Coronary artery disease, reduced serum albumin levels, smoking, patients on immunosuppressants e.g., steroids, diabetes, malnutrition, obesity, past history of infections, radiation, hypoxia (31-38). Proper optimization of patient prior to surgery is important.
Surgery related risk factors are surgical site shaving, improper scrubbing, antiseptic use and perioperative blood transfusions, longer operating times (35,39,40).
Optimization and control of these risk factors is important to prevent infections. Modifiable risk factors should be addressed by following established guidelines and hospital/departmental bases protocols of best practices (41). Smoking cessation will not only reduce SSI risk but also benefit the cardio-pulmonary system. Maintaining normoglycemia and normothermia intra-operatively is beneficial (42). Whenever any prosthesis implantation is planned, proper treatment and complete control of remote infections is paramount before offering surgery. Pre-operative hair removal mainly shaving should be avoided, rather hair clipping be performed if needed (39). Single dose of prophylactic antibiotics half an hour prior to surgery is advisable (43). Intra-operatively, meticulous technique, avoidance of bleeding and shorter operative times reduces SSI risk.
Mesh infection
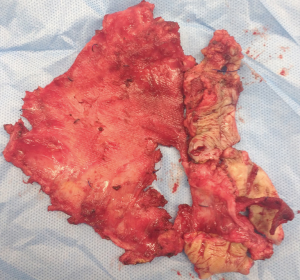
The greatest advantage LVHR offers over open repair is reduced wound related complications mainly wound infections. Mesh infection is one of the most serious complication following a hernia repair, equally challenging to manage. The mesh infection rates following LVHR are ranging between 0–1% following LVHR (15). The sequelae of an infected mesh range from intra-abdominal or abdominal wall abscess, enterocutaneous fistula formation and sepsis. If a polytetrafluoroethylene (PTFE) based mesh is infected it invariably needs removal (Figure 2). With other prosthetic materials, attempts at conservative trials with parenteral and local antibiotics, drainage of the abscess cavity/infected region, debridement of the wound, partial mesh removal and wound vacuum dressings. At any juncture of failure to treat the infection, a complete mesh ex-plantation is mandatory. Counsel patients that post mesh removal, the remaining defect even if closed with suture will inevitably result in a recurrence.
Avoiding blood loss and placement of mesh in subcutaneous position reduces mesh infection rates. While, post-operative seroma formation, deep venous or pulmonary thromboembolism, pneumonia, blood loss and anemia will increase the risk.
Seroma
The sac of hernia is not resected in LVHR and hence seroma is one of the commonest complications following the procedure. The incidence rate in reported literature varies from 3–100%. Commonly seen after few days, its peak presentation is about 7 days after operation. Almost all seromas will invariably resolve by 90 days post-surgery (44-48). Always counsel patients about seroma formation, and most will resolve over time. While aspiration can be attempted, there is a high likelihood of recurrence of the seroma. Also, the risk of inserting infection is real and repeated aspirations should be avoided. Use of abdominal binder has no bearing on the seroma formation. Defect closure has been shown to reduce the seroma formation rates.
Post-operative bulging
A phenomenon more commonly seen following LVHR, is not an uncommon finding. LVHR IPOM technique is commonly used, wherein defect is just bridged. In large hernia, when defect closure is not done, post-operatively patient will still notice the bulge. Here the mesh protrudes through a large defect and at times can be symptomatic. While asymptomatic bulge can be observed, symptomatic bulging will need a second repair. Defect closure reduces the bulging, one of the prime reasons for advent of IPOM Plus wherein defect closure is done before mesh placement. This also improves the mesh to abdominal wall interface, vital for mesh integration and better fixation with possible lesser seroma formation.
Bowel obstruction
Though a rare complication, its occurrence should alert the surgical team to ascertain the right diagnosis. Usually patient will present with distention of abdomen, vague abdominal pain, vomiting. Clinically features of ileus, obstruction or signs of acute abdomen will be seen. Although most cases will resolve with conservative treatment, any suspicion otherwise should warrant a CT scan to assess the entire abdomen. This will demonstrate any obvious obstruction, deep seated abscess or collection, air-fluid levels, significant free air in abdomen and interloop air pockets. Rarely the CT scan may pick up a trocar site herniation. If a mesh is improperly fixed, rarely a loop of bowel may slide in between the mesh and the anterior abdominal wall. Intra-operatively it is important that when we use tackers on the mesh edge, the gap between two tacks should not be too far.
Conservative treatment is on the lines of any sub-acute obstruction i.e., nil orally, nasogastric aspiration, proper hydration and nutrition. At any point if patient clinical condition worsens or we suspect a leak or bowel injury surgical exploration is mandatory. A high index of suspicion is necessary. If need be the team can revisit the recorded surgical procedure to look for any inadvertent injury that was missed during the surgery. Avoiding excessive use of opioid analgesics, avoid overloading with intra-venous fluids, mobilize patient early, correction of electrolyte imbalance are some of the preventive measures to avoid paralytic ileus.
Enterocutaneous fistula
A rare event, it has a significant morbidity associated with it. It may present weeks, to months to years after a hernia repair usually with multiple discharging sinuses on the abdominal wall. An unrecognized bowel injury during surgery, mesh erosion into the bowel, erosion by one of the fixation devices e.g., tacks are some of the reasons for fistula formation.
Management should be on the lines of standard protocols for any enterocutaneous fistula management i.e., source identification and control, proper nutrition and hydration, care of skin around the fistula and ruling out a distal obstruction. CT scan or fistulogram to assess the nature and anatomy of the fistula. For source control antibiotics followed by drainage of abscess cavity if any is done. Once the toxemia is controlled and patient optimized, surgical treatment of the fistula may be needed if conservative management fails. The major worry is that of the fistula being a manifestation of a prosthetic infection. In such case the management should be on the lines of mesh infection as discussed earlier.
At times if instruments are not properly sterilized, sinuses at the port sites due to atypical mycobacteria has been reported in literature. When documented, antibiotics based on culture sensitivity results, excision of sinus with healing with primary or secondary intention is the treatment. With modern sterilization techniques, this is an infrequent complication these days.
Recurrence
Over the years, hernia repair surgery has undergone multiple modifications with regards to type of mesh used, plane in which mesh is placed, fixation technique used etc. with an eye on reducing the recurrence rate. The risk of recurrence rate is dependent on patients as well as surgical factors.
Patient factors are collagen synthesis disorders, inherently weak tissue, COPD, Diabetes, chronic cough, obesity, smoking, large defect size usually >10 cm and history of previous failed repair (15). Surgical factors are suture repair without mesh, inadequate mesh overlap, improper mesh fixation, insufficient coverage of incision scar, SSI, prosthesis infections (15).
Modifiable risk factors need to be controlled before offering patient surgery. Patient optimization for COPD, chronic cough, diabetes, obesity, smoking cessation etc. From surgical point of view the most important steps are adequate mesh overlap of at least 3–5 cm. Any defect >2 cm, always use a mesh. In obese the overlap needs to be at least 5 cm. While selecting mesh size, mesh contraction needs to be accounted for. Improper mesh placement or displacement of mesh will invariably lead to recurrence from one side. For hernia located in the supra-pubic area, it is advisable to create the pre-peritoneal space and fix the mesh to the Cooper’s ligament. For incisional hernias make sure that the mesh covers the entire incision.
Defect closure will increase the mesh to abdominal interface thereby improving mesh integration into tissue in addition to avoiding bulging. Most surgeons prefer using dual fixation methods i.e., a combination of trans-fascial sutures and tacks. The distance between the tacks on the periphery should be 1–1.5 cm apart. If you are using only tackers make sure to perform “double crowning”. Laparoscopy by allowing complete visualization of the entire scar as well as the exact mesh overlap that is needed, lesser SSI should theoretically reduce the recurrence rates, but literature review does not show any significant differences in recurrence rates when compared to open techniques.
Management of recurrent hernia is challenging. In the absence of any evidence-based guidelines for optimal strategy, it is the authors’ opinion that the decision should be based upon the surgeon experience, skill and should be similar to that as for a primary incisional hernia. The author feels that laparoscopy by providing good resolution imaging allows us to completely examine the hernia, ascertain the cause of recurrence mainly due to technical failures. Also, in Laparoscopy as we access the abdomen away from the hernia, we are in a relatively safe zone. As a rule, never remove the old mesh. Uranues et al. in their study demonstrated that laparoscopic repair of recurrent hernia is safe and feasible in experienced hands with acceptable recurrence rates (49). Sharma et al. in their series of 1,242 LVHR reported 203 occult hernias (50). Laparoscopy thus allows us to identify previously missed out hernias which can be a cause of recurrence.
Chronic pain
Chronic pain is defined as pain post-surgery which lasts for 3 months or more (51). The risk factors for post-operative pain are again divided into patient factors and intra-operative and post-operative management factors. Though not evaluate in detail, it is well known that patient factors do contribute to pain perception (52,53). Surgery induced tissue damage, mesh to host reactions based on mesh type, mode of anesthesia, post-operative analgesics cover will all contribute to pain. Recurrence of hernia and non-midline hernia have been reported as risk factors for chronic pain. Similarly, acute pain after surgery is itself a risk factor for chronic pain, and hence adequate analgesic cover should be provided to patient (15). There are no protocols to manage chronic pain, hence involvement of pain management team is advisable. If oral analgesics or analgesic patch do not help, and pain is localized to a particular point e.g., trans-fascial suture site, local anesthetic agent at the suture site can be attempted. When conservative treatment fails and the pain is distressing, attempt at suture removal can be done. Mesh removal is the final option, bearing in mind that the hernia will recur. Hence multimodality treatment should be considered to manage chronic pain.
Other miscellaneous complications
These may be the sequelae of any laparoscopic procedure e.g., pneumonia, respiratory failure, urinary retention, venous thromboembolism etc. Pre-operative optimization, adequate analgesia, thromboembolic prophylaxis based on hospital protocols, early mobilization after surgery will all help in avoiding these complications. Post-operative chest physiotherapy, incentive spirometry is helpful particularly in large hernias.
Occasionally, the trans-fascial suture in subcoastal region can accidentally pass through pleura space leading to pneumothorax. When encountered should be managed like pneumothorax due to any other cause.
Port site hernia though rare can be encountered. With routine closure of all port sites ≥10 mm, the incidence has dropped significantly. On literature review its incidence has ranged from 0.25–3%. Management is based upon standard principles of hernia management.
Conclusions
LVHR has stood the test of time and its popularity is on the rise. More surgeons today readily accept this. With standardization of techniques, we can minimize complications. But despite stringent care and expertise, complications do occur. A high index of suspicion, early recognition and timely management are vital to limit its associated morbidity. Patient counselling prior to hernia repair, optimization of modifiable conditions, protocol-based management, surgical expertise is of critical importance.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Laparoscopic and Endoscopic Surgery for the series “Ventral Hernia”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2019.01.04). The series “Ventral Hernia” was commissioned by the editorial office without any funding or sponsorship. DL served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sarela AI, Miner TJ, Karpeh MS, et al. Clinical outcomes with laparoscopic stage M1, unresected gastric adenocarcinoma. Ann Surg 2006;243:189-95. [Crossref] [PubMed]
- Sarela AI, Murphy I, Coit DG, et al. Metastasis to the adrenal gland: the emerging role of laparoscopic surgery. Ann Surg Oncol 2003;10:1191-6. [Crossref] [PubMed]
- Poulose BK, Shelton J, Phillips S, et al. Epidemiology and cost of ventral hernia repair: making the case for hernia research. Hernia 2012;16:179-83. [Crossref] [PubMed]
- Mudge M, Hughes LE. Incisional hernia: a 10 year prospective study of incidence and attitudes. Br J Surg 1985;72:70-1. [Crossref] [PubMed]
- Cassar K, Munro A. Surgical treatment of incisional hernia. Br J Surg 2002;89:534-45. [Crossref] [PubMed]
- Luijendijk RW, Hop WC, van den Tol MP, et al. A comparison of suture repair with mesh repair for incisional hernia. N Engl J Med 2000;343:392-8. [Crossref] [PubMed]
- Stoppa RE. The treatment of complicated groin and incisional hernias. World J Surg 1989;13:545-54. [Crossref] [PubMed]
- Wantz GE. Incisional hernioplasty with Mersilene. Surg Gynecol Obstet 1991;172:129-37. [PubMed]
- Cobb WS, Kercher KW, Heniford BT. Laparoscopic repair of incisional hernias. Patel, NA, Bergamaschi, R. 91-103. Surgical Clinics of North America. Laparoscopic Surgery: Beyond Mere Feasibility. Saunders: Philadelphia, 2006.
- Flum DR, Horvath K, Koepsell T. Have outcomes of incisional hernia repair improved with time? A population-based analysis. Ann Surg 2003;237:129-35. [Crossref] [PubMed]
- Heniford BT, Park A, Ramshaw BJ, et al. Laparoscopic repair of ventral hernias: Nine years' experience with 850 consecutive hernias. Ann Surg 2003;238:391-9. [PubMed]
- Novitsky YW, Cobb WS, Kercher KW, et al. Laparoscopic ventral hernia repair in obese patients: a new standard of care. Arch Surg. 2006;141:57-61. [Crossref] [PubMed]
- Moreno-Egea A, Carrillo-Alcaraz A, Aguayo-Albasini JL. Is the outcome of laparoscopic incisional hernia repair affected by defect size? A prospective study. Am J Surg 2012;203:87-94. [Crossref] [PubMed]
- Hesselink VJ, Luijendijk RW, de Wilt JH, et al. An evaluation of risk factors in incisional hernia recurrence. Surg Gynecol Obstet 1993;176:228-34. [PubMed]
- Bittner R, Bingener-Casey J, Dietz U, et al. Guidelines for laparoscopic treatment of ventral and incisional abdominal wall hernias (International Endohernia Society (IEHS)): part 1. Surg Endosc 2014;28:2-29. [Crossref] [PubMed]
- Menzo EL, Hinojosa M, Carbonell A, et al. American Society for Metabolic and Bariatric Surgery and American Hernia Society consensus guideline on bariatric surgery and hernia surgery. Surg Obes Relat Dis 2018;14:1221-32. [Crossref] [PubMed]
- Brummer TH, Jalkanen J, Fraser J, et al. FINHYST, a prospective study of 5279 hysterectomies: complications and their risk factors. Hum Reprod 2011;26:1741-51. [Crossref] [PubMed]
- Swank DJ, Bonjer HJ, Jeekel J. Safe laparoscopic adhesiolysis with optical access trocar and ultrasonic dissection. A prospective study. Surg Endosc 2002;16:1796-801. [Crossref] [PubMed]
- Janssen IM, Swank DJ, Boonstra O, et al. Randomized clinical trial of ultrasonic versus electrocautery dissection of the gallbladder in laparoscopic cholecystectomy. Br J Surg 2003;90:799-803. [Crossref] [PubMed]
- LeBlanc KA, Elieson MJ, Corder JM 3rd. Enterotomy and mortality rates of laparoscopic incisional and ventral hernia repair: a review of the literature. JSLS 2007;11:408-14. [PubMed]
- Sauerland S, Walgenbach M, Habermalz B, et al. Laparoscopic versus open surgical techniques for ventral or incisional hernia repair. Cochrane Database Syst Rev 2011;CD007781 [PubMed]
- Carbajo MA, Martın del Olmo JC, Blanco JI, et al. Laparoscopic approach to incisional hernia-lessons learned from 270 patients over 8 years. Surg Endosc 2003;17:118-22. [Crossref] [PubMed]
- Ramshaw BJ, Esartia P, Schwab J, et al. Comparison of laparoscopic and open ventral herniorrhaphy. Am Surg 1999;65:827-831; discussion 831-2. [PubMed]
- Lederman AB, Ramshaw BJ. A Short-Term Delayed Approach to Laparoscopic Ventral Hernia When Injury Is Suspected. Surg Innov 2005;12:31-5. [Crossref] [PubMed]
- Franklin ME, Gonzalez JJ, Glass JL. Use of porcine small intestinal submucosa as a prosthetic device for laparoscopic repair of hernias in contaminated fields: 2-year follow-up. Hernia 2004;8:186-9. [Crossref] [PubMed]
- Franklin ME, Dorman JP, Glass JL, et al. Laparoscopic Ventral and Incisional Hernia Repair. Surg Laparosc Endosc 1998;8:294-9. [Crossref] [PubMed]
- den Hartog D, Dur AH, Tuinebreijer WE, et al. Open surgical procedures for incisional hernias. Cochrane Database Syst Rev 2008;CD006438 [PubMed]
- Zuvela M, Milićević M, Galun D, et al. Infection in hernia surgery. Acta Chir Iugosl 2005;52:9-26. [Crossref] [PubMed]
- Kensarah AM, Dunne JR, Malone DL, et al. A Long-term Follow-up: Suture versus Mesh Repair for Adult Umbilical Hernia in Saudi Patients. A Single Center Prospective Study. Surgical Science 2011;2:155-8. [Crossref]
- Chowbey PK, Sharma A, Mehrotra M, et al. Laparoscopic repair of ventral/incisional hernias. J Minim Access Surg 2006;2:192-8. [Crossref] [PubMed]
- Razavi SM, Ibrahimpoor M, Sabouri Kashani A, et al. Abdominal surgical site infections: incidence and risk factors at an Iranian teaching hospital. BMC Surg 2005;5:2. [Crossref] [PubMed]
- Dunne JR, Malone DL, Tracy JK, et al. Abdominal wall hernias: risk factors for infection and resource utilization. J Surg Res 2003;111:78-84. [Crossref] [PubMed]
- Malone DL, Genuit T, Tracy JK, et al. Surgical site infections: reanalysis of risk factors. J Surg Res 2002;103:89-95. [Crossref] [PubMed]
- Anaya DA, Dellinger EP. The Obese Surgical Patient. Surg Infect (Larchmt) 2006;7:473-80. [Crossref] [PubMed]
- Kurz A, Sessler DI, Lenhardt R. Perioperative normathermia to reduce the incidence of surgical wound infection and shorten hospitalization. N Engl J Med 1996;334:1209-15. [Crossref] [PubMed]
- Cheadle WG. Risk factors for surgical site infection. Surg Infect (Larchmt) 2006;7:S7-11. [Crossref] [PubMed]
- Mangram AJ, Horan TC, Pearson MLThe Hospital Infection Control Practices Advisory Committee, et al. Guideline for prevention of surgical site infection, 1999. Infect Control Hosp Epidemiol 1999;20:250-78; quiz 279-80. [Crossref] [PubMed]
- Boni L, Benevento A, Rovera F, et al. Infective complications in laparoscopic surgery. Surg Infect (Larchmt) 2006;7:S109-11. [Crossref] [PubMed]
- Seropian R, Reynolds BM. Wound infection after preoperative depilation versus razor preparation. Am J Surg 1971;121:251-4. [Crossref] [PubMed]
- Hill GE, Frawley WH, Griffith KE, et al. Allogeneic blood transfusion increases the risk of postoperative bacterial infection: a meta-analysis. J Trauma 2003;54:908-14. [Crossref] [PubMed]
- Kirby JP, Mazuski JE. Prevention of surgical site infection. Surg Clin North Am 2009;89:365-89. [Crossref] [PubMed]
- Latham R, Lancaster AD, Covington JF, et al. The association of diabetes and glucose control with surgical site infections among cardiothoracic surgery patients. Infect Control Hosp Epidemiol 2001;22:607-12. [Crossref] [PubMed]
- Yerdel MA, Akin EB, Dololan S, et al. Effect of single dose prophylactic ampicillin and sulbactam on wound infection after tension-free inguinal hernia repair with polypropylene mesh. Ann Surg 2001;233:26-33. [Crossref] [PubMed]
- Forbes SS, Eskicioglu C, McLeod RS, et al. Meta-analysis of randomized controlled trials comparing open and laparoscopic ventral and incisional hernia repair with mesh. Br J Surg 2009;96:851-8. [Crossref] [PubMed]
- Kaafarani HM, Hur K, Hirter A, et al. Seroma in ventral incisional herniorrhaphy: incidence, predictors and outcome. Am J Surg 2009;198:639-44. [Crossref] [PubMed]
- Palanivelu C, Jani KV, Senthilnathan P, et al. Laparoscopic sutured closure with mesh reinforcement of incisional hernias. Hernia 2007;11:223-8. [Crossref] [PubMed]
- Susmallian S, Gewurtz G, Ezri T, et al. Seroma after laparoscopic repair of hernia with PTFE patch: is it really a complication? Hernia 2001;5:139-41. [Crossref] [PubMed]
- Sodergren MH, Swift I. Seroma formation and method of mesh fixation in laparoscopic ventral hernia repair--highlights of a case series. Scand J Surg 2010;99:24-7. [Crossref] [PubMed]
- Uranues S, Salehi B, Bergamaschi R. Adverse events, quality of life, and recurrence rates after laparoscopic adhesiolysis and recurrent incisional hernia mesh repair in patients with previous failed repairs. J Am Coll Surg 2008;207:663-9. [Crossref] [PubMed]
- Sharma A, Mehrotra M, Khullar R, et al. Laparoscopic ventral/incisional hernia repair: a single centre experience of 1,242 patients over a period of 13 years. Hernia 2011;15:131-9. [Crossref] [PubMed]
- Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. Prepared by the International Association for the Study of Pain, Subcommittee on Taxonomy. Pain Suppl 1986;3:S1-226. [PubMed]
- Kehlet H, Rathmell JP. Persistent postsurgical pain: the path forward through better design of clinical studies. Anesthesiology 2010;112:514-5. [Crossref] [PubMed]
- Khan RS, Ahmed K, Blakeway E, et al. Catastrophizing: a predictive factor for postoperative pain. Am J Surg 2011;201:122-31. [Crossref] [PubMed]
Cite this article as: Salgaonkar H, Wijerathne S, Lomanto D. Managing complications in laparoscopic ventral hernia. Ann Laparosc Endosc Surg 2019;4:11.