The dilemmas of the transverse colon cancer: segmental or extended right colectomy, laparoscopic hazards for the inexperienced surgeon
Introduction
Laparoscopic colorectal surgery was first described in 1991. Over the years, multiple studies have shown a wide range of patients’ benefits such as decreased pain and morbidity, improved cosmetic outcomes, shorter length of hospital stay (LOS), faster recovery times and return to work. Acceptance and wide spread adoption of minimally invasive colorectal surgery was initially slow due to the fact that it requires surgeons to be comfortable with advanced laparoscopic techniques. When treating patients with colorectal cancer, concern for worsened oncologic outcomes as well as the potential for increased health care costs were weighted heavily during the early stages of the development of this approach (1-3). In 2001, the first cases of colorectal robotic surgeries were described, adding an extra tool for surgeons to apply when trying to manage colorectal diseases in a minimally invasive approach (4,5). However, this contributed to the creation of even further division among the surgical community regarding the role of these techniques, especially as previously mentioned, when oncologic safety and cost were considered in the discussion. Currently, minimally invasive laparoscopic colorectal surgery is considered comparable to open procedures from an oncologic standpoint and maybe even superior to from an overall patient care standpoint (1,3,6,7). Landmark studies such as the Conventional versus Laparoscopic-assisted Surgery in Colorectal Cancer trial (CLASICC), COLOR trial, and the COST trial have shown decreased complication rates, decreased LOS, and similar oncological outcomes between open and laparoscopic procedures (8,9). However, in several of these landmark studies, patients with transverse colon cancer were not included. Factors that contribute to it were the low incidence of lesions located in the transverse colon per se (approximately 10%) and how technically challenging performing an adequate lymph node dissection and division of the middle colic vessels (MCV) is (10,11).
The amount of scientific literature addressing specifically the management of transverse colon lesions is still scarce, comprised mainly of retrospective data-analysis articles, articles including a limited number of patients or data that compares mainly open to laparoscopic resections. Very limited published data actually compares a laparoscopic extended right colectomy with a laparoscopic segmental transverse colectomy. A laparoscopic left colectomy with a colocolonic anastomosis between the transverse colon and the sigmoid colon also constitutes an option for the management of distal transverse colon lesions, such as splenic colon ones. This present chapter, however, focuses in the management of transverse colon lesion either by a segmental transverse colectomy or an extended right resection. Laparoscopic left colectomy is discussed in a subsequent chapter of this special issue of “laparoscopic colon surgery” (12).
Understanding basic technical principles in the management of transverse colon cancer
- Adequate preoperative knowledge of tumor location may allow the surgeon to select patients adequately for either a laparoscopic or an open approach. The surgeon’s experience in minimally invasive colorectal surgery as well as tumor and patient characteristics (i.e., BMI, prior upper abdominal surgeries) may facilitate planning (13).
- There are four anatomic areas that should be kept in mind during this procedure: (I) mobilization of the right colon and hepatic flexure; (II) mobilization of the splenic flexure, these include learning to approach this area by developing the plane under the inferior mesenteric vein (IMV); (III) dissection of the omentum and access to the mid colic vessels from a ventral or caudal approach; (IV) anastomosis construction, either in an extracorporeal or intracorporeal fashion.
- Planning ahead: keeping in mind necessary steps such as port location, the need for hepatic and splenic flexure mobilization, management of the omentum, lymph node dissection and management of vascular structures allows the surgeon to be mentally prepared and able to anticipate problems. This is especially true when the surgeon is at the beginning of his/her learning curve. For example, failure to completely mobilize the hepatic and splenic flexures increases the risk of tension on the anastomosis and places the patient at risk for an anastomotic leak. Trying to complete mobilization of the flexures once an incision has been created for specimen extraction is technically very difficult and may lead to conversion to an open procedure.
- High ligation of the middle colic vessels (MCVs) before it branches can prove challenging laparoscopically. Several techniques have been described that allow for identification of the MCV below the inferior aspect of the pancreas. However, some of the data come from articles where patients’ body mass index (BMI) was an average of 21–23. Venous bleeding can also prove challenging to control laparoscopically, therefore a clear understanding of the vascular anatomy is very important to prevent injuries. Conversion to an open procedure should be kept in mind at all times while dealing with bleeding due to the close proximity of the superior mesenteric artery and vein to some of these vessels (14-17).
- Although a medial to lateral approach is preferred, the surgeon should be able to modify his or her approach to a lateral to medial technique on a case-to-case basis, dependent upon anatomical variants.
- Developing the plane under the IMV and division of this vessel near the Ligament of Treitz can help with identification of the inferior edge of the pancreas, as well as splenic flexure mobilization, thus ensuring adequate colonic mobilization for construction of a tension free anastomosis (18).
- Ensuring that the tumor was tattooed correctly during colonoscopy is very important, as lesions may be difficult to visualize in patients with high BMIs and/or when located near the hepatic or splenic flexures. Intraoperative colonoscopy with CO2 should be employed as needed to verify location of the tumor if the tattoo is not visible. However, bowel distention may occur, despite using CO2, necessitating conversion to an open procedure.
- Division of the specimen and extraction site may vary depending on patient’s characteristics and surgeon experience. Our preferred extraction site is through a Pfannenstiel incision when an intracorporeal anastomosis is performed. A periumbilical excision is otherwise used, although data shows that the rate of hernia development is high.
Laparoscopic extended right colectomy
Extended right colectomy: step by step technical approach
Common initial steps
Room setup and positioning
The patient can be placed on the operating room table in a supine position. A modified lithotomy position has also been described for this procedure.
Changes in the operating room table position during the procedure places the patient at risk for sliding and at risk for injuries. Positioning should be completed in such a way that the patient can be placed in Trendelenburg, reverse Trendelenburg, and lateral positions without sliding. Various methods have been described to prevent patient sliding, such us using bean bags and various styles of straps with various degrees of success. It is our preference to position the patient directly on a large foam mat. This mat is usually secured to the operating table through Velcro straps or tape. The goal of these mats is to provide a “friction hold” and decreased sliding. A second strap, in this case a Velcro belt, similar to the ones commonly used to secure the legs in place, is also used across the chest and secured to the operating table at this level. This way, the risk of both in-line sliding and lateral sliding decreases as the patient is placed in Trendelenburg or reverse Trendelenburg positions as well as in right or left lateral positions. It is our preference to position both arms parallel to the patient, tucked to the operating table.
Preoperative antibiotics are administered per NSQUIP guidelines.
Pneumoperitoneum and port placement
Performing this procedure using a total of 5 ports is the standard technique in our practice. However additional ports may be needed. Port placement is typically as follows: a 5 mm camera port (C) located at the level of the umbilicus, a 5 mm in the left upper quadrant (LUQ), a 5 mm or a 12 mm in the left lower quadrant port (LLQ) as well as a 5 mm in the right upper quadrant (RUQ), and a 5 mm or a 12 mm in the right lower quadrant port (RLQ). Two monitors are usually used, one on the right and the other to the left of the patient. Both surgeon and assistant are standing to the left of the patient while performing the right colon dissection and will move to the right of the patient to perform the splenic flexure mobilization and the intracorporeal anastomosis if it is decided to construct it in this fashion.
Pneumoperitoneum can be achieved in a number of ways. Access to the abdominal cavity through an open technique at the level of the umbilicus or through placement of a Veress needle in the LUQ are valid entry options. It is our preference, however, to create pneumoperitoneum by placing a Veress needle at Palmer’s point, 1–2 cm below the left costal margin at the left mid-clavicular line (MCL).
Insertion and placement of ports is decided after pneumoperitoneum has been created. Routinely, our approach to port placement is as follows:
- A 5-mm camera (C) port at the level of the umbilicus;
- The following description is a very practical and “easy to remember” approach for trocar placement. Once the C port is in place, the next 4 ports are placed in the right upper and lower quadrants and left upper and lower quadrants. Ports are placed four fingerbreadths apart from the camera port and from each other. Avoiding placement of the ports too far from the umbilical port is very important, especially in patients with high BMI. Ports placed too far laterally may prevent regular length instruments from reaching areas such as the splenic flexure.
Initial operative steps
Both surgeon and assistant stand on the left side of the patient. The procedure starts by inspecting the abdominal cavity, which is especially important to rule out metastatic disease when treating cancer. Confirming tumor location is an initially important step especially when dealing with transverse colon lesion, as the colonoscopy report may not be accurate, and it could potentially change the required procedure altogether. Subsequently, the patient is rotated to the left (partial left lateral decubitus) to facilitate displacement of the small bowel away from the right colon.
Atraumatic bowel graspers are used at all times to avoid bowel injury to intra-abdominal structures.
Laparoscopic medial to lateral dissection of the right colon and vessel ligation
The LLQ and LUQ ports are the operative ports during this portion of the operation. The camera is controlled by the assistant. A medial to lateral mobilization of the right colon is our preferred approach. Dissection usually starts by grasping the ileocecal valve area and retracting it laterally and upward toward the abdominal wall. This creates tension in the mesocolon allowing for easy identification of the ileocolic pedicle. The peritoneum is divided using electrocautery just inferior and adjacent to the pedicle. Subsequently, the avascular plane between the mesocolon and the retroperitoneum is developed, progressing from a caudal to a cranial direction. As this plane is bluntly developed, several structures are identified. It is our preference to not divide the pedicle too early in the dissection, as it aids with traction and countertraction. The duodenum and the head of the pancreas are encountered in the most medial aspect of this plane and are gently dissected down and away from the mesocolon. As dissection progresses, the mesocolon is dissected of Gerota’s fascia. The ureter may be identified in some patients. It is our preference to develop this plane as far as possible because it simplifies takedown of the hepatic flexure later on in the case. Subsequently, a high ligation of the ileocolic pedicle is performed either using an advanced bipolar device, clips, or a vascular stapler that could be introduced either through a RLQ or LLQ 12 mm port. The right colic vessel (when present) is divided next.
We prefer not to address the MCV at all until later in the procedure, once the omentum has been either dissected from the colon or divided from the stomach (if it is decided to remove the omentum during the procedure), so that vascular control of the MCV can be obtained both from a caudal and a ventral approach if needed. This is a very important aspect to keep in mind. Running into bleeding while attempting to divide the MCV can be challenging to control. This is even more challenging when the surgeon is not able to access the vascular pedicle from the ventral aspect as is the case if the omentum has not been previously dissected off the colon. This creates a situation that may be difficult to control laparoscopically.
Therefore, and as mentioned above, the next step is to proceed with dissecting the omentum off of the colon. If the surgeon so chooses to remove the omentum with the specimen rather than dissecting off of the colon, the omentum can be divided along the greater curvature of the stomach. Dissection of the omentum off of the colon can be performed with hook cautery, otherwise an advanced bipolar device is used if the omentum is divided.
Working from the left sided ports allows the surgeon to takedown the hepatic flexure. Taking down the hepatic flexure is usually straightforward, as most of the dissection has been performed earlier while dissecting the mesocolon from the retroperitoneum. Due to the fact that these planes are connected, the surgeon can continue the dissection of the right colon using a top-down approach. In this case, the lateral attachments of the right colon can be taken down progressing from the hepatic flexure towards the RLQ. By placing the patient in the Trendelenburg position, the small bowel is usually retracted away from the RLQ and the peritoneal attachments of the terminal ileum mesentery to the pelvis are easily freed. This step is important to ensure that the ileum is fully mobile and will reach the colon with no tension.
Subsequently, both assistant and surgeon switch to the left of the patient. The patient is now placed in the right lateral position. Slight reverse Trendelenburg may help with further dissection of the omentum. If the transverse colon is redundant and the tumor is centrally located, it may not be necessary to mobilize the splenic flexure. However, extensive mobilization of the omentum allows for appropriate exposure of the MCV. At the same time, ensuring adequate colonic mobilization is very important, especially when planning to construct the anastomosis extracorporeally requiring extraction of the colon through the incision.
Various techniques have been described to identify and divide the middle colic vessels. These include (I) finding the ileocolic and superior mesenteric arteries directly and continuing medial dissection until the MCV is identified, (II) a method described as the “window technique” and (III) by rotating the transverse mesocolon. We generally proceed by gently grasping the transverse colon at opposite ends, separating them and gently putting the mesocolon under tension. The MCV can then be identified and the vascular pedicle divided just distal to the inferior aspect of the pancreas but before it bifurcates, allowing for a complete lymphadenectomy. Care must be taken to avoid excessive traction that could lead to venous injury. Understanding the venous anatomy and the relationship of the right and middle colic vein to the gastrocolic trunk of Henle is important. Traction injury to these vessels may be difficult to control laparoscopically. Aggressive maneuvers to try to control bleeding are discouraged due to the proximity of the superior mesenteric vein. Conversion to open surgery may be required in these situations (11,14-16).
Once the MCV are divided, the next steps depend on whether the anastomosis is going to be performed intra or extra corporeally.
Splenic flexure mobilization
Laparoscopic mobilization of the splenic flexure can be accomplished in a number of ways
These include:
- Starting the dissection by taking down the lateral attachments of the descending colon progressing in a caudal to cranial direction towards the spleen. As one approaches the flexure, attention usually shifts to taking down the omentum from its attachments to the transverse colon, advancing towards the splenic flexure. As dissection progresses, the colon can be safely mobilized off of the pancreas as well as Gerota’s fascia. These steps can be performed with electrocautery or an advanced bipolar device. When following this approach, division of the mesocolon is performed last, continuing in the same level or plane that was created while dividing the MCV. This step is usually performed using an advanced bipolar device.
- Dissection can also start by taking down the omentum from the colon towards the splenic flexure, and then either taking the lateral attachments of the descending colon in a cranial to caudal direction or vice versa. Once again, the mesocolon is divided at the end, once the flexure is completely mobilized.
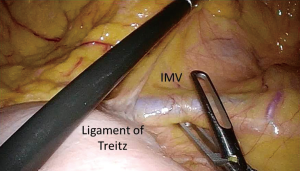
- A third approach, which is this author’s preference, begins with opening the peritoneum just inferior to the IMV at the level of the ligament of Treitz (Figure 1). Starting the dissection at the level of the IMV requires excellent exposure of the fourth portion of the duodenum and the ligament of Treitz. This exposure allows for easy identification of the IMV which travels in the mesocolon just superiorly (1–3 cm) to the ligament of Treitz. This approach requires the small bowel to be displaced away from the LUQ, while the transverse colon should be gently grasped by the assistant and reflected towards the stomach and spleen. Monopolar cautery is usually employed to open the peritoneum just below and along the course of the IMV towards the inferior mesenteric artery. Once the peritoneum is opened, an avascular plane between the mesocolon and the retroperitoneum is encountered. This plane can be easily developed in a medial to lateral fashion in the same manner the plane is developed under the ileocolic pedicle during a right colectomy. Blunt dissection is used to elevate the IMV and mesocolon off of the retroperitoneum. As dissection advances laterally and towards the splenic flexure, Gerota’s fascia is encountered and dissected away from the mesocolon. This is, again, an avascular plane that is easily developed. At this point, the IMV can be divided with an advanced bipolar device, clips or using an endostapler. Progressing with the dissection towards the splenic flexure, as well as entering the lesser sac can be accomplished by continuing to develop this plane even further (19).
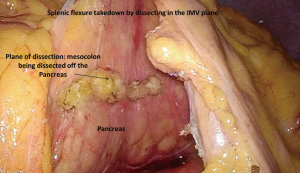
It is key to understand, however, that continuing dissection in this plane will put a surgeon not familiar with this approach in a complex technical scenario. This plane continues cranially under the pancreas towards the splenic vessels. In order to avoid lifting the distal portion of the pancreas along with the mesocolon during this step, the inferior edge of the pancreas needs to be identified prior to continuing the dissection. Once the inferior edge of the pancreas is clearly visualized, the attachments of the mesocolon to the pancreas can be divided (Figure 2). At this point, access to the lesser sac is gained and the splenic flexure can be taken down this way. However, if the pancreas cannot be identified clearly, access to the lesser sac should be accomplished while taking down the splenic flexure in a more traditional manner as described above. The benefit of this approach is that it allows the surgeon to work with the colon in a fixed position as it is still anchored to the abdominal wall by its lateral attachments. The attachments of the omentum to the colon and the lateral attachments of the colon to the abdominal wall are taken down last (20).
Extracorporeal versus intracorporeal ileocolonic anastomosis
Extracorporeal ileocolonic anastomosis
In order to proceed with an extracorporeal anastomosis, it is usually recommended to have mobilized both the hepatic and splenic flexures.
A small periumbilical or supraumbilical midline incision can be used. A wound protector usually aids with exposure. The small bowel and colon are usually divided with a stapler. The anastomosis can be constructed in a number of ways that include a side-to-side end functional stapled ileocolonic anastomosis, a side-to-side isoperistaltic anastomosis or by constructing an end-to-end or end-to-side hand-sewn anastomosis. A side-to-side end functional anastomosis is commonly used. An enterotomy is created in the antimesenteric border of the small bowel and colon and a stapler is used to create the common channel. Subsequently, the common enterotomy is closed with sutures or another load of a stapler.
Intracorporeal isoperistaltic ileocolonic anastomosis
Surgeon and assistant stand on the right side of the patient. If an intracorporeal anastomosis is planned, both the colon and terminal ileum are divided intracorporeally with an endoscopic stapler. The small bowel and the colon are aligned in an isoperistaltic fashion. A stay suture can be used to maintain both bowel segments in the appropriate position. If used, a stay suture is placed close to the stapled end of the small bowel and on the antimesenteric border of the colon. This suture is placed in the colon at least 10 cm distal to the stapled line, in order to have enough length for the laparoscopic stapler that will be used to create the common channel. Subsequently, an enterotomy is performed with hook cautery in the antimesenteric border of the small bowel and of the colon. Careful attention is employed to ensure that the colostomy is several centimeters away from the staple line in order to avoid creating an area of ischemia or having difficulty with closure of the common enterotomy. An endovascular stapler is introduced through the RLQ port and a side to side end functional anastomosis is created. The common enterotomy can be closed in a number of ways with intracorporeal sutures per surgeon’s preference. In these cases, our preferred extraction site is the suprapubic area through a Pfannestiel incision.
Technical steps will be described below.
Segmental transverse colectomy: technical approach
If the decision was to perform a segmental transverse colectomy, the following steps should be considered:
- Both the hepatic flexure and the splenic flexure need to be mobilized to ensure a tension free anastomosis.
- Starting the dissection using the IMV approach described above allows for partial mobilization of the splenic flexure. By keeping the splenic flexure and the descending colon in their anatomic position until the lateral attachments are taken down, it allows the surgeon to avoid the struggles associated with the colon being too mobile too early in the procedure.
- Mobilizing the omentum along the entire length of the transverse colon or dividing it close to the greater curve of the stomach allows for direct visualization of the transverse colon vessels. This is a recommended step that needs to be performed prior to dividing the MCV as mentioned above.
- Dividing the MCV just distal to the inferior edge of the pancreas allows for a complete lymphadenectomy as described above. It is important to avoid traction injuries during these steps. Early division of the IMV allows the surgeon to have a clear target when dividing the transverse mesocolon. Previous studies have described several techniques to safely control the MCV; however the average BMI of the patients included in those studies was around 23 (21,22). This BMI may not be representative of the current patient population that is being treated in some western countries. Identification of the vascular anatomy and division of these vessels with energy devices may prove challenging in patients with a high BMI. Using stapling devices with vascular loads or clips may be an alternative to advanced bipolar devices to control the vascular pedicle. However, it must be noted that the use of clips may require extensive dissection in order for the clips to be placed safely, which once again can prove challenging in obese patients. Venous traction injuries are especially difficult to control laparoscopically and may require conversion to open surgery.
Extracorporeal or intracorporeal isoperistaltic colocolonic anastomosis
- Steps to complete the resection of the bowel and to create an anastomosis were described above. A small periumbilical or supraumbilical midline incision can be used. A wound protector usually aids with exposure. The colon is usually divided with a stapler. The anastomosis is usually constructed in an isoperistaltic fashion versus the typical antiperistaltic anastomosis that is commonly used for ileocolonic anastomosis. A colotomy is created in the antimesenteric border of the proximal segment of the colon, 8 to 10 cm proximal to the stapled end. A second colotomy is created in the distal segment of the colon, a few centimeters away from the staple line. As mentioned above, this is to avoid creating an area of ischemia or having difficulty with closure of the common enterotomy. A stapler is used to create the common channel and the common enterotomy is then closed using sutures or a stapler.
- If an intracorporeal anastomosis is planned, the colon is divided intracorporeally with an endoscopic stapler. The anastomosis is created in the same fashion as described above. Our preferred extraction site is, as mentioned before, the suprapubic area through a Pfannestiel incision.
Discussion
Several key questions have been addressed over the years when trying to decide the best treatment option for patients with transverse colon cancer. The decision-making process to treat lesions located near the hepatic flexure is, in general, straightforward. A laparoscopic right colectomy with ligation of the ileocolic pedicle, right colic artery (when present), and the right branch of the MCV is generally performed and allows for an adequate lymphadenectomy.
However, when the tumor is located in the mid portion of the transverse colon or near the splenic flexure, there are several options.
Historically, these lesions were treated by a left colectomy or by a segmental transverse resection with construction of a colocolonic anastomosis. When compared to ileocolonic anastomosis, the colocolonic anastomosis formed after a segmental transverse colon resection is suggested by some authors as having a higher risk of anastomotic leak. Furthermore, they may also be technically more challenging (23-25). In 1985, the first report of performing an extended right colectomy for tumors located in the distal transverse colon or proximal descending colon was published. Some technical advantages such as being able to perform the anastomosis between a mobile segment of bowel such as the ileum, that could easily reach the colon without tension, was accepted at the expense of resecting a longer segment of colon (26-29). However, data regarding differences in anastomotic leakage needs to be interpreted with caution. Odermatt et al. describe a 3.3% rate of anastomotic leaks after a left colectomy versus 10.5% after an extended right colectomy. This directly contradicts the above-mentioned statement. However, in his article, 73.7% of extended right colectomies were performed emergently (i.e., obstructing distal transverse colon cancer). On the other hand, only 20% of left colectomies were performed under emergent conditions. Furthermore, over the years numerous articles have shown similar complication rates, independently of the type of resection and anastomosis performed (i.e., ileocolonic vs. colocolonic anastomosis) (11,12).
From an oncologic standpoint, a large number of published articles have shown comparable oncologic outcomes independently of whether an open extended right colectomy, an open segmental transverse colectomy or an open left colectomy is performed. Chong et al. reported the oncologic outcome of 1,066 patients who underwent either an extended right colectomy or left colectomy versus those who underwent a transverse colectomy. A total of 750 patients (70%) underwent an extended right colectomy, 127 (11.9%) underwent a transverse colectomy and 189 (17.7%) underwent a left colectomy. Analysis with a propensity-matched cohort between the transverse and extended colectomy groups showed no significant difference in disease free survival and overall survival (30).
As surgeons become more and more proficient in laparoscopic colon resection, the questions then became:
- Is a laparoscopic extended right colectomy or a segmental transverse colectomy feasible from a laparoscopic standpoint?
- Are these minimally invasive procedures comparable to an open resection from an oncologic standpoint?
As mentioned above, literature is scarce when discussing laparoscopic management of transverse colon cancers. However, more and more evidence is now available to support the fact that both, an extended right colectomy or a segmental transverse colectomy, can safely be performed laparoscopically by experienced minimally invasive surgeons.
Zeng et al. analyzed a series of 278 patients that underwent surgery for transverse colon cancer. A total of 156 underwent a laparoscopic resection versus 122 who underwent an open procedure. Morbidity, mortality and five year survival rates were similar between groups. Rate of conversion to an open procedure was 5% in their series (10). Published conversion rates show a wide range, ranging from 1.9% to as high as 16% (31). However, when comparing return of bowel function and LOS, laparoscopic procedures were superior - this data is similar to most published data that evaluate the role of laparoscopic surgery in colon and rectal pathologies. More importantly, Zeng et al. showed similar oncologic outcomes between these procedures (10,11,31-33).
Yamaguchi et al. presented data from 1,830 patients who underwent resection for transverse and descending colon cancer (34). A laparoscopic resection was performed in 958 (52.3%) patients versus an open resection in 872 (47.7%) patients. Conversion rate was 4.5%. Operative time was longer in the open group, while complications rate and LOS were shorter in the laparoscopic group. Oncologic outcomes at 3 years when all stages were considered were similar between groups. However, it was statistically higher for Stage I patients who underwent a laparoscopic resection versus those who underwent an open procedure. Data from several other articles showed similar oncologic outcomes between open and laparoscopic resections.
Zhao et al. compared oncologic outcomes from 157 consecutive patients who underwent resection for transverse colon cancer (74 laparoscopic resections versus 83 open ones). The 5-year survival rate was 73% vs. 71% (P=0.3) for the laparoscopic and open groups, respectively (22).
Yamamoto et al. compared the 5-year overall survival of patients undergoing an open versus a laparoscopic resection for transverse colon cancer (n=245). For stage II patients, the 5-year overall survival was 84.9% vs. 93.7% for patients in the open and laparoscopic groups, respectively. For stage III patients, the 5-year overall survival was 63.4% vs. 66.7% for the open and laparoscopic groups, respectively (21).
Hirasaki et al. compared survival per stage between patients who underwent a laparoscopic transverse colectomy versus patients who underwent a laparoscopic right or sigmoid colectomy. The 5-year overall survival was 93.7% for Stage II and 81.4% for stage III patients in the laparoscopic group. There was no statistical difference in survival between groups. This showed that oncologic outcomes were not negatively affected by treating transverse colon lesions laparoscopically. Survival rates, stage per stage, were similar to that reported for patients with lesions in other parts of the colon, for which a laparoscopic approach has already been proven safe (35).
Matsuda et al. compared patients who underwent a laparoscopic extended right colectomy versus a laparoscopic transverse colectomy. Five-years overall survival was 92.4% and 95.7% respectively. This demonstrates similar oncologic outcomes between these procedures. They described, however, fewer complications after a laparoscopic extended right colectomy (morbidity 29% vs. 10.5%). There were no anastomotic leaks in the laparoscopic extended right colectomy group compared to 2 (5.8%). However, the number of patients was small, with 38 and 34 patients in each group, respectively (36).
Conclusions
Colorectal surgeons are currently routinely trained in advanced minimally invasive surgery and commonly face complex laparoscopic situations. As minimally invasive surgery is gaining popularity, laparoscopic right colectomies are routinely performed. Laparoscopic takedown of the splenic flexure is a very common technical step performed almost routinely during sigmoid colectomies or low anterior resections. It may still be a challenging part of these operations; however, this is a step that surgeons perform on a regular basis. Colorectal surgeons are routinely required to perform complex laparoscopic technical steps. What sets a laparoscopic extended right colectomy or a segmental transverse colectomy apart from some of the above-mentioned procedures is performing a high ligation of the MCV. Both division of the middle colic artery as well as of the venous drainage of the transverse colon can prove challenging; with bleeding being difficult to control in many cases. Therefore, understanding what scenarios can be dealt with laparoscopically and when to convert to an open operation is possibly the biggest challenge when performing this operation.
In summary, resection of transverse colon cancer by means of an extended right colectomy, a segmental transverse resection or even a left colectomy is feasible from a laparoscopic standpoint. Longer operative times are common for laparoscopic cases; however complication rates and length of hospital stay favor a laparoscopic approach. Furthermore, from an oncologic standpoint, a laparoscopic extended or segmental resection appears as safe as an open procedure.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Michael J Stamos and Mehraneh Dorna Jafari) for the series “Laparoscopic Colon Surgery” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2018.12.11). The series “Laparoscopic Colon Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Laparoscopically assisted colectomy is as safe and effective as open colectomy in people with colon cancer Abstracted from: Nelson H, Sargent D, Wieand HS, et al; for the Clinical Outcomes of Surgical Therapy Study Group. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med 2004; 350: 2050-2059. Cancer Treat Rev 2004;30:707-9.
- Colon Cancer Laparoscopic or Open Resection Study Group. Survival after laparoscopic surgery versus open surgery for colon cancer: long-term outcome of a randomised clinical trial. Lancet Oncol 2009;10:44-52. [Crossref] [PubMed]
- Veldkamp R, Kuhry E, Hop WC, et al. Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomised trial. Lancet Oncol 2005;6:477-84. [Crossref] [PubMed]
- Baik SH. Robotic colorectal surgery. Yonsei Med J 2008;49:891-6. [Crossref] [PubMed]
- Bianchi PP, Luca F, Petz W, et al. The role of the robotic technique in minimally invasive surgery in rectal cancer. Ecancermedicalscience 2013;7:357. [PubMed]
- Jayne DG, Thorpe HC, Copeland J, et al. Five-year follow-up of the Medical Research Council CLASICC trial of laparoscopically assisted versus open surgery for colorectal cancer. Br J Surg 2010;97:1638-45. [Crossref] [PubMed]
- McKay GD, Morgan MJ, Wong SK, et al. Improved short-term outcomes of laparoscopic versus open resection for colon and rectal cancer in an area health service: a multicenter study. Dis Colon Rectum 2012;55:42-50. [Crossref] [PubMed]
- Hazebroek EJColor Study Group. COLOR: a randomized clinical trial comparing laparoscopic and open resection for colon cancer. Surg Endosc 2002;16:949-53. [Crossref] [PubMed]
- Fleshman J, Sargent DJ, Green E, et al. Laparoscopic colectomy for cancer is not inferior to open surgery based on 5-year data from the COST Study Group trial. Ann Surg 2007;246:655-62; discussion 662-4. [Crossref] [PubMed]
- Zeng WG, Liu MJ, Zhou ZX, et al. Outcome of Laparoscopic Versus Open Resection for Transverse Colon Cancer. J Gastrointest Surg 2015;19:1869-74. [Crossref] [PubMed]
- Kim HJ, Lee IK, Lee YS, et al. A comparative study on the short-term clinicopathologic outcomes of laparoscopic surgery versus conventional open surgery for transverse colon cancer. Surg Endosc 2009;23:1812-7. [Crossref] [PubMed]
- Odermatt M, Siddiqi N, Johns R, et al. Short- and long-term outcomes for patients with splenic flexure tumours treated by left versus extended right colectomy are comparable: a retrospective analysis. Surg Today 2014;44:2045-51. [Crossref] [PubMed]
- Zmora O, Bar-Dayan A, Khaikin M, et al. Laparoscopic colectomy for transverse colon carcinoma. Tech Coloproctol 2010;14:25-30. [Crossref] [PubMed]
- Lee YS, Lee IK, Kang WK, et al. Surgical and pathological outcomes of laparoscopic surgery for transverse colon cancer. Int J Colorectal Dis 2008;23:669-73. [Crossref] [PubMed]
- Fujita J, Uyama I, Sugioka A, et al. Laparoscopic right hemicolectomy with radical lymph node dissection using the no-touch isolation technique for advanced colon cancer. Surg Today 2001;31:93-6. [Crossref] [PubMed]
- Baća I, Perko Z, Bokan I, et al. Technique and survival after laparoscopically assisted right hemicolectomy. Surg Endosc 2005;19:650-5. [Crossref] [PubMed]
- Ichihara T, Takada M, Fukumoto S, et al. Lymphadenectomy along the middle colic artery in laparoscopic resection of transverse colon. Hepatogastroenterology 2004;51:454-6. [PubMed]
- Matsuda T, Iwasaki T, Hirata K, et al. A Three-Step Method for Laparoscopic Mobilization of the Splenic Flexure. Ann Surg Oncol 2015;22:S335. [Crossref] [PubMed]
- Bosio RM, Pigazzi A. Emerging and evolving technology in colon and rectal surgery. Clin Colon Rectal Surg 2015;28:152-7. [Crossref] [PubMed]
- Bosio RM, Pigazzi A. Hybrid laparoscopic-robotic low anterior resection. In: Kim J, Garcia-Aguilar J. editors. Surgery for cancers of the gastrointestinal tract: a step-by-step approach. New York: Springer, 2014;23:247-62.
- Yamamoto M, Okuda J, Tanaka K, et al. Clinical outcomes of laparoscopic surgery for advanced transverse and descending colon cancer: a single-center experience. Surg Endosc 2012;26:1566-72. [Crossref] [PubMed]
- Zhao L, Wang Y, Liu H, et al. Long-term outcomes of laparoscopic surgery for advanced transverse colon cancer. J Gastrointest Surg 2014;18:1003-9. [Crossref] [PubMed]
- Gravante G, Elshaer M, Parker R, et al. Extended right hemicolectomy and left hemicolectomy for colorectal cancers between the distal transverse and proximal descending colon. Ann R Coll Surg Engl 2016;98:303-7. [Crossref] [PubMed]
- Bakker IS, Grossmann I, Henneman D, et al. Risk factors for anastomotic leakage and leak-related mortality after colonic cancer surgery in a nationwide audit. Br J Surg 2014;101:424-32; discussion 432. [Crossref] [PubMed]
- Krarup PM, Jorgensen LN, Andreasen AH, et al. A nationwide study on anastomotic leakage after colonic cancer surgery. Colorectal Dis 2012;14:e661-7. [Crossref] [PubMed]
- Delannoy E, Gautier P, Devambez J, et al. (Extended right hemicolectomy for cancer of the right colon). Lille Chir 1954;9:243-5. [PubMed]
- Gallagher HW. Extended right hemicolectomy; the treatment of advanced carcinoma of the hepatic flexure and malignant duodenocolic fistula. Br J Surg 1960;47:616-21. [Crossref] [PubMed]
- Bouasakao N, Druart R, Dupres M, et al. Colo-duodenal fistula caused by cancer of the right colonic flexure treated by right extended hemicolectomy associated with a mucosal patch using a terminal ileal pedicled graft. Apropos of a case) J Chir (Paris) 1984;121:757-63. [PubMed]
- Chew SS, Adams WJ. Laparoscopic hand-assisted extended right hemicolectomy for cancer management. Surg Endosc 2007;21:1654-6. [Crossref] [PubMed]
- Chong CS, Huh JW, Oh BY, et al. Operative Method for Transverse Colon Carcinoma: Transverse Colectomy Versus Extended Colectomy. Dis Colon Rectum 2016;59:630-9. [Crossref] [PubMed]
- Akiyoshi T, Kuroyanagi H, Fujimoto Y, et al. Short-term outcomes of laparoscopic colectomy for transverse colon cancer. J Gastrointest Surg 2010;14:818-23. [Crossref] [PubMed]
- Fernández-Cebrián JM, Gil Yonte P, Jiminez-Toscano M, et al. Laparoscopic colectomy for transverse colon carcinoma: a surgical challenge but oncologically feasible. Colorectal Dis 2013;15:e79-83. [Crossref] [PubMed]
- Mistrangelo M, Allaix ME, Cassoni P, et al. Laparoscopic versus open resection for transverse colon cancer. Surg Endosc 2015;29:2196-202. [Crossref] [PubMed]
- Yamaguchi S, Tashiro J, Araki R, et al. Laparoscopic versus open resection for transverse and descending colon cancer: Short-term and long-term outcomes of a multicenter retrospective study of 1830 patients. Asian J Endosc Surg 2017;10:268-75. [Crossref] [PubMed]
- Hirasaki Y, Fukunaga M, Sugano M, et al. Short- and long-term results of laparoscopic surgery for transverse colon cancer. Surg Today 2014;44:1266-72. [Crossref] [PubMed]
- Matsuda T, Iwasaki T, Hirata K, et al. Optimal Surgery for Mid-Transverse Colon Cancer: Laparoscopic Extended Right Hemicolectomy Versus Laparoscopic Transverse Colectomy. World J Surg 2018;42:3398-404. [Crossref] [PubMed]
Cite this article as: Angel CA, Bosio RM. The dilemmas of the transverse colon cancer: segmental or extended right colectomy, laparoscopic hazards for the inexperienced surgeon. Ann Laparosc Endosc Surg 2019;4:4.