
Early rectal cancer treated by endoscopic submucosal dissection (ESD), endoscopic mucosal resection (EMR) or transanal endoscopic microsurgery (TEM)
Introduction
Colorectal cancer is the second cause of death in the western world and about 35% of these tumors are located in the rectum (1). Early rectal cancer (ERC) is defined as an adenocarcinoma of the rectum that involves the rectal wall up to the submucosa (T1–T2 according to WHO classification), regardless of the presence of lymph-node metastases. Prognosis primarily depends on tumor stage at the time of the diagnosis; only about 50% of adenocarcinoma have been diagnosed at an early stage (2,3). Mortality exponentially increases as the stage becomes higher. Five years survival rate is over 90% for adenocarcinoma staged as T1N0 but it decrease to 75–80% when lymph-node metastases are involved (4,5). The data primarily impose the necessity of early diagnosis. Common surgical resection allows resection of the involved specimen and related lymph-nodes because mesorectum and sigmoid mesum with lymph-nodes along inferior mesenteric artery are removed, but on the other side it is burned by high morbidity and mortality related to surgery. Tumors of the lower third of the rectum impose coloanal anastomoses that have higher morbidity rate (in particular higher dehiscence rate, impaired continence), higher temporary stoma rate that in a not negligible number of cases will not be closed and, when coloanal anastomosis is not technically feasible or contraindicated, abdominoperineal amputation with definitive colostomy can be the only therapeutic choice (6-8).
In order to reduce invasiveness and morbidity of surgery efforts have been spent to define which lesions are amenable of local excision, how early tumors can be diagnosed with acceptable accuracy and which surgical technique is the best therapeutic option.
Preoperative assessment of ERC
The term local excision includes several surgical and endoscopic procedures, ranging from mucosectomy to full-thickness local excision with partial resection of mesorectal fat (9).
The role of local excision in the treatment of ERC is still controversial, mainly because of the absence of adequate lymphadenectomy (10).
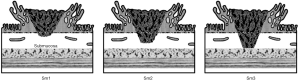
Kikuchi classification
The role of Kikuchi Classification for depth of invasion is crucial, consisting of the division of the submucosal layer in three parts: sm1, sm2 and sm3, initially denoting the upper, middle and lower thirds of the submucosa respectively (11), then restricting sm1 to those lesions deepening <1 mm into the submucosal layer. Reported risks of lymph-node metastases are 0–3% for sm1 invasion, 8–10% for sm2, and 23–25% for sm3 (9,12-25) (Figure 1).
Therefore, appropriate patient and tumor selection remain a major obstacle to these treatments for rectal cancer but morphological and histopathologic features can guide the selection of patients with ERC amenable of local excision.
Kudo classification
The Kudo classification is a morphological classification based on pit pattern, the appearance of the glandular orifices and the surface structures, combined with the visualization of capillary design, which reflects the structural atypia and represents a histological prediction index. Kudo et al. (26) have proposed a description in 5 different classes, to which Fujii et al. (27) gave a clinical value, now widely accepted. Type I, with small and round pit, is suggestive of normal mucosa; Type II, with asteroid pit, for non-neoplastic lesions, i.e., hyperplastic. The Type III, distinguished in a -L form, with tubular or round pit larger than the normal pit (type 1), and a -S form, characterized by tubular or round pit smaller than the normal pit, it is suggestive of non-invasive neoplastic lesions. Type IV, with dendritic or gyrus-like pit, and Type V, with irregular pit, completely unstructured, in turn divided in a form Vi and a Vn, they suggest neoplastic lesions, up to an invasive malignant evolution (Vi and Vn).
Paris classification
The Paris Classification of superficial neoplastic lesions is another useful morphological instrument, and allows a stratification the risk of submucosal invasion and lymph-node metastasis (28-30) (Table 1). A polypoid lesion may be pedunculated (0-Ip), sessile (0-Is) or with a mixed pattern (0-Isp). Non-polypoid lesions are either slightly elevated (0-IIa, with elevation of <2.5 mm above the level of the mucosa), completely flat (0-IIb) or slightly depressed (0-IIc). The mixed types include elevated and depressed lesions (0-IIa + IIc), depressed and elevated (0-IIc + IIa) and sessile and depressed (0-Is + IIc) (31-34). The non-depressed types (i.e., 0-IIa, 0-IIb) might progress to polypoid or laterally spreading tumors (LST). LSTs are at least 10 mm in diameter lesions that typically extend laterally and circumferentially rather than vertically along the colonic wall (11,30). They are further classified based on their granular (G) or non-granular (NG) appearance (33). Type 0-IIc has a greater risk of submucosal infiltration and lymph-node metastasis than 0-IIa, 0-IIb and polypoid lesions. The risk is also higher in the non-granular LST compared to the granular surface LST. A greater risk of submucosal infiltration and lymph-node metastasis also correlates with the presence of large nodules in granular LST and depression in non-granular LST (35).
Table 1
| Endoscopic features | Type | Description |
|---|---|---|
| Polypoid lesions | 0-Ip | Pedunculated polyp |
| 0-Is | Sessile polyp | |
| Non-polypoid lesions | 0-IIa | Superficial-elevated polyp |
| 0-IIb | Completely fat polyp | |
| 0-IIc | Superficial-depressed lesion without ulceration | |
| Non-polypoid depressed lesions | 0-III | Depressed and ulcerated lesion |
Regarding histopathological features, four items should be assessed to determine the risk of nodes metastases, and to define a curative or non-curative local excision: grade of differentiation of the cancer, lymphatic invasion, venous invasion and tumor budding (9,14,15,20-22,36-52). If these histological finding are present the local resection should be considered non-curative and will be necessary surgical radicalization (9).
Staging
In order to select which lesion is amenable for local excision it is important to select diagnostic tools that define a tumor staging with acceptable sensitivity and specificity. Rectal cancer staging influences more than for colon cancer subsequent management because many tools can be associated to surgical resection depending on stage, ranging from local excision to neoadjuvant radio and chemotherapy.
Primary tumor
The first tool in order to define the depth of invasion of rectal wall is clinical examination and digital rectal exploration. During digital examination, consistence and motility of the tumor on the deeper floors have to be evaluated. Accuracy of digital exploration, when performed by colorectal surgeons, ranges from 58% to 88% but literature shows a high inter-observer variability, results are highly influenced by surgeon experience (53).
Magnetic resonance offers an accurate imaging of extraperitoneal space, and it allows a complete exploration of mesorectum, but its major drawback is the tendency to overestimate T1 and T2 tumors interpreting irregularity of muscular layer and perirectal fat as cancer invasion (54).
Endorectal ultrasound allows direct visualization of the layers of the rectal wall. With respect to magnetic resonance endorectal ultrasound demonstrate a higher specificity in detecting muscolaris propria invasion (differentiating between T1 and T2) and a higher both sensitivity and specificity in distinguishing muscolaris mucosae invasion (54). About 20% of tumors EUS staged as T3 were T2 tumors at pathologic assessment. Despite that, endorectal ultrasound is actually the most accurate staging system for ERC, where distinguishing the depth of invasion is essential to define subsequent surgical treatment. Major drawback of endorectal ultrasound are the difficulty to obtain adequate imaging when large occluding tumors have been found and to visualize the mesorectal space near mesorectal fascia, particularly in the upper third of the rectum where mesorectal fat is wider (55,56). Consequently, endorectal ultrasound is less accurate in staging T category in advanced rectal tumors.
Supplementary information that can help in defining a probability of deep submucosal invasion is the analysis of pit pattern during magnified chromoendoscopy. Irregular and distorted pits suggest deep (>1,000 micron) invasion of the submucosal layer.
Lymph-node status
Radiologic assessment of lymph-node status employs endorectal ultrasound and magnetic resonance. The accuracy of both the techniques ranges from 60% to 80% with the mayor difference that magnetic resonance allows a more accurate visualization of mesorectal fascia (57,58). Criteria to define lymph-node involvement are a decreased echogenicity and a round rather than oval shape or a larger size and the presence of irregular contour. Magnetic resonance adds the possibility to analyze homogeneity of the signal with a sensitivity for lymph-nodes with a cutoff of 3 mm 78%.
When an early cancer is suspected, the combination of diagnostic information (biopsy specimen, magnification chromoendoscopy) and staging systems (clinical evaluation and endorectal ultrasound) have to be employed to define lesions amenable of local excision rather than radical resection, but even if endorectal ultrasound is the most accurate staging option, it allows a relative low accuracy. A retrospective study that compared preoperative and postoperative staging of more than 7,000 patients on 384 hospitals, demonstrated a correspondence rate of about 64% (57). That is why it is important to obtain an en bloc resection of rectal lesion when a local excision is performed, in order to achieve a complete postoperative staging and select tumors that require subsequent radical surgery. If a rectal adenocarcinoma is resected piece meal, it is not possible to define correct staging and define if it is a lesion for which local excision is oncologically correct (Tis and T1 up to sm1) and subsequent radical surgery is mandatory in any case. So the choice of the surgical technique for local excision has to guarantee the highest rate of en bloc resection.
Endoscopic and surgical treatment
Endoscopic resection techniques available for local excision for rectal tumors are endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD). About 30 years ago a new transanal surgical resection technique, transanal endoscopic microsurgery (TEM), was introduced demonstrating a higher rate of en bloc resection rate with disease free margins when compared to standard transanal excision (58). It consisted in an operative resectoscope with magnified stereoscopic vision that allows dissection and suturing.
EMR
EMR is an endoscopic technique that consists in the use of a snare advanced in the operative channel of the endoscope. The lesion is caught by the opened snare and cut below mucosal surface during snare closure. For non-pedunculated lesions it is suggested to inject a solution into the submucosal space to separate a mucosal lesion from the underlying muscularis propria. The submucosal cushion theoretically reduces the risk of thermal or mechanical injury to the underlying muscularis propria. Normal saline can be used but is absorbed quickly. Hypertonic saline is available and a longer standing submucosal cushion has been reported. A small amount of adrenaline can be used to decrease the risk of bleeding. Snare cut can be done without electrocautery (cold snare mucosectomy), but it is suggested to use electrocautery for lesion greater than 10 mm (59-64). Major advantage of this technique is primarily its limited invasiveness; it does not require general anesthesia, allows lower postoperative morbidity (bleeding rate is reported to be about 5% for lesions greater than 20 mm) and a relatively low operative time (literature reports a mean operative time of about 15 minutes) (63). Mayor drawback is the low rate of en bloc resection if the lesion is greater in size. En bloc resection rate is about 84% for lesions <20 mm and 50% for lesions greater than 20 mm. Consequently this technique is contraindicated when rectal lesion is considered a high risk lesion (pit pattern >3 sec kudo, biopsy of adenocarcinoma, diameter greater than 20 mm) (65-68).
ESD
Endoscopic submucosa dissection employs a modified needle knife to dissect lesions through the submucosa. It raised about 15 years ago as an endoscopic technique to obtain en bloc resection of LST of the gastrointestinal tract. The technique consists in marking the margin of the lesion at about 5 mm with electrocautery. Submucosal injection is than performed. A circumferential incision is performed with the ESD knife in order to create a flap that is gradually lifted dissecting the submucosal space. Different electrocautery setting are available and advancement of the endoscope under the flap can be easily performed if a transparent flap is attached on the tip of the endoscope (69-71).
With respect to other endoscopic techniques, ESD requires highest operative time (ranging from 70 to 130 min) and sedation techniques. When lesions greater than 20 mm are treated morbidity rate is reported to be of about 10% (primarily bleeding and perforation). En bloc resection rates range from 86% to 90% and R0 resection rates range from 72% to 80% (60,72,73).
TEM
TEM is a transanal resection technique performed for the first time in 1986 by Buess. The original TEM proctoscope has a diameter of 40 mm, a length of either 12 or 20 cm, and an end that is either flat or beveled. The proctoscope is inserted through the anus using a blunt obturator. Once the obturator is removed, the proctoscope is sealed with a faceplate containing ports for insertion of an angled stereoscopic optics system and surgical instruments. An insufflation system then generates and maintains a constant pneumorectum, while a suction and irrigation system driven by a roller pump evacuates smoke from the operative field.
This platform allows triangulation of the instrument and has been assessed as a safe and effective technique to perform local excision. It allows excision of rectal tumors with a full thickness bowel of rectal wall. Triangulation of instruments allows an easier manipulation of tissue, the control of bleeding with electrocautery techniques and the possibility to suture tissues. Patient positioning is of critical importance in TEM. Patients are placed in an appropriate position according to the orientation of the lesion. A better visualization and manipulation of the lesion can be obtained if it is placed at the bottom of the visual field (at 6 o’clock). Therefore patient will be placed prone for lesions of the anterior rectal wall and supine for lesion of the lateral or posterior rectal wall. The tissue surrounding the lesion is marked with electrocautery at 1 cm of distance, than incision is performed and a full thickness excision of the tumor down to the mesorectum is performed. The defect can be closed with absorbable sutures. TEM is performed for lesion situated below peritoneal reflection but in case of peritoneal perforation during dissection, the defect can be closed during the same procedure, using the TEM platform, with an absorbable suture.
The procedure is performed under general or spinal anesthesia. Literature shows an en bloc resection rate ranging from 96% to 100% and an R0 resection rate of about 90% for lesion greater than 20 mm. Morbidity rate ranges from 6% to 12% (bleeding, fistulas, suture leaks being the more frequent). Local recurrence after TEM is reported to be about 5% when large (>2 cm) non-pedunculated lesions preoperatively assessed as non-invasive by digital examination or confined to the mucosal layer by endoscopic ultrasound were considered. Operative time is reported to range from 30 to 110 minutes (74-77).
Comparative studies in literature
A review of the literature has been taken including comparative studies. A recent review compares EMR and ESD in the treatment of large colorectal lesions. ESD demonstrated a higher en bloc resection rate of 83% if compared to EMR (48% of en bloc resection rate). ESD was associated with a greater risk of perforation (5.9% vs. 0%) (68). These results were confirmed by an analysis of 17 case series in which the risk of perforation was 0.2% (78) while a large series of ESD reports a risk of perforation up to 18% (79). A recent systematic review comparing piece EMR and TEM for large colorectal lesions (>2 cm) concluded than, although recurrence rate after EMR was 11%, overall recurrence after repeated treatments was comparable with recurrence after TEM (80). However these result should be carefully analyzed because the two groups were not homogeneous; lesions treated with TEM were significantly greater in diameter, a significantly higher carcinoma rate was found in the TEM group and tumors treated with EMR were both colon and rectal lesions. Data from these comparisons suggest that EMR allows an acceptable en bloc resection rate for lesion <2 cm and, since piece meal and R1 resections are directly related to recurrence rate, this technique in the rectum where other techniques are available, can be indicated for lesions less than 20 mm in diameter and when clinical and endoscopic data are not suspected for submucosal invasion.
As regard ESD and TEM two comparative retrospective studies are available. Both reports no difference in terms of R0 resection and recurrence rate but the number of patients included in the studies is limited (81,82). A wider population was included in a systematic review and meta-analyses comparing ESD and TEM for the treatment of extraperitoneal lesion greater than 2 cm preoperatively assessed as non-invasive (83). 2,077 patients were included. Operative time was significantly longer in the TEM group, but en bloc resection rate and R0 resections were significantly higher when patients were treated by TEM (99% vs. 88% and 89% vs. 74% respectively). Even if the difference between recurrences was not statistically different, the need of further surgery for oncological reasons was higher in the ESD group with a statistically significant difference (9% vs. 2%).
Consequently in case of extraperitoneal rectal lesion supposed to be non-invasive TEM is the treatment of choice because of the limited need of further surgery for oncological reason as a consequence of higher R0 resections.
New perspectives
Beside the need to increase the accuracy of preoperative staging, in order to better select the rate of ERC without lymph-node metastases amenable of local excision, many effort have been spent in define techniques to analyze mesorectal lymphatic pattern.
Sentinel lymph node navigation surgery tried to evaluate the presence of a reproducible and sequential distribution of lymph node drainage in the mesorectum by excising the first enhancing lymph node after injection of a tracer, but further studies are needed to define the impact of this technique in the rectal pathology (84,85).
Endoscopic posterior mesorectal resection (EPMR) consists in excising the posterior aspect of the mesorectum through a precoccygeal access to the presacral space. Even if only preliminary data are available, this technique can be an option if combined to local excision to evaluate the presence of lymph-node metastases in the mesorectum (86,87) although more data and more information on the lymphatic drainage in the mesorectum are needed.
Another option can be the employment of neoadjuvant therapy for rectal tumors that exceed the standard indication for local resection (T1–T2 N0 rectal adenocarcinoma) followed by local excision by TEM. Literature shows conflicting results mainly due to high postoperative complication (88-90).
Conclusions
In conclusion, when a lesion of the rectum is found and at clinical examination appears to be an early rumor, diagnostic colonoscopy has to be performed, in order to exclude the presence of metachronous lesion and to assess characteristics of the tumor (extension, histological features from bioptic samples, pit pattern and Kudo classification).
If it is supposed to be non-invasive, local staging with echoendoscopy offers the highest accuracy and if non-invasiveness is confirmed than local excision is indicated. Because of the relatively low correspondence between preoperative and postoperative stadiation, en bloc and R0 excision is fundamental in order to reduce further surgery for oncological reason. If the lesion is inferior of 2 cm in diameter and does not have malignant features, en bloc mucosectomy can be performed. Otherwise TEM or ESD is indicate, literature demonstrate that TEM have higher en bloc and R0 resection rate and fewer necessity of further surgery for oncological reason, so it is indicated when available, ESD can be a valid alternative in specialized centers with high endoscopic experience.
Definitive histologic assessment will allow to select which tumor need further surgery on the base of local stadiation and tumor characteristics. Local excision may be considered oncologically adequate for ERC invading up to 1 mm of the submucosal layer (up to T1sm1). Other risk factors for lymph node metastases such as tumor differentiation, tumor budding and lymphovascular invasion have to be taken into account in order to select patients that need radical surgery.
We hope that with technological improvement new techniques may rise, in order to better select among early rectal lesions the ones with lowest probability of lymph node metastases, and extend indication of local excision.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Eduardo Ma Targarona and Andrea Balla) for the series “Rectal Cancer” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2018.08.01). The series “Rectal Cancer” was commissioned by the editorial office without any funding or sponsorship. AA serves as an unpaid editorial board member of Annals of Laparoscopic and Endoscopic Surgery from Jul 2016 to Jun 2018. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Global Burden of Disease Cancer Collaboration. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol 2017;3:524-48. [Crossref] [PubMed]
- Jemal A, Ward EM, Johnson CJ, et al. Annual Report to the Nation on the Status of Cancer, 1975-2014, Featuring Survival. J Natl Cancer Inst 2017;109: [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Tarantino I, Müller SA, Warschkow R, et al. Baseline mortality-adjusted survival in resected rectal cancer patients. J Gastrointest Surg 2014;18:1837-44. [Crossref] [PubMed]
- Akhavan A, Binesh F, Soltani A. Survival of rectal cancer in Yazd, Iran. Asian Pac J Cancer Prev 2014;15:4857-60. [Crossref] [PubMed]
- Steele SR, Bleier J, Champagne B, et al. Improving outcomes and cost-effectiveness of colorectal surgery. J Gastrointest Surg 2014;18:1944-56. [Crossref] [PubMed]
- Daher R, Chouillard E, Panis Y. New trends in colorectal surgery: single port and natural orifice techniques. World J Gastroenterol 2014;20:18104-20. [Crossref] [PubMed]
- Lee CM, Huh JW, Yun SH, et al. Laparoscopic versus open reintervention for anastomotic leakage following minimally invasive colorectal surgery. Surg Endosc 2015;29:931-6. [Crossref] [PubMed]
- Morino M, Risio M, Bach S, et al. Early rectal cancer: the European Association for Endoscopic Surgery (EAES) clinical consensus conference. Surg Endosc 2015;29:755-73. [Crossref] [PubMed]
- Heafner TA, Glasgow SC. A critical review of the role of local excision in the treatment of early (T1 and T2) rectal tumors. J Gastrointest Oncol 2014;5:345-52. [PubMed]
- Kudo S. Endoscopic mucosal resection of flat and depressed types of early colorectal cancer. Endoscopy 1993;25:455-61. [Crossref] [PubMed]
- Kikuchi R, Takano M, Takagi K, et al. Management of early invasive colorectal cancer. Risk of recurrence and clinical guidelines. Dis Colon Rectum 1995;38:1286-95. [Crossref] [PubMed]
- Williams JG, Pullan RD, Hill J, et al. Management of the malignant colorectal polyp: ACPGBI position statement. Colorectal Dis 2013;15:1-38. [Crossref] [PubMed]
- Nascimbeni R, Burgart LJ, Nivatvongs S, et al. Risk of lymph node metastasis in T1 carcinoma of the colon and rectum. Dis Colon Rectum 2002;45:200-6. [Crossref] [PubMed]
- Yamamoto S, Watanabe M, Hasegawa H, et al. The risk of lymph node metastasis in T1 colorectal carcinoma. Hepatogastroenterology 2004;51:998-1000. [PubMed]
- Smith KJ, Jones PF, Burke DA, et al. Lymphatic vessel distribution in the mucosa and submucosa and potential implications for T1 colorectal tumors. Dis Colon Rectum 2011;54:35-40. [Crossref] [PubMed]
- Saraste D, Gunnarsson U, Janson M. Predicting lymph node metastases in early rectal cancer. Eur J Cancer 2013;49:1104-8. [Crossref] [PubMed]
- Casadesus D. Surgical resection of rectal adenoma: a rapid review. World J Gastroenterol 2009;15:3851-4. [Crossref] [PubMed]
- Suppiah A, Maslekar S, Alabi A, et al. Transanal endoscopic microsurgery in early rectal cancer: time for a trial? Colorectal Dis 2008;10:314-27; discussion 327-9. [Crossref] [PubMed]
- Kaneko I, Tanaka S, Oka S, et al. Lymphatic vessel density at the site of deepest penetration as a predictor of lymph node metastasis in submucosal colorectal cancer. Dis Colon Rectum 2007;50:13-21. [Crossref] [PubMed]
- Sakuragi M. Predictive factors for lymph node metastasis in T1 stage colorectal carcinomas. Dis Colon Rectum 2003;46:1626-32. [Crossref] [PubMed]
- Tominaga K, Nakanishi Y, Nimura S, et al. Predictive histopathologic factors for lymph node metastasis in patients with nonpedunculated submucosal invasive colorectal carcinoma. Dis Colon Rectum 2005;48:92-100. [Crossref] [PubMed]
- Puli SR, Bechtold ML, Reddy JB, et al. How good is endoscopic ultrasound in differentiating various T stages of rectal cancer? Meta-analysis and systematic review. Ann Surg Oncol 2009;16:254-65. [Crossref] [PubMed]
- Marusch F, Ptok H, Sahm M, et al. Endorectal ultrasound in rectal carcinoma--do the literature results really correspond to the realities of routine clinical care? Endoscopy 2011;43:425-31. [Crossref] [PubMed]
- Ashraf S, Hompes R, Slater A, et al. A critical appraisal of endorectal ultrasound and transanal endoscopic microsurgery and decision-making in early rectal cancer. Colorectal Dis 2012;14:821-6. [Crossref] [PubMed]
- Kudo S, Rubio CA, Teixeira CR, et al. Pit pattern in colorectal neoplasia: endoscopic magnifying view. Endoscopy 2001;33:367-73. [Crossref] [PubMed]
- Fujii T, Hasegawa RT, Saitoh Y, et al. Chromoscopy during colonoscopy. Endoscopy 2001;33:1036-41. [PubMed]
- Bianco MA, Cipolletta L, Rotondano G, et al. Prevalence of nonpolypoid colorectal neoplasia: an Italian multicenter observational study. Endoscopy 2010;42:279-85. [Crossref] [PubMed]
- Saitoh Y, Obara T, Watari J, et al. Invasion depth diagnosis of depressed type early colorectal cancers by combined use of videoendoscopy and chromoendoscopy. Gastrointest Endosc 1998;48:362-70. [Crossref] [PubMed]
- Uraoka T, Saito Y, Matsuda T, et al. Endoscopic indications for endoscopic mucosal resection of laterally spreading tumours in the colorectum. Gut 2006;55:1592-7. [Crossref] [PubMed]
- The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc 2003;58:S3-43. [Crossref] [PubMed]
- Endoscopic Classification Review Group. Update on the paris classification of superficial neoplastic lesions in the digestive tract. Endoscopy 2005;37:570-8. [Crossref] [PubMed]
- Kudo S, Lambert R, Allen JI, et al. Nonpolypoid neoplastic lesions of the colorectal mucosa. Gastrointest Endosc 2008;68:S3-47. [Crossref] [PubMed]
- Lambert R, Kudo SE, Vieth M, et al. Pragmatic classification of superficial neoplastic colorectal lesions. Gastrointest Endosc 2009;70:1182-99. [Crossref] [PubMed]
- Oka S, Tanaka S, Kanao H, et al. Therapeutic strategy for colorectal laterally spreading tumor. Digestive Endoscopy 2009;21:S43-6. [Crossref] [PubMed]
- Kitajima K, Fujimori T, Fujii S, et al. Correlations between lymph node metastasis and depth of submucosal invasion in submucosal invasive colorectal carcinoma: a Japanese collaborative study. J Gastroenterol 2004;39:534-43. [Crossref] [PubMed]
- Bretagnol F, Rullier E, George B, et al. Local therapy for rectal cancer: still controversial? Dis Colon Rectum 2007;50:523-33. [Crossref] [PubMed]
- Yasuda K, Inomata M, Shiromizu A, et al. Risk factors for occult lymph node metastasis of colorectal cancer invading the submucosa and indications for endoscopic mucosal resection. Dis Colon Rectum 2007;50:1370-6. [Crossref] [PubMed]
- Ueno H, Mochizuki H, Hashiguchi Y, et al. Risk factors for an adverse outcome in early invasive colorectal carcinoma. Gastroenterology 2004;127:385-94. [Crossref] [PubMed]
- Suzuki T, Sadahiro S, Mukoyama S, et al. Risk of lymph node and distant metastases in patients with early invasive colorectal cancer classified as Haggitt's level 4 invasion: image analysis of submucosal layer invasion. Dis Colon Rectum 2003;46:203-8. [Crossref] [PubMed]
- Choi PW, Yu CS, Jang SJ, et al. Risk factors for lymph node metastasis in submucosal invasive colorectal cancer. World J Surg 2008;32:2089-94. [Crossref] [PubMed]
- Tanaka S, Yokota T, Saito D, et al. Clinicopathologic features of early rectal carcinoma and indications for endoscopic treatment. Dis Colon Rectum 1995;38:959-63. [Crossref] [PubMed]
- Gopaul D, Belliveau P, Vuong T, et al. Outcome of local excision of rectal carcinoma. Dis Colon Rectum 2004;47:1780-8. [Crossref] [PubMed]
- Gao JD, Shao YF, Bi JJ, et al. Local excision carcinoma in early stage. World J Gastroenterol 2003;9:871-3. [Crossref] [PubMed]
- Bleday R, Breen E, Jessup JM, et al. Prospective evaluation of local excision for small rectal cancers. Dis Colon Rectum 1997;40:388-92. [Crossref] [PubMed]
- Watanabe T, Itabashi M, Shimada Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2010 for the treatment of colorectal cancer. Int J Clin Oncol 2012;17:1-29. [Crossref] [PubMed]
- Ueno H, Hashiguchi Y, Kajiwara Y, et al. Proposed objective criteria for "grade 3" in early invasive colorectal cancer. Am J Clin Pathol 2010;134:312-22. [Crossref] [PubMed]
- Sohn DK, Chang HJ, Park JW, et al. Histopathological risk factors for lymph node metastasis in submucosal invasive colorectal carcinoma of pedunculated or semipedunculated type. J Clin Pathol 2007;60:912-5. [Crossref] [PubMed]
- Quirke P, Risio M, Lambert R, et al. Quality assurance in pathology in colorectal cancer screening and diagnosis - European recommendations. Virchows Arch 2011;458:1-19. [Crossref] [PubMed]
- Suzuki A, Togashi K, Nokubi M, et al. Evaluation of venous invasion by Elastica van Gieson stain and tumor budding predicts local and distant metastases in patients with T1 stage colorectal cancer. Am J Surg Pathol 2009;33:1601-7. [Crossref] [PubMed]
- Hase K, Shatney CH, Mochizuki H, et al. Long-term results of curative resection of "minimally invasive" colorectal cancer. Dis Colon Rectum 1995;38:19-26. [Crossref] [PubMed]
- Puppa G, Senore C, Sheahan K, et al. Diagnostic reproducibility of tumour budding in colorectal cancer: a multicentre, multinational study using virtual microscopy. Histopathology 2012;61:562-75. [Crossref] [PubMed]
- Schaffzin DM, Wong WD. Endorectal ultrasound in the preoperative evaluation of rectal cancer. Clin Colorectal Cancer 2004;4:124-32. [Crossref] [PubMed]
- Muthusamy VR, Chang KJ. Optimal methods for staging rectal cancer. Clin Cancer Res 2007;13:6877s-84s. [Crossref] [PubMed]
- Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351:1731-40. [Crossref] [PubMed]
- Kwok H, Bissett IP, Hill GL. Preoperative staging of rectal cancer. Int J Colorectal Dis 2000;15:9-20. [Crossref] [PubMed]
- Brown G, Richards CJ, Bourne MW, et al. Morphologic predictors of lymph node status in rectal cancer with use of high-spatial-resolution MR imaging with histopathologic comparison. Radiology 2003;227:371-7. [Crossref] [PubMed]
- Kim JH, Beets GL, Kim MJ, et al. High-resolution MR imaging for nodal staging in rectal cancer: are there any criteria in addition to the size? Eur J Radiol 2004;52:78-83. [Crossref] [PubMed]
- Nakajima T, Saito Y, Tanaka S, et al. Current status of endoscopic resection strategy for large, early colorectal neoplasia in Japan. Surg Endosc 2013;27:3262-70. [Crossref] [PubMed]
- Saito Y, Uraoka T, Yamaguchi Y, et al. A prospective, multicenter study of 1111 colorectal endoscopic submucosal dissections (with video). Gastrointest Endosc 2010;72:1217-25. [Crossref] [PubMed]
- Kiriyama S, Saito Y, Matsuda T, et al. Comparing endoscopic submucosal dissection with transanal resection for non-invasive rectal tumor: a retrospective study. J Gastroenterol Hepatol 2011;26:1028-33. [Crossref] [PubMed]
- Kiriyama S, Saito Y, Yamamoto S, et al. Comparison of endoscopic submucosal dissection with laparoscopic-assisted colorectal surgery for early-stage colorectal cancer: a retrospective analysis. Endoscopy 2012;44:1024-30. [Crossref] [PubMed]
- Saito Y, Fukuzawa M, Matsuda T, et al. Clinical outcome of endoscopic submucosal dissection versus endoscopic mucosal resection of large colorectal tumors as determined by curative resection. Surg Endosc 2010;24:343-52. [Crossref] [PubMed]
- Ferlitsch M, Moss A, Hassan C, et al. Colorectal polypectomy and endoscopic mucosal resection (EMR): European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy 2017;49:270-97. [Crossref] [PubMed]
- Moss A, Williams SJ, Hourigan LF, et al. Long-term adenoma recurrence following wide-field endoscopic mucosal resection for advanced colonic mucosal neoplasia is infrequent: results and risk factors in 1000 cases from the Australian Colonic EMR study. Gut 2015;64:57-65. [Crossref] [PubMed]
- Buchner AM, Guarner-Argente C, Ginsberg GG. Outcomes of EMR of defiant colorectal lesions directed to an endoscopy referral center. Gastrointest Endosc 2012;76:255-63. [Crossref] [PubMed]
- Lee EJ, Lee JB, Lee SH, et al. Endoscopic treatment of large colorectal tumors: comparison of endoscopic mucosal resection, endoscopic mucosal resection-precutting, and endoscopic submucosal dissection. Surg Endosc 2012;26:2220-30. [Crossref] [PubMed]
- Tajika M, Niwa Y, Bhatia V, et al. Comparison of endoscopic submucosal dissection and endoscopic mucosal resection for large colorectal tumors. Eur J Gastroenterol Hepatol 2011;23:1042-9. [PubMed]
- Yamamoto H, Yahagi N, Oyama T. Mucosectomy in the colon with endoscopic submucosal dissection. Endoscopy 2005;37:764-8. [Crossref] [PubMed]
- Oyama T, Tomori A, Hotta K, et al. Endoscopic submucosal dissection of early esophageal cancer. Clin Gastroenterol Hepatol 2005;3:S67-70. [Crossref] [PubMed]
- Fujishiro M, Yahagi N, Nakamura M, et al. Successful outcomes of a novel endoscopic treatment for GI tumors: endoscopic submucosal dissection with a mixture of high-molecular-weight hyaluronic acid, glycerin, and sugar. Gastrointest Endosc 2006;63:243-9. [Crossref] [PubMed]
- Probst A, Ebigbo A, Märkl B, et al. Endoscopic submucosal dissection for early rectal neoplasia: experience from a European center. Endoscopy 2017;49:222-232. [PubMed]
- Tang XW, Ren YT, Zhou JQ, et al. Endoscopic submucosal dissection for laterally spreading tumors in the rectum ≥40 mm. Tech Coloproctol 2016;20:437-43. [Crossref] [PubMed]
- Moore JS, Cataldo PA, Osler T, et al. Transanal endoscopic microsurgery is more effective than traditional transanal excision for resection of rectal masses. Dis Colon Rectum 2008;51:1026-30; discussion 1030-1. [Crossref] [PubMed]
- de Graaf EJ, Burger JW, van Ijsseldijk AL, et al. Transanal endoscopic microsurgery is superior to transanal excision of rectal adenomas. Colorectal Dis 2011;13:762-7. [Crossref] [PubMed]
- Langer C, Liersch T, Süss M, et al. Surgical cure for early rectal carcinoma and large adenoma: transanal endoscopic microsurgery (using ultrasound or electrosurgery) compared to conventional local and radical resection. Int J Colorectal Dis 2003;18:222-9. [PubMed]
- Christoforidis D, Cho HM, Dixon MR, et al. Transanal endoscopic microsurgery versus conventional transanal excision for patients with early rectal cancer. Ann Surg 2009;249:776-82. [Crossref] [PubMed]
- Panteris V, Haringsma J, Kuipers EJ. Colonoscopy perforation rate, mechanisms and outcome: from diagnostic to therapeutic colonoscopy. Endoscopy. 2009;41:941-51. [Crossref] [PubMed]
- Farhat S, Chaussade S, Ponchon T, et al. Endoscopic submucosal dissection in a European setting. A multi-institutional report of a technique in development. Endoscopy 2011;43:664-70. [Crossref] [PubMed]
- Barendse RM, van den Broek FJ, Dekker E, et al. Systematic review of endoscopic mucosal resection versus transanal endoscopic microsurgery for large rectal adenomas. Endoscopy 2011;43:941-9. [Crossref] [PubMed]
- Kawaguti FS, Nahas CS, Marques CF, et al. Endoscopic submucosal dissection versus transanal endoscopic microsurgery for the treatment of early rectal cancer. Surg Endosc 2014;28:1173-9. [Crossref] [PubMed]
- Park SU, Min YW, Shin JU, et al. Endoscopic submucosal dissection or transanal endoscopic microsurgery for nonpolypoid rectal high grade dysplasia and submucosa-invading rectal cancer. Endoscopy 2012;44:1031-6. [Crossref] [PubMed]
- Arezzo A, Passera R, Saito Y, et al. Systematic review and meta-analysis of endoscopic submucosal dissection versus transanal endoscopic microsurgery for large noninvasive rectal lesions. Surg Endosc 2014;28:427-38. [Crossref] [PubMed]
- Saha S, Dan AG, Viehl CT, et al. Sentinel lymph node mapping in colon and rectal cancer: its impact on staging, limitations, and pitfalls. Cancer Treat Res 2005;127:105-22. [Crossref] [PubMed]
- Arezzo A, Arolfo S, Mistrangelo M, et al. Transrectal sentinel lymph node biopsy for early rectal cancer during transanal endoscopic microsurgery. Minim Invasive Ther Allied Technol 2014;23:17-20. [Crossref] [PubMed]
- Wałęga P, Kenig J, Richter P. Transanal endoscopic microsurgery combined with endoscopic posterior mesorectum resection in the treatment of patients with T1 rectal cancer - 3-year results. Wideochir Inne Tech Maloinwazyjne 2014;9:40-5. [Crossref] [PubMed]
- Walega P, Kenig J, Richter P, et al. Functional and clinical results of transanal endoscopic microsurgery combined with endoscopic posterior mesorectum resection for the treatment of patients with t1 rectal cancer. World J Surg 2010;34:1604-8. [Crossref] [PubMed]
- Perez RO, Habr-Gama A, Lynn PB, et al. Transanal endoscopic microsurgery for residual rectal cancer (ypT0-2) following neoadjuvant chemoradiation therapy: another word of caution. Dis Colon Rectum 2013;56:6-13. [Crossref] [PubMed]
- Habr-Gama A, São Julião GP, Perez RO. Pitfalls of transanal endoscopic microsurgery for rectal cancer following neoadjuvant chemoradiation therapy. Minim Invasive Ther Allied Technol 2014;23:63-9. [Crossref] [PubMed]
- Arezzo A, Arolfo S, Allaix ME, et al. Results of neoadjuvant short-course radiation therapy followed by transanal endoscopic microsurgery for t1-t2 n0 extraperitoneal rectal cancer. Int J Radiat Oncol Biol Phys 2015;92:299-306. [Crossref] [PubMed]
Cite this article as: Verra M, Riente F, Arezzo A. Early rectal cancer treated by endoscopic submucosal dissection (ESD), endoscopic mucosal resection (EMR) or transanal endoscopic microsurgery (TEM). Ann Laparosc Endosc Surg 2018;3:67.


