
Alternative treatment to surgery for rectal cancer
Introduction
In order to avoid the significant rates of postoperative morbidity and mortality, including long-term urinary and sexual dysfunction, fecal incontinence, low anterior resection syndrome and the need for a temporary or a definitive stoma, associated with radical resection in low rectal cancer, organ preservation strategies have been considered with increasing interest.
Rectal cancers may develop significant tumor regression with tumor size reduction (downsizing), decrease in depth of tumor infiltration and even possible nodal clearance (downstaging) setting the “perfect” scenario for organ-preservation strategies such as transanal excision of small and superficial residual tumors (1). In addition, the observation that nCRT may lead to a complete tumor-cell death in the resected specimen [pathological complete response (pCR)] prompted surgeons to an attempt in the identification of these patients before surgical resection, known as complete clinical response (cCR) (2). These patients with complete tumor regression to nCRT would also constitute the ideal candidates to consider organ-preservation strategies such as no immediate surgery and strict surveillance (also known as the “Watch & Wait” strategy—WW) (3). In order to even consider these approaches, colorectal surgeons have to consider several aspects of the assessment of the disease, patients and treatment modalities that may be quite relevant during their clinical decision-making process.
Neoadjuvant chemoradiation (nCRT): indications and options
After the results of the German Trial, nCRT became the preferred approach for most cT3-4 or cN+ rectal cancers patients in an attempt to improve local disease control after radical surgery (4,5). The results of the Mercury study suggested that nCRT would preferably be restricted only to patients with high-risk of local recurrence after TME, also referred to as the “ugly” tumors. High-risk features would include radiological evidence of a threatened or positive circumferential margin (cCRM+), presence of extramural venous invasion (cEMVI+) and ≥3 positive lymph nodes (cN2) (6). In addition, radical surgery in the setting of preoperative radiation has been associated with worse functional outcomes and increased surgical morbidity when compared to surgery alone (7,8). Altogether, these findings suggested that the sole benefit of nCRT would be to improve local disease control in high-risk rectal cancer patients. Since baseline staging may affect rates of response to nCRT, one could anticipate that not many patients with considerably advanced disease would develop cCR and benefit from nCRT in terms of organ-preservation.
Instead, the idea of delivering nCRT with the intent of achieving a cCR and the possibility of avoiding radical surgery (particularly among patients otherwise candidates for abdominal-perineal resections or ultra-low intersphincteric anastomosis) with its related comorbidities led colorectal surgeons to consider nCRT to more early stage disease. Patients with cT2N0 or early cT3N0, potentially more likely to develop a complete clinical response following nCRT and could benefit the most from nCRT if organ-preservation is considered (9-11).
Even though one could argue that initial tumor grade could represent a good marker or predictor of tumor response to nCRT, the presence of significant intratumoral heterogeneity has limited the use of this information into clinical practice. Performance of pre-treatment (or even post-treatment) biopsies or macro-biopsies to predict tumor response by assessing tumor differentiation is therefore usually not recommended. Individual fragments from a single rectal cancer have been shown to exhibit significant morphological/pathological differences, despite being spatially very close to each other. In addition, these individual fragments may share less the 1/3 of all genetic mutations. Ultimately, single fragments obtained by endoscopic biopsies or macro-biopsies are rarely representative of the entirety of the cancer and therefore are simply not reliable for treatment decision purposes (12).
Therefore, when nCRT is required for local disease control purposes after total mesorectal excision (TME), it should probably be restricted to patients with high-risk features (threatened-cCRM, cN2 or cEMVI+). However, if organ-preservation is an option, nCRT may be offered more liberally to high and low-risk distal rectal cancers (including stage I disease—mrT2N0M0) (13).
The type of neoadjuvant therapy may affect the chances of developing a complete clinical response and should be considered in the scenario of organ preservation strategies. Long-course CRT was the original strategy implemented to result in significant rates of complete response. However, short-course RT followed by longer interval periods showed similar rates of complete response than long-course regimen (14). In addition, the final dose of radiation therapy and the method of delivered may also influence the odds of developing a cCR. Dose-escalation has demonstrated progressive increase in CR rates with higher doses of RT delivered to the primary tumor (15). The combination of external beam or intensity modulated RT (EBRT or IMRT) with endorectal brachytherapy (HBRT) or even with Contact RT could play a role in maximizing the chances of developing complete clinical response and still avoid major treatment related toxicity (16-18).
Alternative neoadjuvant strategies that could spare patients from the potential detrimental effects of radiation (with the same benefits) are highly warranted. Patients may develop worse functional outcomes after TME in the setting of previous exposure to RT (8). Even patients that develop a cCR and avoid radical surgery may not have perfect function (19,20). In this setting, the use of chemotherapy alone is an attractive option and has been used to restrict standard CRT to patients showing poor response to chemotherapy alone and therefore decreasing the number of patients receiving RT (21).
Finally, the incorporation of additional chemotherapy cycles in standard nCRT has been suggested. The incorporation of additional chemotherapy during the interval between RT completion and assessment of response using 5FU-based chemotherapy (consolidation CRT regimens) demonstrated an increase of CR rates to more than half of consecutive patients with T2/T3 rectal cancer (22,23). Although the observation that chemotherapy may have an important role in tumor regression, the incorporation of additional drugs to 5FU has been disappointing. The addition of oxaliplatin did not improve pCR rates in most studies. Instead, it resulted in significantly higher toxicity rates. Also, the incorporation of biological agents including anti-EGFR or anti-VEGF have been tested in the neoadjuvant setting of patients with rectal cancer. Even though these agents have demonstrated good safety profiles, their real benefits in terms of tumor regression have been even more disappointing with pCR rates even lower than usually observed with standard CRT regimens (24-26).
Assessing tumor response to nCRT
When considering patients for organ-preserving strategy, assessment of tumor response to nCRT is crucial. However, two issues remain controversial: the optimal timing for assessment and clinical/radiological tools for this purpose.
Assessment of tumor response is also highly recommended in patients with an incomplete tumor response. Even if the patient is not being considered for an organ-preserving strategy, significant changes in tumor and surrounding anatomy may be anticipated. Knowing potential anatomical changes between pre and post-treatment status ahead of time may aid in optimization of intraoperative surgical strategies and anticipate surgical challenges during the procedure (27). Therefore, the reassessment of tumor response should be preferably routinely performed.
Intervals after nCRT
Tumor-regression after nCRT may be time-dependent. The influence of distinct intervals on the response to nCRT was considered firstly by the French study comparing 2 versus 6 weeks from CRT. Patients underwent radical surgery after being randomly allocated to one of these two time intervals. Patients in the 6-week interval group presented significantly more tumor regression after nCRT (28). Following this contribution, 6-week interval from nCRT completion became the standard of care for many years in assessment of tumor response and final surgical management. However, retrospective data suggested that longer intervals, as long as 12 weeks from treatment completion, were more likely to develop pCR (29). On one hand, these considerably longer intervals could increase response to CRT. On the other hand, they could lead tissue fibrosis and increased technical difficulties and postoperative morbidity after radical surgery. A prospective, non-randomized study evaluated patients in nCRT regimens with progressively longer interval periods prior to surgical resection (30). While the first group of patients underwent surgery after a 6-week interval, the following three groups underwent surgery after longer intervals of 12, 16 and even 20 weeks, with additional chemotherapy cycles during the longer intervals (mFOLFOX) (31). The study showed that longer intervals were associated with significantly higher rates of pCR. In addition, the study suggested that longer intervals time did not have a negative effect on overall postoperative complications or surgical technical difficulty. Finally, another recently published randomized study showed different conclusions. In the GRECCAR-6 trial, the authors did not find differences in pCR rates between patients undergoing 7- or 11-week intervals. Moreover, the trial observed that more postoperative complications and worse quality of the mesorectum were associated with the 11-week interval group, suggesting the potentially negative effects of prolonged time-intervals after nCRT associated with fibrotic changes in the surgical and previously irradiated fields (32).
The optimal interval after nCRT remains undetermined, and additional ongoing trials will provide more data to allow us to understand the benefits and risks of waiting extended intervals after treatment. It is possible that individual tumors respond differently to nCRT as a function of time. In this setting, responsive tumors may require and benefit from extended intervals, whereas unresponsive tumors may not (33).
Studies for the assessment of response
Clinical & endoscopic findings
Clinical assessment remains as a critical part in the evaluation of tumor response to treatment. Even in the absence of clinical symptoms after nCRT, digital rectal examination (DRE) may be able to detect subtle residual irregularities within the rectal wall, residual masses, ulceration or even stenosis. During DRE, the surface needs to be as much as regular and smooth with only mild induration and subtle loss in the pliability of the rectal wall being acceptable findings consistent with a cCR (2).
Any irregularity or superficial ulcer missed during DRE and detected during endoscopic evaluation should raise the suspicion of an incomplete clinical response. Instead, a flat white scar and telangiectasia are typical findings encountered during endoscopic assessment of patients with a cCR (Figure 1).
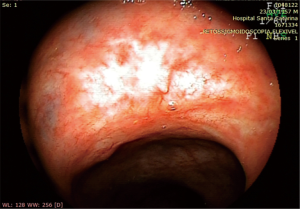
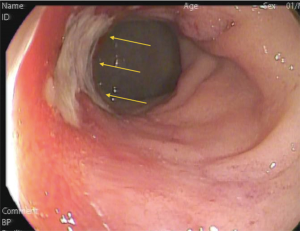
In the context of a cCR (during clinical and endoscopic assessment), endoscopic biopsies to confirm absence of residual tumor are not necessarily required. This means that in the absence of residual ulceration, mass, stenosis, there is no need for a negative biopsy to classify these patients as a complete clinical response. In contrast, in the presence of an incomplete clinical response, the results of endoscopic biopsies should be interpreted with caution. In patients with residual ulceration, mass or stenosis, a POSITIVE endoscopic biopsy is usually diagnostic of residual cancer. A NEGATIVE biopsy in such patients (with INCOMPLETE clinical response) is rarely associated with no residual cancer. Most of these patients will have residual viable cancer in nearly 80% of the cases despite the presence of negative endoscopic biopsies (34) (Figure 2). An interesting study has revealed that after nCRT, the mucosa is the layer of the rectal wall less likely to harbor residual cancer cells (35). Therefore, the presence of a negative biopsy should not be interpreted as a complete clinical response or as a marker of a complete pathological response.
Radiological assessment
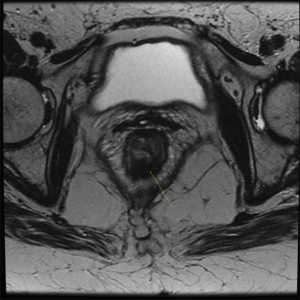
Radiological studies should also be routinely performed. High-resolution magnetic resonance (MR) is considered as the method of choice for the assessment of tumor response. Discriminating between fibrotic changes and viable residual disease has improved with current imaging modalities, placing MR as an integral part in the assessment of response to nCRT (36). Typical findings of complete tumor regression include the presence of low-signal intensity areas in the area previously harboring the rectal cancer with multiple patterns (36) (Figures 3,4). MR may accurately estimate of the pathological tumor regression grade (TRG) by providing similar mrTRG grades. This scoring system may identify patients with poor or good response prior to definitive surgical treatment and with a significant correlation between response and survival (27,37).
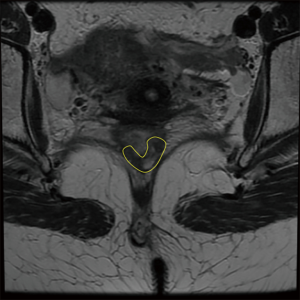
Diffusion-weighted magnetic resonance imaging (DWI-MR) has been suggested to provide additional information to standard MR imaging. The properties of water molecule diffusion may be distinct within areas of high cellularity (common within areas of residual tumor) or fibrotic scarring, and could represent an additional tool to assess tumor response to nCRT (38,39).
PET/CT imaging may also provide additional information to standard radiological features by providing an estimate of tumor metabolism. The variation in mean standard uptake values (SUV) and metabolic tumor volume reduction between pre and post-treatment scans may provide one of the best predictors of a complete tumor regression among patients with rectal cancer (40) in the setting of nCRT.
Finally, the combination of multiple studies may increase the accuracy in the detection of complete tumor response to nCRT (41).
Excisional biopsies or transanal full-thickness local excisions (FTLEs)
Excisional biopsies with the use of currently available transanal endoscopic microsurgical platforms (TEMs) have been considered an attractive tool for the assessment of primary tumor response to nCRT (42). Definitive information on pathological response including final ypT status, TRG, lymphovascular/perineural invasion and resection margins may aid in the decision regarding the need for additional TME. On the other hand, it could provide an objective pathological confirmation of pCR in the primary tumor (ypT0) and obviate the need for additional TME. However, these attractive advantages should be balanced against by several potential disadvantages. First, primary healing of the scars created by local excision after nCRT may be quite difficult and lead to significant anal symptoms. In the setting of a dehiscence, these defects may only completely close after 8 weeks from primary resection. Although, Grade III or IV postoperative complications are not usually seen, pain may be quite significant requiring frequent readmission to the hospital (43). In addition, the significant scarring following delayed healing may result in equivocal clinical and radiological follow-up findings leading to significant challenges in distinguishing postoperative fibrosis or local recurrences (44). Also, anorectal function may be significantly compromised after a FTLE. When patients with cCR managed by WW were compared to patients with “near-complete” response managed by FTLE following nCRT, functional outcomes were significantly better among patients managed non-operatively (45). In this setting, even though organ preservation has been achieved with FTLE, anorectal function may be far from normal in these patients.
Even if patients are found to have incomplete pathological response, FTLE may significant disadvantages. Patients that required additional TME after FTLE (due to the presence of unfavorable pathological features) frequently required an APR, despite the fact that some of these patients were originally candidates for a restorative procedure (46,47). In addition, completion of TME in this setting frequently resulted in a less than perfect mesorectal specimen. A recent review of patients undergoing completion TME indicated that previous TEM was a risk factor for poor quality of the TME specimen (48). Finally, function of patients that required FTLE followed by TME was significantly worse than those that required TME alone (32).
Complete clinical response: Watch & Wait strategy
All patients with a cCR after nCRT that are considered for a non-operative management require a relatively intense surveillance. The importance to adhere to this strict follow-up program is to allow early recognition of any local or systemic recurrence and therefore, increase the chances of successful salvage. Visits have been recommended with 1–2-month intervals in the first, 3-month intervals for the second year and 6-month for the remaining years of follow-up. Complete clinical and endoscopic assessments are recommended in all visits. Even though not yet standardized, radiological assessment of response has been performed at least every 6 months for the first 2 years and yearly thereafter in our practice (49). PET/CT imaging has been reserved for equivocal cases.
Outcomes
Patients undergoing the WW strategy after a cCR following nCRT were compared to patients managed by radical surgery in the presence of a pCR. Both groups had similar long-term oncological outcomes with no apparent benefit of radical surgery in this setting (3). Similar oncological outcomes between these subgroups of patients were further supported by additional retrospective studies (50,51).
Local recurrences after WW are worrisome and have been considered a significant limitation in widespread implementation of such strategy. However, it has been suggested that the majority of local recurrences appears during the first 12 months of follow-up and that the vast majority of these local recurrence (nearly 90%) are usually with an endoluminal component. This means that a strict follow-up and simple clinical assessment will be able to detect the majority of these local recurrences and allow salvage treatment with excellent long-term local disease control (52,53). Patients with more advanced cT stage at baseline staging appear to be at greater risk for local recurrence after initial cCR and should be carefully monitored 11. Ultimately, the pooled local recurrence rate including all published series analyzed in a systematic review suggested to be around 16–22% (50,51).
Systemic recurrences may also develop after non-operative management of patients that achieve a cCR. One series reported 14% systemic recurrence rates after no adjuvant systemic chemotherapy following standard nCRT and cCR managed non-operatively (51). These rates compare favorably with the 11% systemic recurrence rate after radical surgery in patients with pCR with nearly 40% of patients undergoing adjuvant systemic chemotherapy (54).
Perspectives
With the use of nCRT specifically aimed to provide organ-preservation for selected patients (including early stage disease), accurate prediction of tumor response with molecular biology studies will become increasingly relevant. Identification of good responders would allow better selection of candidates for organ-preservation strategies and avoidance of potentially unnecessary treatment to poor responders (13,55). However, the significant inter and intratumoral heterogeneity observed in rectal cancer may have contributed for the lack of clinically useful gene expression signatures in predicting tumor response (12,55,56). Considering this intratumoral heterogeneity within a single rectal cancer, there may be areas of the tumor that are resistant to treatment while others are sensitive to CRT. This means that gene signatures derived from single biopsy specimens may not work simply because these fragments are not representative of the entirety of the tumors they were taken from. Instead of prediction of tumor response, introduction of liquid biopsies for the assessment and monitoring of tumor response may also represent a clinically useful tool for the management and surveillance of patients during this approach (57).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Eduardo Ma Targarona and Andrea Balla) for the series “Rectal Cancer” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2018.05.05). The series “Rectal Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Smith FM, Waldron D, Winter DC. Rectum-conserving surgery in the era of chemoradiotherapy. Br J Surg 2010;97:1752-64. [Crossref] [PubMed]
- Habr-Gama A, Perez RO, Wynn G, et al. Complete Clinical Response After Neoadjuvant Chemoradiation Therapy for Distal Rectal Cancer: Characterization of Clinical and Endoscopic Findings for Standardization. Dis Colon Rectum 2010;53:1692-8. [Crossref] [PubMed]
- Habr-Gama A, Perez RO, Nadalin W, et al. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg 2004;240:711-7; discussion 717-8. [PubMed]
- Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351:1731-40. [Crossref] [PubMed]
- Sauer R, Liersch T, Merkel S, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol 2012;30:1926-33. [Crossref] [PubMed]
- Taylor FG, Quirke P, Heald RJ, et al. Preoperative high-resolution magnetic resonance imaging can identify good prognosis stage I, II, and III rectal cancer best managed by surgery alone: a prospective, multicenter, European study. Ann Surg 2011;253:711-9. [Crossref] [PubMed]
- Peeters KC, van de Velde CJ, Leer JW, et al. Late side effects of short-course preoperative radiotherapy combined with total mesorectal excision for rectal cancer: increased bowel dysfunction in irradiated patients--a Dutch colorectal cancer group study. J Clin Oncol 2005;23:6199-206. [Crossref] [PubMed]
- Loos M, Quentmeier P, Schuster T, et al. Effect of preoperative radio(chemo)therapy on long-term functional outcome in rectal cancer patients: a systematic review and meta-analysis. Ann Surg Oncol 2013;20:1816-28. [Crossref] [PubMed]
- Perez RO, Habr-Gama A, Sao Juliao GP, et al. Predicting complete response to neoadjuvant CRT for distal rectal cancer using sequential PET/CT imaging. Tech Coloproctol 2014;18:699-708. [Crossref] [PubMed]
- Garcia-Aguilar J, Shi Q, Thomas CR, et al. A Phase II trial of neoadjuvant chemoradiation and local excision for t2n0 rectal cancer: preliminary results of the ACOSOG Z6041 trial. Ann Surg Oncol 2012;19:384-91. [Crossref] [PubMed]
- Habr-Gama A, São Julião GP, Gama-Rodrigues J, et al. Baseline T Classification Predicts Early Tumor Regrowth After Nonoperative Management in Distal Rectal Cancer After Extended Neoadjuvant Chemoradiation and Initial Complete Clinical Response. Dis Colon Rectum 2017;60:586-94. [Crossref] [PubMed]
- Bettoni F, Masotti C, Habr-Gama A, et al. Intratumoral Genetic Heterogeneity in Rectal Cancer: Are Single Biopsies representative of the entirety of the tumor? Ann Surg 2017;265:e4-e6. [Crossref] [PubMed]
- Habr-Gama A, Gama-Rodrigues J, Perez RO. Is tailoring treatment of rectal cancer the only true benefit of long-course neoadjuvant chemoradiation? Dis Colon Rectum 2013;56:264-6. [Crossref] [PubMed]
- Radu C, Berglund Å, Påhlman L, et al. Short-course preoperative radiotherapy with delayed surgery in rectal cancer - A retrospective study. Radiother Oncol 2008;87:343-9. [Crossref] [PubMed]
- Appelt AL, Pløen J, Vogelius IR, et al. Radiation dose-response model for locally advanced rectal cancer after preoperative chemoradiation therapy. Int J Radiat Oncol Biol Phys 2013;85:74-80. [Crossref] [PubMed]
- Appelt AL, Pløen J, Harling H, et al. High-dose chemoradiotherapy and watchful waiting for distal rectal cancer: a prospective observational study. Lancet Oncol 2015;16:919-27. [Crossref] [PubMed]
- Vuong T, Devic S. High-dose-rate pre-operative endorectal brachytherapy for patients with rectal cancer. J Contemp Brachytherapy 2015;7:183-8. [Crossref] [PubMed]
- Gerard JP, Benezery K, Doyen J, et al. Aims of combined modality therapy in rectal cancer (M0). Recent Results Cancer Res 2014;203:153-69. [Crossref] [PubMed]
- Hupkens BJP, Martens MH, Stoot JH, et al. Quality of Life in Rectal Cancer Patients After Chemoradiation: Watch-and-Wait Policy Versus Standard Resection - A Matched-Controlled Study. Dis Colon Rectum 2017;60:1032-40. [Crossref] [PubMed]
- Vailati BB, Habr-Gama A, Mattacheo AE, et al. Quality of Life in Patients With Rectal Cancer After Chemoradiation: Watch-and-Wait Policy Versus Standard Resection-Are We Comparing Apples to Oranges? Dis Colon Rectum 2018;61:e21 [Crossref] [PubMed]
- Schrag D, Weiser MR, Goodman KA, et al. Neoadjuvant chemotherapy without routine use of radiation therapy for patients with locally advanced rectal cancer: a pilot trial. J Clin Oncol 2014;32:513-8. [Crossref] [PubMed]
- Habr-Gama A, Perez RO, Sabbaga J, et al. Increasing the Rates of Complete Response to Neoadjuvant Chemoradiotherapy for Distal Rectal Cancer: Results of a Prospective Study Using Additional Chemotherapy During the Resting Period. Dis Colon Rectum 2009;52:1927-34. [Crossref] [PubMed]
- Habr-Gama A, Sabbaga J, Gama-Rodrigues J, et al. Watch and Wait Approach Following Extended Neoadjuvant Chemoradiation for Distal Rectal Cancer. Dis Colon Rectum 2013;56:1109-17. [Crossref] [PubMed]
- Fornaro L, Caparello C, Vivaldi C, et al. Bevacizumab in the pre-operative treatment of locally advanced rectal cancer: a systematic review. World J Gastroenterol 2014;20:6081-91. [Crossref] [PubMed]
- Borg C, André T, Mantion G, et al. Pathological response and safety of two neoadjuvant strategies with bevacizumab in MRI-defined locally advanced T3 resectable rectal cancer: a randomized, noncomparative phase II study. Ann Oncol 2014;25:2205-10. [Crossref] [PubMed]
- Dellas K, Buller J, Görtz GJ, et al. Analysis of Bevacizumab-based Preoperative Radiochemotherapy in Patients with Locally Advanced Rectal Cancer on Surgeryassociated Spectrum of Complications. Ann Surg Oncol 2014;21:1352-60. [Crossref] [PubMed]
- Patel UB, Brown G, Rutten H, et al. Comparison of magnetic resonance imaging and histopathological response to chemoradiotherapy in locally advanced rectal cancer. Ann Surg Oncol 2012;19:2842-52. [Crossref] [PubMed]
- Francois Y, Nemoz CJ, Baulieux J, et al. Influence of the interval between preoperative radiation therapy and surgery on downstaging and on the rate of sphinctersparing surgery for rectal cancer: the Lyon R90-01 randomized trial. J Clin Oncol 1999;17:2396. [Crossref] [PubMed]
- Kalady MF, de Campos-Lobato LF, Stocchi L, et al. Predictive Factors of Pathologic Complete Response After Neoadjuvant Chemoradiation for Rectal Cancer. Ann Surg 2009;250:582-9. [PubMed]
- Garcia-Aguilar J, Smith DD, Avila K, et al. Optimal timing of surgery after chemoradiation for advanced rectal cancer: preliminary results of a multicenter, nonrandomized phase II prospective trial. Ann Surg 2011;254:97-102. [Crossref] [PubMed]
- Garcia-Aguilar J, Chow OS, Smith DD, et al. Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: a multicentre, phase 2 trial. Lancet Oncol 2015;16:957-66. [Crossref] [PubMed]
- Rullier E, Rouanet P, Tuech JJ, et al. Organ preservation for rectal cancer (GRECCAR 2): a prospective, randomised, open-label, multicentre, phase 3 trial. Lancet 2017;390:469-79. [Crossref] [PubMed]
- Perez RO, Habr-Gama A, São Julião GP, et al. Optimal timing for assessment of tumor response to neoadjuvant chemoradiation in patients with rectal cancer: do all patients benefit from waiting longer than 6 weeks? Int J Radiat Oncol Biol Phys 2012;84:1159-65. [Crossref] [PubMed]
- Perez RO, Habr-Gama A, Pereira GV, et al. Role of biopsies in patients with residual rectal cancer following neoadjuvant chemoradiation after downsizing: can they rule out persisting cancer? Colorectal Dis 2012;14:714-20. [Crossref] [PubMed]
- Duldulao MP, Lee W, Streja L, et al. Distribution of Residual Cancer Cells in the Bowel Wall After Neoadjuvant Chemoradiation in Patients With Rectal Cancer. Dis Colon Rectum 2013;56:142-9. [Crossref] [PubMed]
- Lambregts DM, Maas M, Bakers FC, et al. Long-term follow-up features on rectal MRI during a wait-and-see approach after a clinical complete response in patients with rectal cancer treated with chemoradiotherapy. Dis Colon Rectum 2011;54:1521-8. [Crossref] [PubMed]
- Patel UB, Taylor F, Blomqvist L, et al. Magnetic Resonance Imaging-Detected Tumor Response for Locally Advanced Rectal Cancer Predicts Survival Outcomes: MERCURY Experience. J Clin Oncol 2011;29:3753-60. [Crossref] [PubMed]
- Lambregts DMJ, van Heeswijk MM, Delli Pizzi A, et al. Diffusion-weighted MRI to assess response to chemoradiotherapy in rectal cancer: main interpretation pitfalls and their use for teaching. Eur Radiol 2017;27:4445-54. [Crossref] [PubMed]
- Curvo-Semedo L, Lambregts DM, Maas M, et al. Rectal Cancer: Assessment of Complete Response to Preoperative Combined Radiation Therapy with Chemotherapy— Conventional MR Volumetry versus Diffusion-weighted MR Imaging. Radiology 2011;260:734-43. [Crossref] [PubMed]
- Anjos Dos DA, Perez RO, Habr-Gama A, et al. Semiquantitative Volumetry by Sequential PET/CT May Improve Prediction of Complete Response to Neoadjuvant Chemoradiation in Patients With Distal Rectal Cancer. Dis Colon Rectum 2016;59:805-12. [Crossref] [PubMed]
- Maas M, Lambregts DM, Nelemans PJ, et al. Assessment of Clinical Complete Response After Chemoradiation for Rectal Cancer with Digital Rectal Examination, Endoscopy, and MRI: Selection for Organ-Saving Treatment. Ann Surg Oncol 2015;22:3873-80. [Crossref] [PubMed]
- Smith FM, Ahad A, Perez RO, et al. Local Excision Techniques for Rectal Cancer After Neoadjuvant Chemoradiotherapy. Dis Colon Rectum 2017;60:228-39. [Crossref] [PubMed]
- Perez RO, Habr-Gama A, São Julião GP, et al. Transanal endoscopic microsurgery for residual rectal cancer after neoadjuvant chemoradiation therapy is associated with significant immediate pain and hospital readmission rates. Dis Colon Rectum 2011;54:545-51. [Crossref] [PubMed]
- São Julião GP, Ortega CD, Vailati BB, et al. Magnetic resonance imaging following neoadjuvant chemoradiation and transanal endoscopic microsurgery for rectal cancer. Colorectal Dis 2017;19:O196-O203. [Crossref] [PubMed]
- Habr-Gama A, Lynn PB, Jorge JMN, et al. Impact of Organ-Preserving Strategies on Anorectal Function in Patients with Distal Rectal Cancer Following Neoadjuvant Chemoradiation. Dis Colon Rectum 2016;59:264-9. [Crossref] [PubMed]
- Bujko K, Richter P, Smith FM, et al. Preoperative radiotherapy and local excision of rectal cancer with immediate radical re-operation for poor responders: a prospective multicentre study. Radiother Oncol 2013;106:198-205. [Crossref] [PubMed]
- Morino M, Allaix ME, Arolfo S, et al. Previous transanal endoscopic microsurgery for rectal cancer represents a risk factor for an increased abdominoperineal resection rate. Surg Endosc 2013;27:3315-21. [Crossref] [PubMed]
- Hompes R, McDonald R, Buskens C, et al. Completion surgery following transanal endoscopic microsurgery: assessment of quality and short- and long-term outcome. Colorectal Dis 2013;15:e576-81. [Crossref] [PubMed]
- Habr-Gama A, São Julião GP, Perez RO. Nonoperative Management of Rectal Cancer: Identifying the Ideal Patients. Hematol Oncol Clin North Am 2015;29:135-51. [Crossref] [PubMed]
- Dossa F, Chesney TR, Acuna SA, et al. A watch-and-wait approach for locally advanced rectal cancer after a clinical complete response following neoadjuvant chemoradiation: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2017;2:501-13. [Crossref] [PubMed]
- Dattani M, Heald RJ, Goussous G, et al. Oncological and Survival Outcomes in Watch and Wait Patients With a Clinical Complete Response After Neoadjuvant Chemoradiotherapy for Rectal Cancer: A Systematic Review and Pooled Analysis. Ann Surg 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Habr-Gama A, Gama-Rodrigues J, et al. Local recurrence after complete clinical response and watch and wait in rectal cancer after neoadjuvant chemoradiation: impact of salvage therapy on local disease control. Int J Radiat Oncol Biol Phys 2014;88:822-8. [Crossref] [PubMed]
- Kong JC, Guerra GR, Warrier SK, et al. Outcome and Salvage Surgery Following "Watch and Wait" for Rectal Cancer after Neoadjuvant Therapy: A Systematic Review. Dis Colon Rectum 2017;60:335-45. [PubMed]
- Maas M, Nelemans PJ, Valentini V, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol 2010;11:835-44. [Crossref] [PubMed]
- Perez RO, Habr-Gama A, São Julião GP, et al. Should We Give Up The Search for a Clinically Useful Gene Signature for the Prediction of Response of Rectal Cancer to Neoadjuvant Chemoradiation? Dis Colon Rectum 2016;59:895-7. [Crossref] [PubMed]
- Lopes-Ramos C, Koyama FC, Habr-Gama A, et al. Comprehensive evaluation of the effectiveness of gene expression signatures to predict complete response to neoadjuvant chemoradiotherapy and guide surgical intervention in rectal cancer. Cancer Genet 2015;208:319-26. [Crossref] [PubMed]
- Carpinetti P, Donnard E, Bettoni F, et al. The use of personalized biomarkers and liquid biopsies to monitor treatment response and disease recurrence in locally advanced rectal cancer after neoadjuvant chemoradiation. Oncotarget 2015;6:38360-71. [Crossref] [PubMed]
Cite this article as: Habr-Gama A, Fernandez LM, São Julião GP, Vailati BB, Perez RO. Alternative treatment to surgery for rectal cancer. Ann Laparosc Endosc Surg 2018;3:50.


