Laparoscopic bariatric surgery-current trends and controversies
Introduction
The first laparoscopic procedures for obesity were performed in the early 1990s. Broadbent and colleagues first successfully placed a non-adjustable gastric band in a patient on September 10, 1992 in Australia, and published their preliminary results in 1993 (1). After initial animal experiments in 1992, Belachew and colleagues placed the first laparoscopic adjustable band in a human on September 1, 1993 in Belgium (2).
The first laparoscopic vertical banded gastroplasty (VBG) was performed by Hess and Hess on July 29, 1993 in Bowling Green, Ohio (3). However, only 2 laparoscopic VBGs were performed before changing to the duodenal switch (DS) procedure they were developing. Just 3 months later, Wittgrove and Clark performed the first laparoscopic Roux-en-Y gastric bypass (RYGB) procedure in October, 1993, and published their results on 500 patients in 2000 (4). Gagner performed the first laparoscopic DS procedure in 1999 (5).
Johnston and colleagues from Leeds, United Kingdom, developed a simpler “Magenstrasse and Mill” (M & M) procedure that would avoid the use of implanted foreign material such as bands and reservoirs and overcome the disappointing results of the VBG procedure and the morbidity of the gastric bypass (6). At first, a 40-French bougie was used, but because of unsatisfactory weight loss, the size was reduced gradually, and they found that a 32-French bougie resulted in a 63% excess weight loss at 3 years. In 1999, McMahon performed the M & M operation laparoscopically in Leeds, and in 2000 he performed the first laparoscopic sleeve gastrectomy (SG) in UK while Gagner performed it simultaneously in US (5,7). Modifications were made to the original procedure in subsequent years to simplify the technique, improve weight loss maintenance, and to facilitate the evolution of laparoscopic SG (8).
A worldwide survey on bariatric surgery published in 2015 showed that 468,609 bariatric procedures were performed worldwide in 2013, of which 95.7% were carried out laparoscopically. The most commonly performed procedure in the world was RYGB, 45%; followed by SG, 37%; and adjustable gastric banding (AGB), 10%. Most significant was the rise in popularity of SG from 0 to 37% of the world total from 2003 to 2013, and the decline in AGB of 68% during its peak in 2008 to 10% in 2013 (9). The proportion of SG increased from 3% to 54% between 2008 to 2014, while RYGB decreased from 52% in 2008 to 32% by 2014 (10). Older bariatric surgical procedures, including jejunocolic bypass, jejunoileal bypass, vertical banded gastroplasty, and biliopancreatic diversion, are no longer performed. Biliopancreatic diversion with DS is still performed in select centers in much smaller number (<1.5% of all bariatric procedure) (9).
Over the years many newer laparoscopic bariatric and metabolic procedures have been developed and modified like greater curvature plication (GCP), mini gastric bypass (MGB), duodenojejunal bypass (DJB), ileal interposition (II), transit bipartition (TB), and jejunoileal anastomosis (JIA).
Patient selection
The WHO classifies obesity, as having BMI ≥30 kg/m2 (overweight if BMI ≥25 kg/m2). Caucasians are known to have much lower body fat percentage, waist circumference (WC) and waist-hip ratio (WHR) as compared to Asians, where central obesity is highly prevalent leading to metabolic syndrome even at normal levels of BMI (11). Bariatric surgeries have been standardized worldwide for many years through an influential National Institutes of Health (NIH) consensus statement (12), which has been revalidated in many studies. From the beginning the emphasis has been on BMI as a selection criteria for surgery, whereas worldwide debate suggests that WC, WHR, comorbidities, quality of life indicator especially functional restriction should be important considerations along with BMI (13,14) (Table 1,2).
Table 1
| Eligibility criteria | Age | BMI only | BMI with comorbidities | Additional points |
|---|---|---|---|---|
| NIH guidelines (12) | NA | ≥40 | ≥35 | – |
| IFSO guidelines (14) | ≥18 or ≤60 years | ≥40 | BMI ≥35 with comorbidities; BMI 30–34.9 with recent onset T2DM | Previous non-surgical failed weight loss attempts; patients are motivated and are free of significant psychological disease |
| Asia-Pacific region (13) (ACMOMS) | ≥18 or ≤65 years | ≥35 | BMI ≥32 with comorbidities; BMI ≥30 with central obesity WC ≥80 cm (females) WS ≥90 cm (males) with at least two comorbidities; BMI ≥27.5 kg/m2 with inadequately controlled DM (HbA1c ≥7) | Proven failure of nutritional and behavioral therapy; motivated and able to provide a valid consent, are willing to undergo periodic inspections and follow an established dietary regime; absence of major contraindications (very high operative risk, limited life expectancy due to illness, severe cirrhosis, alcohol abuse, major psychiatric illness); long standing obesity ≥5 years |
| IFSO APC | ≥18 or ≤65 years | ≥35 | BMI ≥30 with comorbidities; BMI ≥27.5 kg/m2 with inadequately controlled DM (HbA1c ≥7) | Failed weight loss attempts; no major psychiatric illness; motivated |
| International Diabetes Organizations | ≥18 or ≤60 years | ≥40 and Asians ≥37.5 | BMI ≥35 (≥32.5 for Asians) with comorbidities; BMI ≥30 (≥27.5 for Asians) kg/m2 with inadequately controlled DM (HbA1c ≥7) | – |
NIH, National Institute of Health; IFSO, International Federation for Surgery of Obesity and Metabolic Disorder; ACMOMS, Asian Consensus Meeting on Metabolic Surgery; WC, Waist circumference.
Table 2
| Hypertension |
| Ischemic heart disease |
| Type 2 diabetes |
| Obstructive sleep apnea syndrome |
| Obesity syndrome/hypoventilation (Pickwickian syndrome) |
| Non-alcoholic fatty liver disease and steatohepatitis |
| Dyslipidemia |
| Gastroesophageal reflux disease |
| Asthma |
| Venous stasis disease |
| Severe urinary incontinence |
| Disabling arthropathy |
| Severely reduced quality of life |
Preoperative evaluation
Patients who are considered for bariatric surgery benefit most from individualized choice of procedure through proper evaluation of medical history and all comorbidities (Table 2); it is important to detect occult pathology through established diagnostic tests (Table 3). Various scoring tools can be used to assess the surgical risk, establish the severity of obesity and predict surgical outcome (15-17) (Table 4).
Table 3
| Investigations | All patients | Selected patients |
|---|---|---|
| Blood | Complete blood picture & blood group; | Anti-GAD for young diabetics (Glutamic acid decarboxylase) antibodies; |
| Kidney function tests (BUN, creatinine, electrolytes); | Islet cell antibodies (ICA); | |
| Liver function tests, lipid profile; | C-peptide-fasting and stimulated | |
| Thyroid function tests; | ||
| Fasting and postprandial blood sugars; | ||
| Fasting serum insulin; | ||
| Serum calcium, vitamin D3 and B12; | ||
| Sr. iron, transferrin saturation, Sr. ferritin, total iron body concentration; | ||
| Clotting screen (PT, APTT, INR); | ||
| Fasting serum cortisol at 8 am; | ||
| Viral markers | ||
| Cardiac | Blood pressure; | – |
| ECG; | ||
| 2-D echo; | ||
| Dobutamine stress test | ||
| Respiratory | Oxygen saturation | Pulmonary function test; |
| Arterial blood gas; | ||
| Polysomnography | ||
| Imaging | Chest X-ray; | Computed tomography; |
| Ultrasonography abdomen; | Doppler for Carotid arteries | |
| Doppler for leg veins | ||
| Endoscopy | Upper GI endoscopy with Rapid urease test (ASMBS does not recommend routine endoscopy but ASGE recommends it in all patients); | – |
| Geographical areas with high incidence of carcinoma stomach; | ||
| Past history of Acid peptic disease; | ||
| High suspicion of GERD or Hiatus hernia |
Table 4
| Scoring tools | Description |
|---|---|
| Assessment of obesity related co-morbidities scale (AORC) (15) | Score 0–5, depending on comorbidities |
| Edmonton obesity scoring system (EOSS) (16) | Classifies obesity in five stages 0–4, considering physical symptoms, psychological symptoms, and limitation of functional aspects. Shown to be more accurate predictor of total mortality than BMI levels |
| Obesity surgery mortality risk score (OS-MRS) (17) | Uses five variables (BMI >50 kg/m2; male gender; hypertension; history or risk of DVT; age >45 years); the presence of ≥4 risk factors has mortality risk of 7.5%, whereas score of 0–1 and 2–3 has mortality risk of 0.31% and 1.9% respectively |
Operating room setup
A steep reverse Trendelenburg position of operating table is adopted with patient lying supine. Some surgeons prefer split leg (French) position to stand between the legs while operating, whereas others prefer to stand on patient’s right side. Prophylaxis against deep vein thrombosis necessitates usage of graduated compression stockings for the lower limb along with a sequential compression device.
Postoperative care
Patients are kept nil by mouth under supervision of critical care specialist and motivated for early ambulation, usually 6–8 hours after surgery. Meanwhile compression stockings and low molecular heparin are continued along with spirometry and chest physiotherapy. On first postoperative day patients are allowed clear sips of liquid once every 10–15 min. Patients are discharged on 2nd or 3rd postoperative day with oral dispersible medicines depending on general condition, hydration, and on drain status.
SG
Introduction
Recently SG has gained worldwide popularity over the gold standard operation, the RYGB due to safety and lesser long term nutritional issues. Barrett’s esophagus is the only absolute contraindication for SG. There is enough long term data to suggest SG is equivalent to RYGB in terms of weight loss and diabetes remission; while some studies show a significant difference in diabetes remission in favor of bypass surgeries (18-20).
Indications
- Morbidly obese patients satisfying the criteria for bariatric surgery;
- First stage or standalone procedure for super obese;
- High risk patient where duration of procedure affects morbidity and mortality;
- Potential/future organ transplant candidates.
Contraindications
- Barrett’s esophagus (Relative contraindication);
- Malignancy;
- Liver cirrhosis with portal hypertension;
- Alcohol abuse.
Surgical anatomy
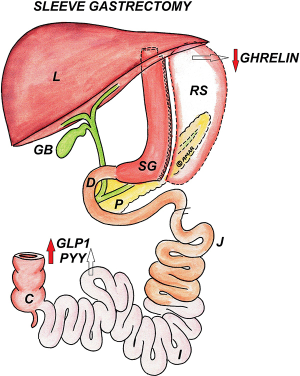
After inserting 32 to 38 French gastric calibration tube (GCT) up to gastroesophageal (GE) junction, a window is created on greater curvature using any energy source like Ligasure or Harmonic just diagonally opposite to “Crow’s foot” at the avascular plane close to stomach wall, so as to enter the lesser sac. This dissection is carried out cranially up to GE junction exposing left crus and caudally up to pylorus. It is very important to free posterior gastric wall so as to avoid twisting or “cork-screw” after SG. The stapler is placed slightly away from bougie maintaining at least 2 cm from incisura edge so as to avoid stricture. The sleeve is created by sequential firing; last fire should be at least 1–2 cm away from GE junction with no left over fundus (Figure 1).
Surgical outcome
Weight loss
The percentage excess weight loss (% EWL) in laparoscopic sleeve gastrectomy (LSG) after first year can vary from 54% to 78% (21,22). This variation in first year outcomes may be due to variations in surgical technique, different bougie sizes, ethnic and/or dietary variations. One study declared 86% EWL at 5 years having a follow up of 90% (Gastroscope used as bougie and first stapler fired at 3 cm from pylorus) (23). There are multiple reports showing >50% EWL at and beyond 5 years (9,24), whereas a recent review reported 62.3%, 53.8%, 43%, and 54.8% EWL at 5, 6, 7 and 8 years respectively (24).
Diabetes resolution/remission
The factors influencing diabetes remission/resolution after bariatric surgery are multiple, like duration of diabetes, elevated glycated hemoglobin (HbA1c), insulin treatment, older age, and poor pancreatic function (Low C-peptide) (25). In a systematic review of 673 patients having a mean follow-up of 13.1 months (range, 3–36 months), 66.2% had remission, 26.9% had improvement, while 13.1% showed no change. Reduction in blood glucose was 88.2 mg/dL while HbA1c decreased by 1.7% (26). In another systematic review (n=402) on long term outcomes after SG in T2DM, 60.8% patients had remission at 5 years with a significant decrease in T2DM prevalence (20.5%). Mean plasma glucose decreased from 170.3 to 112.0 mg/dL and HbA1c from 8.3% to 6.7% at 5 years (27).
Controversial issues
Bougie size
Varying rates of success and long-term weight control seem to be influenced by differing bougie sizes. The 4th international consensus summit on SG recommended 36 French bougie (28), whereas another panel expert concluded that 32–36 French is optimal (29). However, in a large meta-analysis, greater weight loss was seen with a bougie size of less than 40 French during the first six months though the difference in weight loss was not significant at the end of 36 months. Another important outcome of this study showed a leak rate of 2.5%, 1.7% and 0.9% with bougie size of <40 French, 40–49 French and >50 French respectively (30).
Distance from pylorus
There was lack of consensus in the international expert panel regarding distance from pylorus to begin sleeve resection (32% voted for 4–5 cm, 27% for 3–4 cm and 23% for 5–6 cm) (29). There is no strong data to decide optimal antral resection, where some believe that a distance greater than 4 cm from pylorus preserves the antral pump and improves gastric emptying with reduced intraluminal pressure. However, authors recommend SG resection at 3 to 4 cm from the pylorus.
Reinforcement
Chances of bleeding and leaks are expected to decrease with staple line reinforcement. The suggested methods of reinforcement are oversewing, buttressing material, omental flap, and glue along staple line. Publications from some authors report debatable results, with reinforcement leading to higher leak rate (0.96% vs. 0.65%) with expected reduction in rate of bleeding (0.75% vs. 1.00%) (31). A comparison of various methods of reinforcement indicates a clear superiority of absorbable polymer membrane for buttressing, leading to decrease leak rates in comparison to oversewing, bioabsorbable peristrip reinforcement, or no reinforcement (32).
Complications
These are summarized in Table 5 (33-36).
Table 5
| Complication | LSG (33-36) | RYGB (36-40) | LAGB (36,41-43) | BPD (44) /BPD-DS (36,45) | MGB (46-48) | SADI-S/LDJB-SG (49) | II-SG (50,51) | SG-TB/SG-LB (52,53) | LGP & LAGBP (54,55) |
|---|---|---|---|---|---|---|---|---|---|
| Overall mortality rate | 0.11–0.36% | 0.04–0.16% | 0–1.4% | 0.08–0.2% | 0.2% | 0.2% | 0 | ||
| Overall complication rate | 7.75% | 6–12% | LGP:8% | ||||||
| VTE/PE | 0.14% | 0–1.3% | 0.3% | 2% | |||||
| Other complications | Abscess: 0.7% | Internal hernia/obstruction: 5% | Erosion: 1–2%; port related: 6% | Nutrient deficiency: 33%; obstruction: 0.3%-2.4% | Bile reflux:1-2% | Reversal of procedure:2% | Gastric fold herniation: 2.2% | ||
| Port-site hernia | 0.14–1% | 0.2% | 1% | ||||||
| Hemorrhage | 1–6% | 1-2% | 0.3% | 1.9% | 2% | 1.4% | |||
| Leak | Up to 5% | 1-2% | 1–3.5% | 0.6–1.6% | Total: 1.9% (SG: 1.1%, DS: 0.6%, DEA: 0.2%) | 2% from biliary limb | 1.4% | ||
| Marginal ulcers and GG fistula | 4% | 1.4% | 1–6% | 2% | |||||
| Cholelithiasis | 28%#, 12%* | 30–52.8%#, 7–16%* | 26.8%#, 6.8%* | 4-10% | 9.3% | ||||
| Stricture/stenosis | 0.26–4% | GJ:5% | GI:2% | ||||||
| Malabsorption | 2.5–6.6% |
0.5–1% | |||||||
| QOL issues | WLF/regain: 50% | Steatorrhea: up to 1% | Diarrhea: |
Diarrhea: 4% | Vomiting: 2.4% | ||||
| Vitamin B-12 | 3% | 6.5% | 33% | ||||||
| Vitamin D3 | 23% | 45.8% | |||||||
| Folate | 3% | 25% | 25.9% | ||||||
| Iron | 3% | Up to 15% | |||||||
| Ferritin | 14% | 23% | 23% | ||||||
| Zinc | |||||||||
| At 2 years | 18–34% | 20–35% | 74–91% | ||||||
| At 5 years | 12.5% | 21–33% | 45% | ||||||
| Copper | 9.6-18.8% | 50.6% |
#, overall, *, symptomatic. PCM, protein calorie malnutrition; DS, duodenal stump; DEA, duodeno-enteral anastomosis; WLF, weight loss failure. LSG, laparoscopic sleeve gastrectomy; RYGB, Roux-en-Y gastric bypass; LAGB, Laparoscopic Adjustable Gastric Banding; BPD, Biliopancreatic Diversion; BPD-DS, Biliopancreatic Diversion with duodenal switch; MGB, mini gastric bypass; SADI-S, Single Anastomosis Duodeno-Ileal Bypass with sleeve; LDJB-SG, Loop Duodenojejunal Bypass with Sleeve Gastrectomy; IT-SG, Ileal Transposition with Sleeve Gastrectomy; SG-TB, sleeve gastrectomy with transit bipartition; SG-LB, sleeve gastrectomy with loop bipartition; LGP, laparoscopic gastric plication; LAGBP, laparoscopic adjustable gastric band plication.
RYGB
Introduction
RYGB has been considered the “gold standard” in bariatric surgery. In 2013, the most commonly performed bariatric procedure worldwide was RYGB (9). Creation of a small gastric pouch along with bypass of duodenum and proximal jejunum, resulting in a combination of restriction and malabsorption.
Indications
- Morbidly obese patients satisfying the criteria for bariatric surgery;
- First stage or standalone procedure for super obese;
- High risk patient where duration of procedure affects morbidity and mortality;
- Potential/future organ transplant candidates.
Contraindications
- Barrett’s esophagus (Relative contraindication);
- Malignancy;
- Liver cirrhosis with portal hypertension;
- Alcohol abuse.
Surgical anatomy
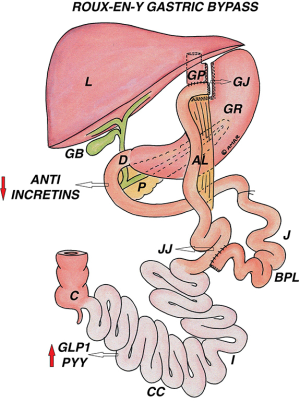
A gastric pouch of 20–30 mL is created by horizontal firing of 45 mm blue cartridge followed by vertical firing of blue 60 or 45 mm; dissection at Angle of His facilitates the final stapler firing to separate the gastric pouch from remnant stomach. It is very important to make sure the GCT is withdrawn before each firing. Keeping an alimentary limb of 75–150 cm and biliopancreatic limb of 50–150 cm, the gastrojejunal anastomosis performed either completely hand sewn or in combination with a linear or circular stapler. It is important to close the Petersen’s space and the mesenteric gap to prevent internal herniation (Figure 2).
Surgical outcome
Weight loss
Most patients have significant weight loss in the early phase, which is sustained with further weight reduction in long term. In 2004, Buchwald et al. in their meta-analysis reported 61.6% (56.7–66.5%) EWL after RYGB (56), while the longest (up to 20 years) matched prospective follow-up data from Swedish Obese Subjects (SOS) study group showed mean percentage weight change of 32%, 25% and 27% at 1, 10 and 20 years, respectively from the baseline in patients of RYGB (57). The short-term outcomes showed 83% EWL at 24 and 77% EWL at 30 months. After 1 year, significance improvement in quality of life (QOL) parameters was reported in 95% of patients (58).
Diabetes resolution/remission
Published data showed RYGB was the most effective procedure after DS in terms of diabetes remission (80% vs. 95%) (56,59). A 3 year comparative study (STAMPEDE Trial) in poorly controlled diabetics concluded that HbA1c <6% was achieved in 5%, 37%, and 24.5% of patients in medical treatment, RYGB, and LSG group respectively (60). More recently the 5-year data of the same study showed diabetic remission rates of 29% with gastric bypass, 23% with SG and 5% with intensive medical therapy alone (61). Diabetes re-emergence has been reported in 24–50% of the patients undergoing RYGB; contributing factors could be tapering off of hormonal effect in foregut and or hindgut, receptor down regulation and persistent stimulation of beta cells by gut incretins, leading to their exhaustion (62).
Controversial issues
Pouch and Gastrojejunostomy (GJ) size
Pouch and GJ size plays a very important role in RYGB, if pouch is really small 15–20 mL and GJ is more than 2.5 cm, then gastric emptying is faster and there is hardly any restrictive element leading to dumping syndrome. On the contrary if pouch size is much bigger and GJ is less than 2 cm then it can lead to severe retrosternal discomfort, reflux, marginal ulcer and GJ narrowing. A recent study found correlation between weight loss maintenance with GJ size but not with pouch size (63). Unfortunately, routine contrast material used to study gastric pouch volume leads to rapid transit with inadequate distension and filling. However, authors recommend a pouch size of 30 mL and GJ diameter of 2.5 cm.
Limb length
The small bowel is elastic and distensible, thus accurate measurement is a practical difficulty. Most studies found no significant difference when the alimentary and biliopancreatic limb lengths (100±50 cm) were correlated to weight loss or nutritional deficiencies, except in super obese (BMI >50) (64).
Closure of potential hernia space
Most authors recommend and agree on closure of mesenteric and Petersen’s defect with non-absorbable sutures to prevent internal hernia, but conclusive evidence is still lacking (37).
Complications
These are summarized in Table 5 (36-40).
Laparoscopic Adjustable Gastric Banding (LAGB)
Introduction
Worldwide trends showed that 24% of patients had banding in 2003, increasing to 42% in 2008, which dropped to 18% by 2011 and finally 10% by 2013 (9). Reasons for the decline could be due to suboptimal weight loss and comorbidity resolution, increasing long term complications and usage of a more effective procedure like SG. Many high volume centers have reported that they are “removing many more bands than they have placed”.
Indications
As previously mentioned.
Contraindications
(I) GE reflux disease;
(II) Hiatal hernia.
Surgical anatomy
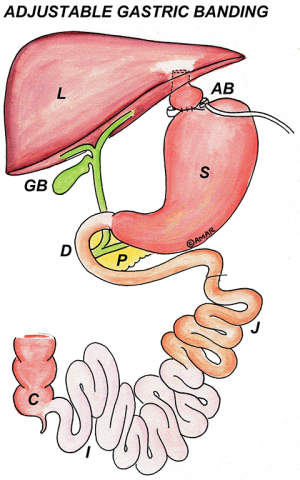
The pars flaccida technique of band placement had been standardized and universally accepted. The band is ideally placed from 2 to 8 o’clock position in an oblique position leaving a very small gastric pouch above it (Figure 3).
Surgical outcome
Weight loss
Buchwald et al. reported 47.5% EWL in their systematic review (56). In another review, percentage excess BMI loss (%EBMIL) at 24 and 36 months was 43.7% and 58.9% respectively (65). LAGB resulted in less weight loss for the first 2 years and although an Australian group has shown equivalent medium term (3–10 years) excess weight loss (47%) compared to RYGB (66), most studies have shown that RYGB results in better weight loss as compared to the band (67).
Diabetes resolution/remission
Buchwald et al. reported 47.5% resolution with LAGB, 71.6% with SG, 83.7% with RYGB, and 98.9% with Biliopancreatic Diversion (BPD) (56). Another study of 122 patients who underwent banding, 93.1% had improved fasting glucose level and 75.4% had reduction in HbA1c at the end of 1 year (65). However, long term results with sustained effects are not reported in literature.
Controversial issues
Surgical technique
The “perigastric” and the “pars-flaccida” techniques have been described in literature. Both techniques are equally effective for weight loss but former is associated with higher slippage and erosion rates (68).
Hiatal hernia repair
A retrospective two year study reported a 1.7% reoperation rate where banding was performed with hiatal hernia repair as compared to 5.6% in standalone banding group (69). Increased pressure of the band on the upper stomach and phrenoesophageal ligament is likely to cause hiatus hernia by itself.
Fixation of band
While most surgeons advocate a suture fixation of the band anteriorly to prevent slippage, some disagree, suggesting that suturing has no advantage and increases operative time (70).
Complications
These are summarized in Table 5 (36,41-43).
BPD and Biliopancreatic Diversion with Duodenal Switch (BPD-DS)
Introduction
BPD gives the best results of weight loss, remission of T2DM and other comorbidities; but it has lost popularity due to nutritional issues following extensive bowel exclusion, causing malabsorption in the longer term. BPD-DS offers comparable results with good quality of life and lesser nutritional issues; however it requires a meticulous technique and lifelong follow-up at regular intervals to take care of impending nutritional problems.
Indications
- BMI >50 kg/m2;
- As a revision surgery for weight regain after other bariatric procedures.
Contraindication
Inflammatory bowel disease.
Surgical anatomy
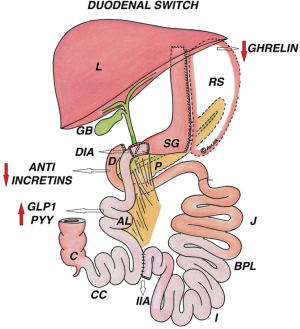
The BPD as described by Scopinaro consists of a large gastric pouch (300–500 mL) with an antecolic alimentary roux limb of 200–250 cm for the gastroileal anastomosis and a common limb of 50 cm. The original BPD has undergone several modifications; the extensive small intestine bypass is retained whereas the partial gastrectomy with gastroileal anastomosis is replaced by SG and a duodenoileal anastomosis, the duodenum being transected a few centimeters beyond the pylorus (Figure 4).
Surgical outcomes
Weight loss
Excellent weight loss was seen in all patients (n=360), who were super obese with a BMI >50 kg/m2, at 5 years with >80% reaching a BMI<35 kg/m2 (44). BPD-DS resulted in 75% EWL as compared to 54% with RYGB at 12 months in super obese patients, as reported by Sovik et al. in their randomized controlled trial (RCT) (71). Sustained weight loss (90% EWL) even up to 5 years is observed due to the stronger incretin response and continued malabsorptive effect of DS (72).
Diabetes resolution/remission
This is more likely to occur with BPD and BPD-DS as compared to any other bariatric procedure. Published literature reports more than 90% of T2DM resolution (72). In a group matched study where patients had mean BMI of 50 kg/m2 diabetic remission was significantly better after BPD-DS as compare to RYGB (82% vs. 64%) (73).
Controversial issues
Operative mortality and morbidity
BPD and BPD-DS are judged as high risk procedures; these procedures were initially advocated for patients with BMI ≥60 kg/m2, which by itself was an independent risk factor for high perioperative mortality (7.2%) (74). However, reported mortality in BPD-DS for BMI ≥60 kg/m2 is 0% as reported by Fazylov et al. (75).
Long term nutritional issues
Protein calorie malnutrition is observed mostly during the first year with an incidence of 3–5% gradually decreasing to 1–3.7% by the 2nd year (72). There is a need to supplement high dosage of vitamins A, D, E, and K (fat soluble) with iron and vitamin-B complex.
Complications
These are summarized in Table 5 (36,44,45).
MGB
Introduction
MGB [also referred to as omega-loop gastric bypass and one anastomosis gastric bypass (OAGB)] has been a hot topic since its inception due to controversial issues like bile reflux and risk of GE cancer. However, surgeons advocating this surgery strongly disagree regarding risk of cancer supported by the long surgical history of Billroth–II procedures, without such evidence.
Indications
All the previously mentioned indications.
Contraindications
- Where gastric surveillance is mandatory;
- Hepatic dysfunction.
Surgical anatomy
MGB consists of a long gastric tube along the lesser curve, starting beyond the Crow’s foot with a wide antecolic gastrojejunostomy, with a biliopancreatic limb (BPL) between 150–250 cm distal to ligament of Treitz’ (Figure 5). The authors recommend 150–200 cm of BPL to avoid excessive malnutrition.
Surgical outcomes
Weight loss
%EWL after 1 year is reported to be from 55% to 91%, maintained at 85% over 6 years (46,76).
Diabetes resolution/remission
Diabetes remission was observed in 83% to 93% of patients. However long term results are still awaited (46,77).
Controversial issues
- Bile reflux;
- Risk of GE junction cancer;
- No standardization regarding Petersen’s defect closure; very few instances of internal hernias have been reported.
Complications
These are summarized in Table 5 (46-48).
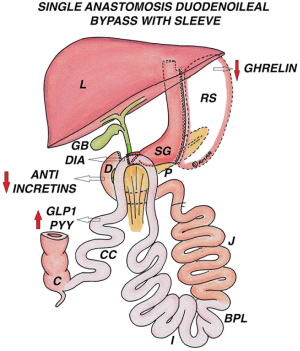
Single Anastomosis Duodeno-Ileal Bypass with Sleeve (SADI-S)
Introduction
This was originally described by Sanchez-Pernaute et al.; it is a loop modification of DS (78). Although theoretical results have been predicted, it has to be tested against mature bariatric operations.
Surgical anatomy
SG is performed using a 54-Fr bougie. first part of duodenum is transected 3 to 4 cm distal to pylorus. Terminal ileum is measured till 200 to 250 or even 300 cm from ileocecal junction and ileal loop is anastomosed to the divided first part of duodenum in an antecolic, end to side fashion (Figure 6).
Advantages
Endoscopic access to the sleeved stomach is maintained without any gastric remnant. Other advantages are shorter operating time, only one anastomosis and no mesenteric openings. Risk of marginal ulcers is minimal since the anastomosis is to the duodenum, rather than to stomach. Since undigested food directly enters terminal ileum, hind gut hormonal changes are comparable to those in DS.
Disadvantages
Endoscopic access to the biliary tract is lost because first part of duodenum is divided.
Surgical outcomes
Weight loss
Sanchez-Pernaute et al. reported 95% EWL in 19 patients at the end of 3 years (79). In 2015 they reported that 25 patients maintained 63% EWL at 5 years; although 6 out of the 97 obese diabetics (6.1%) failed to achieve even a 50% EWL (80). When SADI was performed as a second stage procedure after SG by the same group, they reported 72% mean EWL in 16 patients at 2 years (81).
Diabetes resolution/remission
Recently Nelson et al. reported 50% resolution and 33.3% improvement in T2DM between 6–12 months after SADI-S (82), while Sanchez-Pernaute et al. reported 88% remission (first 100 patients) (79); in the same unit when used as a second procedure after SG, remission was lower at 60% with improvement in 30% of patients (81).
As revisional surgery
One reason for the increasing interest in SADI-S is its potential usefulness in case of SG failure, which is a growing concern. With SADI-S performed in two stages, Sanchez-Pernaute et al. reported 72% EWL at 21 months (81), which compares favorably when SG was resleeved (43–58% EWL) or revised to BPD/DS (73–80% EWL) or RYGB (65% EWL) (49) .
Controversial issues
- Severe malnutrition even when the common channel is kept at 200–250 cm;
- No standardization regarding closure of Petersen’s space.
Complications
These are summarized in Table 5 (49).
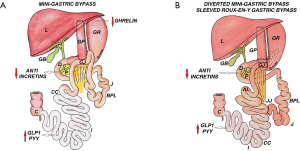
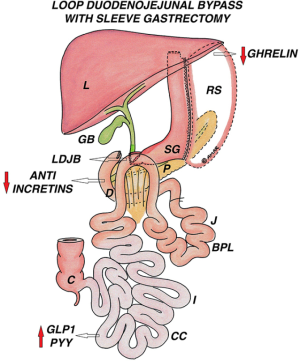
Loop Duodenojejunal Bypass with Sleeve Gastrectomy (LDJB-SG)
Introduction
In 2013 Chih-Kun Huang introduced the LDJB-SG as a modification of SADI-S (83).
Surgical anatomy
SG is performed using a 38-Fr bougie with duodenal transection, 2–4 cm beyond the pylorus. A side to side duodenojejunal anastomosis (aprrox. 1.5 cm) is performed totally hand sewn with 3-0 absorbable sutures at 200–300 cm from ligament of Treitz by bringing up the jejunal loop in a iso-peristaltic and antecolic fashion (Figure 7).
Surgical outcomes
Huang et al. studied 22 diabetic patients (Mean duration 8 years) with mean BMI 28.4 kg/m2, where all patients were on oral hypoglycemic agents (OHA) and 3 (14%) were on insulin also. 11 patients (50%) had complete remission of T2DM while 20 (91%) achieved glycemic control with HbA1c <7% without medication. Mean HbA1c dropped from 8.6% to 6.2%, fasting blood sugar (FBS) from 147 to 110 mg/dL and C-peptide from 2.4 to 1.3 ng/mL at 6 months (83). In a group matched study by the same author comparing RYGB and LDJB-SG (n=30 in each group), both procedures proved to be equally effective with respect to mean BMI, FBS and HbA1c at 1 year; both showing a significant reduction in those parameters from their preoperative levels (P<0.01). Both groups had similar comorbidity resolution, however the LDJB-SG group had better β-cell function (estimated by HOMA-II calculator) compared to the RYGB group (P=0.004); morbidity was higher in the RYGB group (P=0.08) (84).
As revisional surgery
In a case report of two T2DM patients, LDJB-SG was performed as revisional surgery after RYGB to overcome intractable dumping syndrome. Six months postsurgery, the Sigstad's score decreased to 2 points (85).
Controversial issues
- This procedure is like a shorter DS where malabsorption is expected to be much less and efficacy is likely to be much lower;
- Biliary access is lost even in LDJB-SG.
Complications
These are summarized in Table 5 (49).
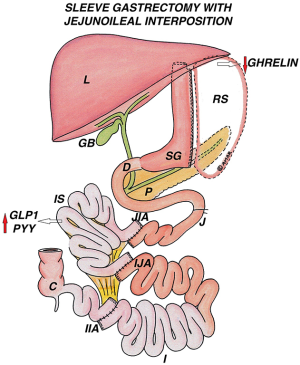
Ileal Interposition with Sleeve Gastrectomy (II-SG)
Introduction
This procedure has gained a lot of popularity as “diabetes surgery” or “metabolic surgery”, having been introduced by Aureo Depaula from Brazil in 2003 (86).
Indications
- Uncontrolled diabetes inspite of optimal medical treatment;
- Gradually worsening T2DM with family history of diabetes related complications;
- Stimulated C-peptide >1 ng/mL.
Contraindications
- T1DM, Latent autoimmune diabetes of adult (LADA) [by estimating Glutamic acid decarboxylase (GAD) antibody, islet cell antibody (ICA), and insulin auto-antibody (IAA2)];
- Beta cell burn out, indicated by fasting C-peptide <0.5ng/mL and/or stimulated C-peptide <1 ng/mL.
Surgical anatomy
II with a BMI adjusted SG is performed either completely laparoscopic or hybrid (SG by laparoscopy and interposition by open approach) or robotically, where a 170 cm segment of terminal ileum is interposed into the jejunal or the duodenal area.
Jejunal ileal interposition with sleeve gastrectomy (JII-SG)
After SG, the ileal segment is interposed into the proximal jejunum, at 20–50 cm from ligament of Treitz, without any bowel exclusion (Figure 8).
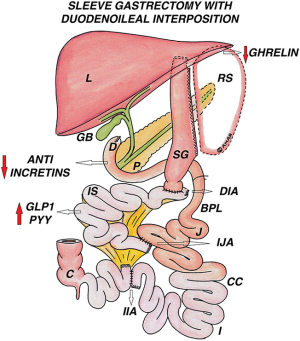
Duodenal ileal interposition with sleeve gastrectomy (DII-SG)
After SG, the ileal segment is interposed between the divided first part of duodenum proximally, with the distal end attached to the jejunum at 50 cm from DJ flexure. This results in a bypass of the duodenum and proximal 50 cm of jejunum, eliminating the foregut anti-incretin factor (Rubino factor) (Figure 9).
Surgical outcomes
In a study conducted by the author, 490 patients underwent II (JII-SG 10.2%, DII-SG 89.8%) at two different centers. 63% of the patients had BMI <35 kg/m2 (mean BMI 29.5 kg/m2), mean HbA1c was 9.8% and duration of T2DM 9.5 years. With a mean follow-up of 24 months (range, 10–72 months), complete remission was observed in 72% of patients and partial remission in 81.5%. These findings were supported by similar results by different authors in their respective studies (50).
Controversial issues
Complex surgical anatomy and long learning curve.
Complications
These are summarized in Table 5 (50,51).
SG with Bipartition (Transit or Loop)
Introduction
SG with TB was first described by Santoro et al. (52). This was later modified by Mui from Hongkong, into SG with Loop Bipartition (SG-LB) [also known as Single Anastomosis Sleeve Ileal (SASI) Bypass as reported by Mahdy] (87).
Surgical anatomy
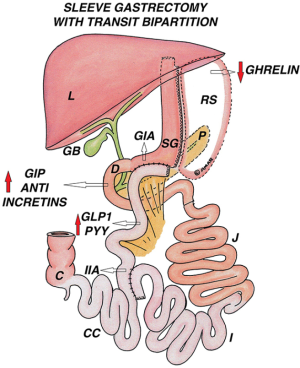
Sleeve gastrectomy with transit bipartition (SG-TB)
After performing a SG, the ileum is transected at 250 cm proximal to ileocaecal junction. The distal ileal end is anastomosed to the antrum in an antecolic fashion, with a stapler or completely hand sewn (anastomosis up to 3 cm is advocated to avoid excess food transit and malabsorption). This creates two potential routes for the transit of food; through the gastroileal anastomosis into distal ileum, and also through the intact duodenum; thus minimizing malnutrition and malabsorption. The proximal ileal end of the transection is anastomosed side to side at 80–130 cm proximal to the ileocaecal junction (depending on length of common channel required), to create the ileo-ileal anastomosis (Figure 10).
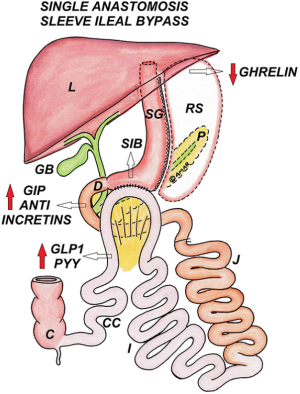
SG-LB/SASI
SG is performed keeping sufficient length of the antrum (approximately 6cm proximal to pylorus) with a loop gastroileostomy at 250 cm proximal to the ileocecal valve, using 2 layers of hand-sewn sutures/stapled anastomosis. In SASI bypass, compared to SADI-S, duodenum is not transected and the anastomosis is gastro-ileal instead of duodeno-ileal (Figure 11).
Surgical outcomes
Santoro et al. studied 1,020 obese patients (BMI 33–72 kg/m2), with a follow-up rate of 59.1% (range, 4 months to 5 years) and reported excellent weight loss (91% EBMIL at 1 year; 94% at 2 years, 85% at 3 years, 78% at 4 years, and 74% at 5 years). Partial diabetes remission was seen in 86%, with a complication rate of 6%, including two deaths (0.2%) (52).
Mui et al. published a case report of SG-LB, with 97% EWL at 12 months follow up in a 46-year-old obese diabetic patient who achieved normo-glycemia without medication within 2 months (87). Mahdy et al. published results of SASI bypass in 50 patients suffering from obesity and diabetes. They have shown %EWL of 90% at one year, normo-glycemia in 100% of patients at 3 months, and resolution of hypertension (86%), hypercholesterolemia (100%) and hypertriglyceridemia (97%) (53).
Controversial issues
Marginal ulcer in transit bypass and ileal contents in stomach, in loop bipartition.
Complications
These are summarized in Table 5 (52,53).
Laparoscopic Gastric Plication (LGP) and Laparoscopic Adjustable Gastric Band Plication (LAGBP)
Introduction
LGP was introduced by Talebpour et al. from Iran with promising results in a 12-year study (88). This technique was further modified by Huang et al. by adding an adjustable gastric band to the plication, creating a dual restriction. Weight loss is brought about by plication and long term weight maintenance is ensured by the adjustable band. A group-matched study of patients undergoing either SG or LAGBP showed that weight loss, comorbidity resolution and complications were similar in both groups at 2 years (89).
Surgical anatomy
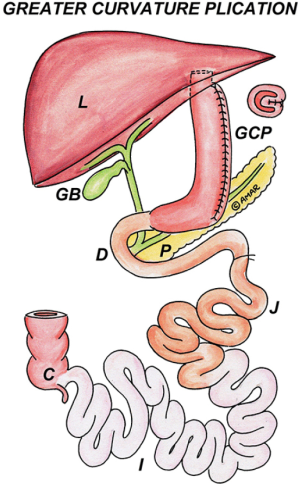
LGP
After devascularization of greater curvature till GE junction, the stomach is plicated from the fundus up to 3 cm proximal to the pylorus, using 2-0 non-absorbable sutures, interrupted at 2 cm intervals to invert the greater curvature, followed by a continuous sero-muscular suture to reinforce it; this prevents the herniation of gastric wall through the interrupted sutures (Figure 12).
LAGBP
After completing the plication, a gastric band is placed using the pars flaccida technique without gastric sutures (Figure 13).
Surgical outcomes
In a 12-year study of 800 cases, LGP resulted in 70% EWL at 2 years and 55% at 5 years, though 31% had weight regain (88). The %EWL in all studies of LGP is comparable to SG (around 50% in 6 months, 60–65% at 12 and 24 months) (90). In another study, SG showed a greater and statistically significant %EWL up to 18 months when compared with LAGBP, though there was no difference at 2 years (89). Multicentric international publications suggest that the efficacy of LGP and its metabolic effects on T2DM lie between AGB and SG (91).
Controversial issues
Even though staplers are not used in gastric plication, bleeding and leaks have been known to occur.
Complications
These are summarized in Table 5 (54,55).
Revisional Bariatric surgeries
There has been a marked increase in revisional bariatric surgeries in the last few years, ranging from 5–43%, probably due to poor choice of the primary procedure with unsatisfactory weight loss or weight regain, recurrence of diabetes and chronic complications requiring intervention. However, there is no clarity regarding its indications, based on evidence.
LAGB
It is well documented that weight loss with LAGB is less than other procedures. Conversions to RYGB, SG, DS or other modifications can be done to improve results or treat complications like slippage, dilatation, migration, erosion, port/tube problems or band intolerance. Some centers report fewer complications when the conversion is done in 2 stages (definitive procedure 2–6 months after band removal). Conversion from LAGB to RYGB has been reported in 2–28.8% of cases (92). In a review of 588 patients from 15 studies evaluating conversion of LAGB to RYGB, the overall complication rate was 8.5% (anastomotic leaks −0.9%; bleeding −1.8%) with 23–74% EWL and follow-up ranging from 7–44 months (93). In conversions of LAGB to SG (286 patients from 8 studies in the same review), there was 31–60% EWL with 12.2% complication rate (staple-line leak −5.6%, probably due to scar tissue caused by the band near GE junction) (93). Small case series report better outcomes when LAGB is converted to a more malabsorptive procedure.
RYGB
Revision of RYGB may be required to deal with complications (marginal ulcer, gastro-gastric fistula, intractable dumping syndrome, and malnutrition), inadequate weight loss, weight regain, or recurrence of diabetes. Factors like pouch or stoma dilation, gastro-gastric fistula or a persistent marginal ulcer can be treated by refashioning the pouch and GJ (63). Alternately, increasing the biliopancreatic limb length or banding the gastric pouch can be used to improve results. However, the reported leak rates of such revisional procedures are very high ranging from 8.5% to 22% (94). There are few case reports of RYGB conversion to SG or LDJB-SG for intractable dumping, severe neuroglycopenia, or malnutrition (95).
Laparoscopic SG
Indication for revisional surgery after SG can be weight regain, leak (acute or chronic), stricture or cork screw deformity of sleeve, and severe GERD not responding to medical management. Different options available to treat weight regain and metabolic recidivism are conversion to MGB, SADI-S, LDJB-SG, bipartition, or ileal transposition. RYGB is a better option in case of severe GERD, chronic leak and stricture. Laparoscopic seromyotomy for stenosis at incisura has been another method in presence of severe reflux and dysphagia but is associated with high leak rate of up to 35% (96). The utilization of a roux limb to create an internal sump proximal to the stricture and to treat chronic leaks has been reported (97).
Conclusions
Although it has been conclusively documented through RCTs that bariatric surgery has a definite, long-term and significant advantage over medical management (along with lifestyle interventions) in treatment of morbid obesity, the majority of the people still shy away from surgery. Lack of sufficient support from physicians, fear of complications, few reports of mishaps, social prejudices or misunderstandings and financial considerations are usually responsible, whereby globally, only 1–2% of the eligible persons get surgery done. Rapid advances in bio-medical technology and refinement of procedures and techniques, to make surgery safer with lesser side-effects, are making these treatments more acceptable for the morbidly obese patients with or without diabetes. Many studies have shown great benefit in uncontrolled diabetics, even with lower BMIs of 30–39 kg/m2.
Greater acceptance amongst patients is likely, if the non-surgical fraternity and society, are convinced of very high surgical and long-term nutritional safety and better quality of life, through clear-cut guidelines and protocols, standardisation of all procedures, individualisation for each patient to get good outcomes and better counselling and nutritional follow-up.
Acknowledgments
The authors would like to appreciate Akshan Ugale (Deccan Medical College, Hyderabad, India) for his revision on the paper.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2017.07.02). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Broadbent R, Tracey M, Harrington P. Laparoscopic Gastric Banding: a preliminary report. Obes Surg 1993;3:63-7. [Crossref] [PubMed]
- Belachew M, Legrand MJ, Defechereux TH, et al. Laparoscopic adjustable silicone gastric banding in the treatment of morbid obesity. Surg Endosc 1994;8:1354-6. [Crossref] [PubMed]
- Fobi MA. Surgical treatment of obesity: a review. J Natl Med Assoc 2004;96:61-75. [PubMed]
- Wittgrove AC, Clark GW. Laparoscopic gastric bypass, Roux-en-Y-500 patients: technique and results, with 3-60 month follow-up. Obes Surg 2000;10:233-9. [Crossref] [PubMed]
- Ren CJ, Patterson E, Gagner M. Early results of laparoscopic biliopancreatic diversion with duodenal switch: a case series of 40 consecutive patients. Obes Surg 2000;10:514-23; discussion 24. [Crossref] [PubMed]
- Johnston D, Dachtler J, Sue-Ling HM, et al. The Magenstrasse and Mill operation for morbid obesity. Obes Surg 2003;13:10-6. [Crossref] [PubMed]
- Sarela AI, Dexter SP, O'Kane M, et al. Long-term follow-up after laparoscopic sleeve gastrectomy: 8-9-year results. Surg Obes Relat Dis 2012;8:679-84. [Crossref] [PubMed]
- Deitel M, Gagner M, Erickson AL, et al. Third International Summit: Current status of sleeve gastrectomy. Surg Obes Relat Dis 2011;7:749-59. [Crossref] [PubMed]
- Angrisani L, Santonicola A, Iovino P, et al. Bariatric Surgery Worldwide 2013. Obes Surg 2015;25:1822-32. [Crossref] [PubMed]
- Abraham A, Ikramuddin S, Jahansouz C, et al. Trends in Bariatric Surgery: Procedure Selection, Revisional Surgeries, and Readmissions. Obes Surg 2016;26:1371-7. [Crossref] [PubMed]
- Gierach M, Gierach J, Ewertowska M, et al. Correlation between Body Mass Index and Waist Circumference in Patients with Metabolic Syndrome. ISRN Endocrinol 2014;2014:514589.
- NIH conference. Gastrointestinal surgery for severe obesity. Consensus Development Conference Panel. Ann Intern Med 1991;115:956-61. [Crossref] [PubMed]
- Lakdawala M, Bhasker A. Asian Consensus Meeting on Metabolic S. Report: Asian Consensus Meeting on Metabolic Surgery. Recommendations for the use of Bariatric and Gastrointestinal Metabolic Surgery for Treatment of Obesity and Type II Diabetes Mellitus in the Asian Population: August 9th and 10th, 2008, Trivandrum, India. Obes Surg 2010;20:929-36. [Crossref] [PubMed]
- De Luca M, Angrisani L, Himpens J, et al. Indications for Surgery for Obesity and Weight-Related Diseases: Position Statements from the International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO). Obes Surg 2016;26:1659-96. [Crossref] [PubMed]
- Ali MR, Maguire MB, Wolfe BM. Assessment of obesity-related comorbidities: a novel scheme for evaluating bariatric surgical patients. J Am Coll Surg 2006;202:70-7. [Crossref] [PubMed]
- Sharma AM, Kushner RF. A proposed clinical staging system for obesity. Int J Obes (Lond) ;33:289-95. [Crossref] [PubMed]
- Orłowski M, Janik MR, Pasnik K, et al. Usefulness of the Obesity Surgery Mortality Risk Score (OR-MRS) in choosing the laparoscopic bariatric procedure. Wideochir Inne Tech Maloinwazyjne 2015;10:233-6. [Crossref] [PubMed]
- Bohdjalian A, Langer FB, Shakeri-Leidenmuhler S, et al. Sleeve gastrectomy as sole and definitive bariatric procedure: 5-year results for weight loss and ghrelin. Obes Surg 2010;20:535-40. [Crossref] [PubMed]
- Diamantis T, Apostolou KG, Alexandrou A, et al. Review of long-term weight loss results after laparoscopic sleeve gastrectomy. Surg Obes Relat Dis 2014;10:177-83. [Crossref] [PubMed]
- Major P, Wysocki M, Pedziwiatr M, et al. Laparoscopic sleeve gastrectomy for the treatment of diabetes mellitus type 2 patients-single center early experience. Gland Surg 2016;5:465-72. [Crossref] [PubMed]
- Hoogerboord M, Wiebe S, Klassen D, et al. Laparoscopic sleeve gastrectomy: perioperative outcomes, weight loss and impact on type 2 diabetes mellitus over 2 years. Can J Surg 2014;57:101-5. [Crossref] [PubMed]
- Jacobs M, Bisland W, Gomez E, et al. Laparoscopic sleeve gastrectomy: a retrospective review of 1- and 2-year results. Surg Endosc 2010;24:781-5. [Crossref] [PubMed]
- Rawlins L, Rawlins MP, Brown CC, et al. Sleeve gastrectomy: 5-year outcomes of a single institution. Surg Obes Relat Dis 2013;9:21-5. [Crossref] [PubMed]
- Eid GM, Brethauer S, Mattar SG, et al. Laparoscopic sleeve gastrectomy for super obese patients: forty-eight percent excess weight loss after 6 to 8 years with 93% follow-up. Ann Surg 2012;256:262-5. [Crossref] [PubMed]
- Lee WJ, Almulaifi A, Tsou JJ, et al. Laparoscopic sleeve gastrectomy for type 2 diabetes mellitus: predicting the success by ABCD score. Surg Obes Relat Dis 2015;11:991-6. [Crossref] [PubMed]
- Gill RS, Birch DW, Shi X, et al. Sleeve gastrectomy and type 2 diabetes mellitus: a systematic review. Surg Obes Relat Dis 2010;6:707-13. [Crossref] [PubMed]
- Switzer NJ, Prasad S, Debru E, et al. Sleeve Gastrectomy and Type 2 Diabetes Mellitus: a Systematic Review of Long-Term Outcomes. Obes Surg 2016;26:1616-21. [Crossref] [PubMed]
- Gagner M, Deitel M, Erickson AL, et al. Survey on laparoscopic sleeve gastrectomy (LSG) at the Fourth International Consensus Summit on Sleeve Gastrectomy. Obes Surg 2013;23:2013-7. [Crossref] [PubMed]
- Rosenthal RJ. International Sleeve Gastrectomy Expert Panel Consensus Statement: best practice guidelines based on experience of >12,000 cases. Surg Obes Relat Dis 2012;8:8-19. [Crossref] [PubMed]
- Parikh M, Issa R, McCrillis A, et al. Surgical strategies that may decrease leak after laparoscopic sleeve gastrectomy: a systematic review and meta-analysis of 9991 cases. Ann Surg 2013;257:231-7. [Crossref] [PubMed]
- Berger ER, Clements RH, Morton JM, et al. The Impact of Different Surgical Techniques on Outcomes in Laparoscopic Sleeve Gastrectomies: The First Report from the Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program (MBSAQIP). Ann Surg 2016;264:464-73. [Crossref] [PubMed]
- Gagner M, Buchwald JN. Comparison of laparoscopic sleeve gastrectomy leak rates in four staple-line reinforcement options: a systematic review. Surg Obes Relat Dis 2014;10:713-23. [Crossref] [PubMed]
- Sarkhosh K, Birch DW, Sharma A, et al. Complications associated with laparoscopic sleeve gastrectomy for morbid obesity: a surgeon’s guide. Can J Surg 2013;56:347-52. [Crossref] [PubMed]
- Chiu S, Birch DW, Shi X, et al. Effect of sleeve gastrectomy on gastroesophageal reflux disease: a systematic review. Surg Obes Relat Dis 2011;7:510-5. [Crossref] [PubMed]
- Weiner RA, El-Sayes IA, Weiner SR. LSG: Complications—Diagnosis and Management. In: Agrawal S. editor. Obesity, Bariatric and Metabolic Surgery: A Practical Guide. Cham: Springer International Publishing, 2016;259-76.
- Kumar S. Gallstone Disease Before and After Bariatric Surgery. In: Kumar S, Gomes RM. editors. Bariatric Surgical Practice Guide: Recommendations. Singapore: Springer Singapore, 2017;115-22.
- Higa KD, Ho T, Boone KB. Internal hernias after laparoscopic Roux-en-Y gastric bypass: incidence, treatment and prevention. Obes Surg 2003;13:350-4. [Crossref] [PubMed]
- Wyles SM, Ahmed AR . LRYGB: Complications-diagnosis and management. Springer 2016; 736:207-29.
- Weng TC, Chang CH, Dong YH, et al. Anaemia and related nutrient deficiencies after Roux-en-Y gastric bypass surgery: a systematic review and meta-analysis. BMJ Open 2015;5:e006964 [Crossref] [PubMed]
- Wyles SM. LRYGB: Complications-diagnosis and management. In: Agarwal S. editor. Obesity, Bariatric and Metabolic surgery: A Practical Guide. Cham: Springer International Publishing, 2016;11.
- Brown WA, Egberts KJ, Franke-Richard D, et al. Erosions after laparoscopic adjustable gastric banding: diagnosis and management. Ann Surg 2013;257:1047-52. [Crossref] [PubMed]
- Tog CH, Halliday J, Khor Y, et al. Evolving pattern of laparoscopic gastric band access port complications. Obes Surg 2012;22:863-5. [Crossref] [PubMed]
- Leeder PC. LAGB: Complications–Diagnosis and Management. In: Agrawal S. editor. Obesity, Bariatric and Metabolic Surgery: A Practical Guide. Cham: Springer International Publishing; 2016. p. 307-19.
- Tacchino RM. Laparoscopic Biliopancreatic Diversion (BPD) Surgery. Springer 2016; 437-45.
- Kerrigan DD, Leuratti L, Khwaja HA, et al. Laparoscopic Biliopancreatic Diversion with Duodenal Switch (BPD-DS) Surgery. In: Agrawal S, editor. Obesity, Bariatric and Metabolic Surgery: A Practical Guide. Cham: Springer International Publishing, 2016;425-35.
- Kular KS, Manchanda N, Rutledge R. A 6-year experience with 1,054 mini-gastric bypasses-first study from Indian subcontinent. Obes Surg. 2014;24:1430-5. [Crossref] [PubMed]
- Lee WJ, Yu PJ, Wang W, et al. Laparoscopic Roux-en-Y versus mini-gastric bypass for the treatment of morbid obesity: a prospective randomized controlled clinical trial. Ann Surg. 2005;242:20-8. [Crossref] [PubMed]
- Rutledge R, Kular KS, Deitel M. Laparoscopic Mini-Gastric (One-Anastomosis) Bypass Surgery. In: Agrawal S. editor. Obesity, Bariatric and Metabolic Surgery: A Practical Guide. Cham: Springer International Publishing, 2016;415-23.
- Martini F, Paolino L, Marzano E, et al. Single-Anastomosis Pylorus-Preserving Bariatric Procedures: Review of the Literature. Obes Surg 2016;26:2503-15. [Crossref] [PubMed]
- De Paula AL, Stival AR, Macedo A, et al. Prospective randomized controlled trial comparing 2 versions of laparoscopic ileal interposition associated with sleeve gastrectomy for patients with type 2 diabetes with BMI 21–34 kg/m2. Surg Obes Relat Dis 2010;6:296-304. [Crossref] [PubMed]
- Ugale SM, Celik A. Ileal Interposition with Sleeve Gastrectomy for Type 2 Diabetes Mellitus and Metabolic Syndrome. In: Agrawal S. editor. Obesity, Bariatric and Metabolic Surgery: A Practical Guide. Cham: Springer International Publishing, 2016;547-54.
- Santoro S, Castro LC, Velhote MC, et al. Sleeve gastrectomy with transit bipartition: a potent intervention for metabolic syndrome and obesity. Ann Surg 2012;256:104-10. [Crossref] [PubMed]
- Mahdy T, Al Wahedi A, Schou C. Efficacy of single anastomosis sleeve ileal (SASI) bypass for type-2 diabetic morbid obese patients: Gastric bipartition, a novel metabolic surgery procedure: A retrospective cohort study. Int J Surg 2016;34:28-34. [PubMed]
- Chang PC, Dev A, Katakwar A, et al. Management of gastric fold herniation after laparoscopic adjustable gastric banded plication: a single-center experience. Surg Obes Relat Dis 2016;12:849-55. [Crossref] [PubMed]
- Huang CK, Katakwar A, Ahluwalia JS, et al. Technical Considerations of Laparoscopic Gastric Plication with or Without a Band. In: Kumar S, Gomes RM. editors. Bariatric Surgical Practice Guide: Recommendations. Singapore: Springer Singapore, 2017;73-80.
- Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA 2004;292:1724-37. [Crossref] [PubMed]
- Sjöström L. Review of the key results from the Swedish Obese Subjects (SOS) trial - a prospective controlled intervention study of bariatric surgery. J Intern Med 2013;273:219-34. [Crossref] [PubMed]
- Schauer PR, Ikramuddin S, Gourash W, et al. Outcomes After Laparoscopic Roux-en-Y Gastric Bypass for Morbid Obesity. Ann Surg 2000;232:515-29. [Crossref] [PubMed]
- Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med 2012;366:1577-85. [Crossref] [PubMed]
- Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes--3-year outcomes. N Engl J Med 2014;370:2002-13. [Crossref] [PubMed]
- Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric Surgery versus Intensive Medical Therapy for Diabetes - 5-Year Outcomes. N Engl J Med 2017;376:641-51. [Crossref] [PubMed]
- DiGiorgi M, Rosen DJ, Choi JJ, et al. Re-emergence of diabetes after gastric bypass in patients with mid- to long-term follow-up. Surg Obes Relat Dis 2010;6:249-53. [Crossref] [PubMed]
- Heneghan HM, Yimcharoen P, Brethauer SA, et al. Influence of pouch and stoma size on weight loss after gastric bypass. Surg Obes Relat Dis 2012;8:408-15. [Crossref] [PubMed]
- Orci L, Chilcott M, Huber O. Short versus long Roux-limb length in Roux-en-Y gastric bypass surgery for the treatment of morbid and super obesity: a systematic review of the literature. Obes Surg 2011;21:797-804. [Crossref] [PubMed]
- Singhal R, Bryant C, Kitchen M, et al. Band slippage and erosion after laparoscopic gastric banding: a meta-analysis. Surg Endosc 2010;24:2980-6. [Crossref] [PubMed]
- O'Brien PE, McPhail T, Chaston TB, et al. Systematic review of medium-term weight loss after bariatric operations. Obes Surg 2006;16:1032-40. [Crossref] [PubMed]
- Colquitt JL, Pickett K, Loveman E, et al. Surgery for weight loss in adults Cochrane Database Syst Rev 2014;CD003641 [PubMed]
- O'Brien PE, Dixon JB, Laurie C, et al. A prospective randomized trial of placement of the laparoscopic adjustable gastric band: comparison of the perigastric and pars flaccida pathways. Obes Surg 2005;15:820-6. [Crossref] [PubMed]
- Gulkarov I, Wetterau M, Ren CJ, et al. Hiatal hernia repair at the initial laparoscopic adjustable gastric band operation reduces the need for reoperation. Surg Endosc. 2008;22:1035-41. [Crossref] [PubMed]
- Avsar FM, Sakcak I, Yildiz BD, et al. Is gastro-gastric fixation suture necessary in laparoscopic adjustable gastric banding? A prospective randomized study. J Laparoendosc Adv Surg Tech A 2011;21:953-6. [Crossref] [PubMed]
- Søvik TT, Taha O, Aasheim ET, et al. Randomized clinical trial of laparoscopic gastric bypass versus laparoscopic duodenal switch for superobesity. Br J Surg 2010;97:160-6. [Crossref] [PubMed]
- Magee CJ, Barry J, Brocklehurst J, et al. Outcome of laparoscopic duodenal switch for morbid obesity. Br J Surg 2011;98:79-84. [Crossref] [PubMed]
- Dorman RB, Rasmus NF, al-Haddad BJ, et al. Benefits and complications of the duodenal switch/biliopancreatic diversion compared to the Roux-en-Y gastric bypass. Surgery 2012;152:758-65; discussion 65-7. [Crossref] [PubMed]
- Mason RJ. Duodenal switch for morbid obesity: is it safe? Adv Surg 2013;47:153-76. [Crossref] [PubMed]
- Fazylov RM, Savel RH, Horovitz JH, et al. Association of super-super-obesity and male gender with elevated mortality in patients undergoing the duodenal switch procedure. Obes Surg 2005;15:618-23. [Crossref] [PubMed]
- Georgiadou D, Sergentanis TN, Nixon A, et al. Efficacy and safety of laparoscopic mini gastric bypass. Surg Obes Relat Dis 2014;10:984-91. [Crossref] [PubMed]
- Lee WJ, Ser KH, Lee YC, et al. Laparoscopic Roux-en-Y vs. mini-gastric bypass for the treatment of morbid obesity: a 10-year experience. Obes Surg 2012;22:1827-34. [Crossref] [PubMed]
- Sánchez-Pernaute A, Rubio Herrera MA, Pérez-Aguirre E, et al. Proximal duodenal-ileal end-to-side bypass with sleeve gastrectomy: proposed technique. Obes Surg 2007;17:1614-8. [Crossref] [PubMed]
- Sánchez-Pernaute A, Rubio MA, Pérez-Aguirre E, et al. Single-anastomosis duodenoileal bypass with sleeve gastrectomy: metabolic improvement and weight loss in first 100 patients. Surg Obes Relat Dis 2013;9:731-5. [Crossref] [PubMed]
- Sánchez-Pernaute A, Rubio MA, Cabrerizo L, et al. Single-anastomosis duodenoileal bypass with sleeve gastrectomy (SADI-S) for obese diabetic patients. Surg Obes Relat Dis 2015;11:1092-8. [Crossref] [PubMed]
- Sánchez-Pernaute A, Rubio MA, Conde M, et al. Single-anastomosis duodenoileal bypass as a second step after sleeve gastrectomy. Surg Obes Relat Dis 2015;11:351-5. [Crossref] [PubMed]
- Nelson L, Moon RC, Teixeira AF, et al. Safety and Effectiveness of Single Anastomosis Duodenal Switch Procedure: Preliminary Result from a Single Institution. Arq Bras Cir Dig 2016;80-4. [Crossref] [PubMed]
- Huang CK, Goel R, Tai CM, et al. Novel metabolic surgery for type II diabetes mellitus: loop duodenojejunal bypass with sleeve gastrectomy. Surg Laparosc Endosc Percutan Tech 2013;23:481-5. [PubMed]
- Huang CK, Tai CM, Chang PC, et al. Loop Duodenojejunal Bypass with Sleeve Gastrectomy: Comparative Study with Roux-en-Y Gastric Bypass in Type 2 Diabetic Patients with a BMI <35 kg/m(2), First Year Results. Obes Surg 2016;26:2291-301. [Crossref] [PubMed]
- Huang CK, Wang MY, Das SS, et al. Laparoscopic conversion to loop duodenojejunal bypass with sleeve gastrectomy for intractable dumping syndrome after Roux-en-Y gastric bypass-two case reports. Obes Surg 2015;25:947. [Crossref] [PubMed]
- DePaula AL, Macedo AL, Mota BR, et al. Laparoscopic ileal interposition associated to a diverted sleeve gastrectomy is an effective operation for the treatment of type 2 diabetes mellitus patients with BMI 21-29. Surg Endosc 2009;23:1313-20. [Crossref] [PubMed]
- Mui WL, Lee DW, Lam KK. Laparoscopic sleeve gastrectomy with loop bipartition: A novel metabolic operation in treating obese type II diabetes mellitus. Int J Surg Case Rep 2014;5:56-8. [Crossref] [PubMed]
- Talebpour M, Motamedi SM, Talebpour A, et al. Twelve year experience of laparoscopic gastric plication in morbid obesity: development of the technique and patient outcomes. Ann Surg Innov Res 2012;6:7. [Crossref] [PubMed]
- Huang CK, Chhabra N, Goel R, et al. Laparoscopic adjustable gastric banded plication: a case-matched comparative study with laparoscopic sleeve gastrectomy. Obes Surg 2013;23:1319-23. [Crossref] [PubMed]
- Kourkoulos M, Giorgakis E, Kokkinos C, et al. Laparoscopic Gastric Plication for the Treatment of Morbid Obesity: A Review. Mini Invas Surg 2012;2012:7.
- Bradnova O, Kyrou I, Hainer V, et al. Laparoscopic greater curvature plication in morbidly obese women with type 2 diabetes: effects on glucose homeostasis, postprandial triglyceridemia and selected gut hormones. Obes Surg 2014;24:718-26. [Crossref] [PubMed]
- Ardestani A, Lautz DB, Tavakkolizadeh A. Band revision versus Roux-en-Y gastric bypass conversion as salvage operation after laparoscopic adjustable gastric banding. Surg Obes Relat Dis 2011;7:33-7. [Crossref] [PubMed]
- Coblijn UK, Verveld CJ, van Wagensveld BA, et al. Laparoscopic Roux-en-Y gastric bypass or laparoscopic sleeve gastrectomy as revisional procedure after adjustable gastric band--a systematic review. Obes Surg 2013;23:1899-914. [Crossref] [PubMed]
- Brethauer SA, Kothari S, Sudan R, et al. Systematic review on reoperative bariatric surgery: American Society for Metabolic and Bariatric Surgery Revision Task Force. Surg Obes Relat Dis 2014;10:952-72. [Crossref] [PubMed]
- Vilallonga R, Van de Vrande S, Himpens J. Laparoscopic reversal of Roux-en-Y gastric bypass into normal anatomy with or without sleeve gastrectomy. Surg Endosc 2013;27:4640-8. [Crossref] [PubMed]
- Vilallonga R, Himpens J, Van de Vrande S. Laparoscopic management of persistent strictures after laparoscopic sleeve gastrectomy. Obes Surg 2013;23:1655-61. [Crossref] [PubMed]
- Iannelli A, Tavana R, Martini F, et al. Laparoscopic roux limb placement over a fistula defect without mucosa-to-mucosa anastomosis: a modified technique for surgical management of chronic proximal fistulas after laparoscopic sleeve gastrectomy. Obes Surg 2014;24:825-8. [Crossref] [PubMed]
Cite this article as: Ugale S, Vennapusa A, Katakwar A, Ugale A. Laparoscopic bariatric surgery-current trends and controversies. Ann Laparosc Endosc Surg 2017;2:154.