The current status of magnetic sphincter augmentation in the management of gastroesophageal reflux disease
Introduction
Gastroesophageal reflux disease (GERD) is among the most common gastrointestinal diseases with a prevalence of 10–20% (1). GERD significantly impairs patient quality of life, often due to symptoms of heartburn and regurgitation. Reflux disease can also cause complications including erosive esophagitis, peptic stricture and Barrett’s esophagus, a precursor of esophageal adenocarcinoma. The first line of treatment of GERD consists of lifestyle modifications and gastric acid suppression. However, these measures do not address the underlying physiologic cause of reflux in many patients, namely ineffective lower esophageal sphincter (LES) function, and breakthrough acid reflux symptoms occur in 17–44% of patients while taking proton pump inhibitors (PPI) (2).
Following its initial description in 1956 and its subsequent refinement, Nissen fundoplication has been the gold-standard surgical treatment for GERD (3). Surgical fundoplication creates a supraphysiologic antireflux valve that is highly effective in controlling acid reflux and eliminating GERD symptoms, but is also associated with significant postoperative side-effects including dysphagia, bloating, and the inability to vomit or belch. Also, the outcomes of laparoscopic fundoplication may vary significantly due to a center’s case volume and surgeon’s expertise. Currently, it is estimated that only 1% of patients with GERD receive a fundoplication procedure (4). In response to the need for highly efficacious minimally invasive treatment options, several laparoscopic and endoscopic procedures have been developed including laparoscopic magnetic sphincter augmentation (MSA) using the LINX® Reflux Management System (Torax Medical, St Paul, MN, USA). The purpose of this article is to review the technique, outcomes and complications of MSA.
The device
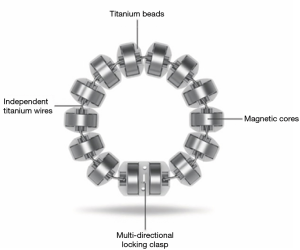
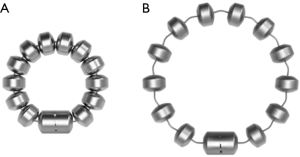
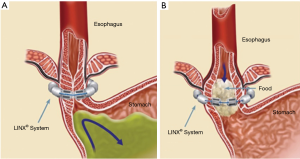
The LINX® Reflux Management System is a band of magnetic beads that forms a ring around the lower esophagus (Figure 1). Each bead has a magnetic core that is covered by a titanium casing. The beads are interconnected by titanium wires that allow each bead to move independently. This structure maintains the ring in the contracted form at rest and allows it to expand during the passage of a food bolus (Figure 2). At rest, the short distance between the magnetic beads results in a higher attraction force. This force augments the barrier function of the LES and prevents the gastric juice from refluxing proximally. When a food bolus passes through the LES the ring expands and the attraction force decreases allowing unimpeded passage of the bolus (Figure 3). The same happens when intragastric pressure increases during belching or vomiting. The device is available in multiple sizes which are determined by the number of beads in the ring (13 to 17 beads). The appropriate device is selected using a sizing instrument that is passed around the esophagus and correlates the esophageal size with the appropriately sized device.
The placement of a prosthetic ring around the GE junction is not a novel idea. The Angelchik device, introduced in 1979, was a silicone ring that was surgically placed around the GE junction in patients with GERD and secured in place with Dacron tape. This device fell out of favor after poor long-term outcomes were shown including dysphagia rates of up to 45% and high reoperation, removal and complication rates (5). In contrast to the Angelchik device, MSA using the LINX® Reflux Management System allows the dynamic changes of the diameter of the magnetic ring with the closed configuration preventing reflux and the open configuration allowing passage of food boluses. Patients who underwent esophageal manometry before and after MSA were noted to have improvement (increase) in LES resting pressure, LES residual pressure and distal esophageal contraction amplitude (6).
Surgical technique
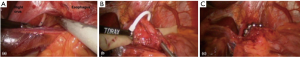
MSA is performed using a 4-port laparoscopic technique similar to that utilized for laparoscopic Nissen fundoplication (LNF). After obtaining laparoscopic access, the pars flaccida of the gastrohepatic ligament is opened to expose the right crus of the diaphragm. Any sliding hiatal hernia is reduced and a circumferential esophageal dissection is completed at the level of the hiatus. If necessary, a posterior cruroplasty is performed using interrupted non-absorbable sutures. When this is complete, the posterior vagus nerve is dissected free from the posterior wall of the distal esophagus. The appropriate position of the MSA device is two centimeters proximal to the gastroesophageal (GE) junction as determined laparoscopically by the gastric fat pad. A Penrose drain is placed around the esophagus excluding the posterior vagus nerve. The sizing instrument is then used to determine the appropriate size of the device, and the device is introduced and passed around the esophagus. The magnetized ends are mated and the device is locked in place around the lower esophagus (Figures 4,5).

Patient selection
The preoperative evaluation for MSA device placement is the same as that for laparoscopic fundoplication. Objective documentation of GERD is needed in the form of ambulatory pH testing. Barium esophagogram and upper endoscopy are helpful in evaluating the anatomy including the identification of large hiatal hernias, severe esophagitis or Barrett’s esophagus. Esophageal manometry is essential to ensure that the distal esophageal peristalsis is sufficient to overcome the 20–25 mmHg device opening pressure. There are no specific manometric parameters that determine the appropriate use of MSA. MSA has not been evaluated when distal esophageal peristaltic amplitude is less than 35 mmHg on wet swallows or when less than 70% of swallows demonstrate peristaltic contractions. Furthermore, MSA should be avoided in the presence a known motility disorder such as achalasia, nutcracker esophagus, diffuse esophageal spasm or hypertensive LES.
The ideal patient for MSA is a patient with:
- Partially PIP responsive typical GERD symptoms;
- Positive ambulatory pH testing;
- Absent or small (<3 cm) hiatal hernia.
Exclusion criteria for MSA include:
- Large hiatal hernia (>3 cm);
- Severe esophagitis;
- Long-segment Barrett’s esophagus;
- Significant abnormality in esophageal manometry;
- Allergy to stainless steel, titanium, nickel or iron.
The manufacturer reports the device to be MRI scanning compatible up to 0.7 or 1.5 Tesla depending on the version of the device used (8).
Outcomes
The initial feasibility studies of MSA showed its safety and feasibility in porcine models (9). These were shortly followed by human feasibility studies from Italy showing that the device can be placed laparoscopically with minimal dissection and a short learning curve compared to LNF (10). Receiving the approval from the US Food and Drug Administration (FDA) in 2012 has facilitated the use of the device in the USA (11).
The feasibility trial evaluated 44 patients who underwent MSA at four centers in Italy, USA and Netherlands between 2007 and 2008. This single arm trial, where patients serve as their own controls, compared the outcomes following MSA implantation to patient’s baseline characteristics. The outcomes were reported at 1 to 2 years, 3 to 4 years and more than 5 years postoperatively. GERD health-related quality of life (HRQL) scores improved from 25.7 at baseline to 3.8 at 1 year, 2.4 at 2 years, 3.3 at 4 years and 2.9 at more than 5 years. All patients were taking PPIs at baseline. The rate of PPI cessation was 90% at 1 year, 85% at 2 years, 80% at 3 to 4 years and 88% at more than 5 years. The percentage of time the pH was less than 4 was 11.9% at baseline and this improved to 3.1% at 1 year, 2.4% at 2 years, 3.8% at 3 years and 4.6% at more than 5 years. More than 86% of patients reported satisfaction with their current condition while off-PPIs at 1, 2, 4 and more than 5 years compared to 0% at baseline (12-14).
These sustained outcomes were replicated in the pivotal trial. This multi-center prospective trial evaluated 100 patients who underwent laparoscopic MSA at 14 centers (13 in USA and one in the Netherlands). The median time to implant the device was 36 minutes (range, 7–125 minutes). All patients were discharged within 1 day after surgery. Postoperative outcomes were evaluated at 3 and 5 years. GERD HRQL scores improved from 11 on-PPI and 27 off-PPI to 2 at 3 years and 4 at 5 years postoperatively. More than 50% improvement in GERD HRQL scores was achieved in 92% of patients at 3 years. More than 50% improvement in PPI use was achieved in 93% of patients at 3 years and PPI use decreased from 100% preoperatively to 15% at 5 years. Normalization of ambulatory pH testing or more than 50% improvement in pH testing was achieved in 64% of patients at 3 years. No device erosions, migrations of malfunctions were reported at 5 years. Table 1 summarizes the studies evaluating MSA outcomes.
Table 1
| Source | N | Follow-up | Description |
|---|---|---|---|
| Bonavina, 2010 (12)* | 44 | 1 and 2 years | Multi-institutional prospective study. Participating institutions from Italy, USA and the Netherlands |
| Lipham, 2012 (13)* | 44 | 3–4 years | Multi-institutional prospective study. Participating institutions from Italy, USA and the Netherlands |
| Bonavina, 2013 (15) | 100 | Median of 3 years | Single center prospective study of consecutive cases from Italy |
| Ganz, 2013 (16)** | 100 | 3 years | Multi-institutional prospective study. 14 participating institutions from USA and the Netherlands |
| Reynolds, 2014 (17) | 67 | Median of 5 months | Prospective study from two institutions |
| Riegler, 2015 (18) | 202 | 1 year | Multicenter prospective study (22 institutions) from four countries (Austria, Germany, Italy and the UK). Also included 47 patients who underwent laparoscopic Nissen fundoplication |
| Saino, 2015 (14)* | 44 | 33 patients followed ≥5 years | Multi-institutional prospective study. Participating institutions from Italy, USA and the Netherlands |
| Czosnyka, 2016 (19) | 102 | Mean 7.6 months | Two community-based health systems in USA |
| Ganz, 2016 (20)** | 100 | 85 patients followed ≥5 years | Multi-institutional prospective study. 14 participating institutions from USA and the Netherlands |
*, feasibility trial reporting short-term, mid-term & 4-year outcomes; **, piroted trial reporting 3- & 5-year outcomes.
Comparison to other surgical GERD treatments
To date, there have been no randomized controlled trials comparing laparoscopic MSA to laparoscopic antireflux surgery. However, several retrospective studies have compared the outcomes of laparoscopic MSA to laparoscopic fundoplication. Warren et al. performed a retrospective review of 201 patient who underwent MSA and 214 patients who underwent LNF for a total of 415 patients. The procedures were performed at three high-volume esophageal centers in the USA. Propensity score matching was performed and 114 patients were analyzed in each arm. The mean follow up was 11 months for MSA and 16 months for LNF (P<0.001). There was no statistically significant difference in preoperative GERD HRQL (21 vs. 19; P=0.56) or postoperative GERD HRQL (6 vs. 5; P=0.54). MSA patients had greater ability to belch (97% vs. 66%; P<0.001) and vomit (88% vs. 40%; P<0.001). The MSA group reported gas-bloat symptoms less frequently than LNF group (41% vs. 59%; P=0.008). Mild dysphagia was more common with MSA (44% vs. 32%; P=0.04). Moderate and severe dysphagia rates were similar in both groups. The MSA group was more likely to require postoperative PPI (24% vs. 12%; P=0.02). Patient satisfaction was similar in both groups (88% vs. 89%; P=0.61) (21). Table 2 summarizes the studies comparing laparoscopic MSA and fundoplication procedures (18,21-26).
Table 2
| Source (year) | Total number | MSA (n) | LNF (n) | Follow-up | Description |
|---|---|---|---|---|---|
| Louie, 2014 (22) | 66 | 34 | 32 | ≥6 months | Retrospective case-controlled study of consecutive patients, included patients with hiatal hernia <3 cm |
| Reynolds, 2015 (23) | 179 | 62 | 117 | 1 year | Retrospective, patients matched using propensity scoring |
| Sheu, 2015 (24) | 24 | 12 | 12 | 7 months | Retrospective, single-center, case-controlled study; matched patients for age, gender and hiatal hernia |
| Riegler, 2015 (18) | 249 | 202 | 47 | 1 year | LNF patients were older, more likely to have Barrett’s esophagus and had larger hiatal hernias |
| Warren, 2016 (21) | 415 | 201 | 214 | 354 patients (MSA 169, LNF 185) were followed for 1 year | Multi-institutional retrospective study (three high-volume centers in USA). Included a propensity matched comparison |
| Reynolds, 2016 (25) | 119 | 52 | 67 | 1 year | Retrospective |
| Asti, 2016 (26) | 238 | 135 | 103 | >1 year | Propensity matched cohort |
MSA, magnetic sphincter augmentation; LNF, laparoscopic Nissen fundoplication.
Skubleny et al. conducted a systematic review of studies comparing MSA to LNF and included three studies in the meta-analysis. These included a total of 688 patients of which 415 underwent MSA and 273 underwent LNF. Operative time was shorter for MSA compared to LNF (63.7 vs. 76.8 minutes). MSA was found to be superior to LNF in maintaining the patient’s ability to belch (95.2% vs. 65.9%; P<0.01) and vomit (93.5% vs. 49.5%; P<0.01). Bloating was more common with LNF compared to MSA but this did not reach statistical significance (53.4% vs. 26.7%; P=0.06). Postoperative dysphagia and PPI elimination rates were similar.
Seeing as reflux is a chronic disease, and long-term outcomes are of paramount importance, studies are needed to compare the long-term efficacy, complications, and need for repeat intervention (endoscopic dilations and reoperations) between MSA and laparoscopic fundoplication procedures. No studies have compared MSA to PPI therapy. The CALIBER study is an ongoing randomized controlled trial designed to compare MSA to double-dose PPIs for the management of GERD symptoms.
Special situations
As experience with MSA procedure accumulates, the indications for this procedure may expand to include patients with larger hiatal hernias (>3 cm) and patients with GERD following bariatric procedures. These situations have been evaluated in small cohort studies.
Hiatal hernia
The presence of a large hiatal hernia was initially thought of as an exclusion criterion for MSA device placement. However, some early data are showing encouraging results of MSA in this patient population. Rona et al. retrospectively reviewed 192 patients who underwent MSA placement in their institution. Fifty-two patients (27%) had a hiatal hernia ≥3 cm (range, 3–7 cm). In these patients their mean GERD-HRQL score decreased from 20.5 preoperatively to 3.6 postoperatively (P<0.01). When compared to patients with smaller hernias, patients with large hiatal hernias had lower postoperative PPI requirement (9.6% vs. 26.6%; P=0.01) and lower mean postoperative GERD HRQL scores (3.6 vs. 5.6; P=0.03). The rates of severe dysphagia and symptom resolution were similar to patients with smaller hiatal hernias (27).
GERD following bariatric procedures
The management of GERD following sleeve gastrectomy is complicated. The altered gastric anatomy makes traditional treatments such as fundoplication impossible. While this issue is controversial, many believe that sleeve gastrectomy can worsen pre-existing reflux disease or create “de novo” GERD (28). Off-label use of MSA to manage GERD in patients with a prior sleeve gastrectomy has been described in a case series (29). Other case reports have also describes the use of MSA to treat GERD following Roux-en-Y gastric bypass (30-32). More studies are needed to further explore the role of MSA procedure in this growing subset of patients. The RELIEF study is an ongoing prospective, multicenter study evaluating the safety and efficacy of MSA in sleeve gastrectomy patients with GERD.
Complications
The most common complication of MSA is dysphagia. Some degree of postoperative dysphagia can be observed in 34% to 79% of patients. Dysphagia is most noticeable shortly after MSA device placement but it tends to resolve over time with conservative management. Significant improvement is usually noticed 8 weeks postoperatively. The reported rates of dysphagia beyond 3 years postoperatively are 4% to 6% compared to 5% rate of dysphagia at baseline (prior to MSA device placement) (10,16,17,20,21,23,33,34).
In a small proportion of patients (6.7%) recurrent reflux symptoms or severe dysphagia can lead to MSA device removal (35). In some patients the device was removed due to the need to obtain an MRI for an unrelated problem. This issue was more common with the first generation devices which were only compatible up to 0.7 Tesla. The device removal can be achieved laparoscopically in the majority of patients (Figure 6). This requires locating the device and incising the fibrous capsule that envelops each of the beads. Once a few beads are freed the connecting wire is divided to facilitate removal. We recommend obtaining a preoperative x-ray as well as consulting the previous operative implant record to allow a count of the beads in order to ensure complete device removal. Laparoscopic fundoplication performed as a concomitant procedure with device removal has been described. Asti et al described the removal of 11 devices out of 164 implants with a 48 month median follow up. A partial or total fundoplication procedure was performed concurrently in all patients. Recurrent heartburn or regurgitation was the most common indication for removal (46%) followed by dysphagia (37%). There were no morbidities associated with the procedure. Follow duration ranged from 12 to 58 months at which time all patients reported satisfaction with their condition and GERD HRQL scores were within normal limits (37).

Device erosion into the esophagus has been described. The exact incidence of this complication is unknown. Eleven cases of device erosion was reported to the FDA Manufacturer’s and User’s Device Experiences (MAUDE) database in 2016 (38). This seemingly uncommon complication mandates removal of the device. The technique for endoscopic removal has been described and follows similar concepts to the endoscopic removal of eroded gastric bands (39). The chronic erosion process allows scarring on the extraluminal portion of the device that prevents a full thickness defect of the esophageal wall when the device is retrieved. Other potential complications include migration and device malfunction (40).
Conclusions
MSA is a novel surgical option for the management of GERD. Short- and long-term data demonstrate excellent control of reflux symptoms, objective GERD control, and improvement in patient quality of life with an acceptable side-effect profile. MSA compares favorably to LNF in retrospective trials, but randomized controlled trials comparing MSA to best medical management and LNF are needed to confirm the findings of the currently available retrospective studies.
Acknowledgments
Funding: KA Perry has received Research funding from Torax medical.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2017.06.13). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dent J, El-Serag HB, Wallander MA, et al. Epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut 2005;54:710-7. [Crossref] [PubMed]
- Becher A, El-Serag H. Systematic review: the association between symptomatic response to proton pump inhibitors and health-related quality of life in patients with gastro-oesophageal reflux disease. Aliment Pharmacol Ther 2011;34:618-27. [Crossref] [PubMed]
- Nissen R. A simple operation for control of reflux esophagitis. Schweiz Med Wochenschr 1956;86:590-2. [PubMed]
- Bonavina L, Saino G, Lipham JC, et al. LINX(®) Reflux Management System in chronic gastroesophageal reflux: a novel effective technology for restoring the natural barrier to reflux. Therap Adv Gastroenterol 2013;6:261-8. [Crossref] [PubMed]
- Thibault C, Marceau P, Biron S, et al. The Angelchik antireflux prosthesis: long-term clinical and technical follow-up. Can J Surg 1994;37:12-7. [PubMed]
- Warren HF, Louie BE, Farivar AS, et al. Manometric Changes to the Lower Esophageal Sphincter After Magnetic Sphincter Augmentation in Patients With Chronic Gastroesophageal Reflux Disease. Ann Surg 2017;266:99-104. [Crossref] [PubMed]
- Al-Mansour MR, Perry KA, Hazey JW. Laparoscopic placement of the LINX® Reflux Management System. Asvide 2017;4:406. Available online: http://www.asvide.com/articles/1720
- Torax. Frequently Asked Questions. cited 2017 Mar 28. Available online: http://www.toraxmedical.com/linx/FAQs.php
- Ganz RA, Gostout CJ, Grudem J, et al. Use of a magnetic sphincter for the treatment of GERD: a feasibility study. Gastrointest Endosc 2008;67:287-94. [Crossref] [PubMed]
- Bonavina L, Saino GI, Bona D, et al. Magnetic augmentation of the lower esophageal sphincter: results of a feasibility clinical trial. J Gastrointest Surg 2008;12:2133-40. [Crossref] [PubMed]
- FDA Approves LINX® System for the Treatment of Reflux Disease. cited 2017 Mar 14. Available online: http://www.toraxmedical.com/news/downloads/PR_LINXFDAApproval.pdf
- Bonavina L, DeMeester T, Fockens P, et al. Laparoscopic sphincter augmentation device eliminates reflux symptoms and normalizes esophageal acid exposure: one- and 2-year results of a feasibility trial. Ann Surg 2010;252:857-62. [Crossref] [PubMed]
- Lipham JC, DeMeester TR, Ganz RA, et al. The LINX® reflux management system: confirmed safety and efficacy now at 4 years. Surg Endosc 2012;26:2944-9. [Crossref] [PubMed]
- Saino G, Bonavina L, Lipham JC, et al. Magnetic Sphincter Augmentation for Gastroesophageal Reflux at 5 Years: Final Results of a Pilot Study Show Long-Term Acid Reduction and Symptom Improvement. J Laparoendosc Adv Surg Tech A 2015;25:787-92. [Crossref] [PubMed]
- Bonavina L, Saino G, Bona D, et al. One hundred consecutive patients treated with magnetic sphincter augmentation for gastroesophageal reflux disease: 6 years of clinical experience from a single center. J Am Coll Surg 2013;217:577-85. [Crossref] [PubMed]
- Ganz RA, Peters JH, Horgan S, et al. Esophageal sphincter device for gastroesophageal reflux disease. N Engl J Med 2013;368:719-27. [Crossref] [PubMed]
- Reynolds JL, Zehetner J, Bildzukewicz N, et al. Magnetic sphincter augmentation with the LINX device for gastroesophageal reflux disease after U.S. Food and Drug Administration approval. Am Surg 2014;80:1034-8. [PubMed]
- Riegler M, Schoppman SF, Bonavina L, et al. Magnetic sphincter augmentation and fundoplication for GERD in clinical practice: one-year results of a multicenter, prospective observational study. Surg Endosc 2015;29:1123-9. [Crossref] [PubMed]
- Czosnyka NM, Buckley FP, Doggett SL, et al. Outcomes of magnetic sphincter augmentation - A community hospital perspective. Am J Surg 2017;213:1019-23. [Crossref] [PubMed]
- Ganz RA, Edmundowicz SA, Taiganides PA, et al. Long-term Outcomes of Patients Receiving a Magnetic Sphincter Augmentation Device for Gastroesophageal Reflux. Clin Gastroenterol Hepatol 2016;14:671-7. [Crossref] [PubMed]
- Warren HF, Reynolds JL, Lipham JC, et al. Multi-institutional outcomes using magnetic sphincter augmentation versus Nissen fundoplication for chronic gastroesophageal reflux disease. Surg Endosc 2016;30:3289-96. [Crossref] [PubMed]
- Louie BE, Farivar AS, Shultz D, et al. Short-term outcomes using magnetic sphincter augmentation versus Nissen fundoplication for medically resistant gastroesophageal reflux disease. Ann Thorac Surg 2014;98:498-504; discussion 504-5. [Crossref] [PubMed]
- Reynolds JL, Zehetner J, Wu P, et al. Laparoscopic Magnetic Sphincter Augmentation vs Laparoscopic Nissen Fundoplication: A Matched-Pair Analysis of 100 Patients. J Am Coll Surg 2015;221:123-8. [Crossref] [PubMed]
- Sheu EG, Nau P, Nath B, et al. A comparative trial of laparoscopic magnetic sphincter augmentation and Nissen fundoplication. Surg Endosc 2015;29:505-9. [Crossref] [PubMed]
- Reynolds JL, Zehetner J, Nieh A, et al. Charges, outcomes, and complications: a comparison of magnetic sphincter augmentation versus laparoscopic Nissen fundoplication for the treatment of GERD. Surg Endosc 2016;30:3225-30. [Crossref] [PubMed]
- Asti E, Bonitta G, Lovece A, et al. Longitudinal comparison of quality of life in patients undergoing laparoscopic Toupet fundoplication versus magnetic sphincter augmentation: Observational cohort study with propensity score analysis. Medicine (Baltimore) 2016;95:e4366 [Crossref] [PubMed]
- Rona KA, Reynolds J, Schwameis K, et al. Efficacy of magnetic sphincter augmentation in patients with large hiatal hernias. Surg Endosc 2017;31:2096-102. [Crossref] [PubMed]
- Mahawar KK, Jennings N, Balupuri S, et al. Sleeve gastrectomy and gastro-oesophageal reflux disease: a complex relationship. Obes Surg 2013;23:987-91. [Crossref] [PubMed]
- Desart K, Rossidis G, Michel M, et al. Gastroesophageal Reflux Management with the LINX® System for Gastroesophageal Reflux Disease Following Laparoscopic Sleeve Gastrectomy. J Gastrointest Surg 2015;19:1782-6. [Crossref] [PubMed]
- Hawasli A, Phillips A, Tarboush M. Laparoscopic management of reflux after Roux-en-Y gastric bypass using the LINX system and repair of hiatal hernia: a case report. Surg Obes Relat Dis 2016;12:e51-4. [Crossref] [PubMed]
- Hawasli A, Tarakji M, Tarboush M. Laparoscopic management of severe reflux after sleeve gastrectomy using the LINX(®) system: Technique and one year follow up case report. Int J Surg Case Rep 2017;30:148-51. [Crossref] [PubMed]
- Muñoz-Largacha JA, Hess DT, Litle VR, et al. Lower Esophageal Magnetic Sphincter Augmentation for Persistent Reflux After Roux-en-Y Gastric Bypass. Obes Surg 2016;26:464-6. [Crossref] [PubMed]
- Schwameis K, Schwameis M, Zörner B, et al. Modern GERD treatment: feasibility of minimally invasive esophageal sphincter augmentation. Anticancer Res 2014;34:2341-8. [PubMed]
- Skubleny D, Switzer NJ, Dang J, et al. LINX® magnetic esophageal sphincter augmentation versus Nissen fundoplication for gastroesophageal reflux disease: a systematic review and meta-analysis. Surg Endosc 2017;31:3078-84. [PubMed]
- Harnsberger CR, Broderick RC, Fuchs HF, et al. Magnetic lower esophageal sphincter augmentation device removal. Surg Endosc 2015;29:984-6. [Crossref] [PubMed]
- Al-Mansour MR, Perry KA, Hazey JW. Laparoscopic removal of the LINX® Reflux Management System. Asvide 2017;4:407. Available online: http://www.asvide.com/articles/1721
- Asti E, Siboni S, Lazzari V, et al. Removal of the Magnetic Sphincter Augmentation Device: Surgical Technique and Results of a Single-center Cohort Study. Ann Surg 2017;265:941-5. [Crossref] [PubMed]
- Bielefeldt K. Adverse Events After Implantation of a Magnetic Sphincter Augmentation Device for Gastroesophageal Reflux. Clin Gastroenterol Hepatol 2016;14:1507-8. [Crossref] [PubMed]
- Bauer M, Meining A, Kranzfelder M, et al. Endoluminal perforation of a magnetic antireflux device. Surg Endosc 2015;29:3806-10. [Crossref] [PubMed]
- Lipham JC, Taiganides PA, Louie BE, et al. Safety analysis of first 1000 patients treated with magnetic sphincter augmentation for gastroesophageal reflux disease. Dis. Esophagus 2015;28:305-11. [Crossref] [PubMed]
Cite this article as: Al-Mansour MR, Perry KA, Hazey JW. The current status of magnetic sphincter augmentation in the management of gastroesophageal reflux disease. Ann Laparosc Endosc Surg 2017;2:146.