The minimal open preperitoneal (MOPP) approach to treat the groin hernias, with the history of the preperitoneal approach
Introduction
In terms of hernia surgery, since our collaboration with René Stoppa (1-4), our preference is the pre-peritoneal route. Which is known not only for is efficiency, but also for the low postoperative morbidity, in particular the low rate of severe chronic pain and the good quality of life (5-13). The latter criterion is recognized as essential if we compare with other surgical techniques which have also a very low recurrence rate, but a higher rate of chronic pain. Here is a brief chronology of this preperitoneal route history from the pioneers of the 19th century to the modern techniques of the 21th century.
This article is the reflect of my opinion in favor of not only the preperitoneal approach, but also the really minimal access and invasive surgery in opposition with the endoscopic surgery which is a minimal access but not minimal invasive surgery.
About the preperitoneal approach, it seems correct to choose the René Stoppa principle as a symbol of the preperitoneal route: the Giant Prosthesis for the Reinforcement of the Visceral Sac (Figure 1).
Historical review
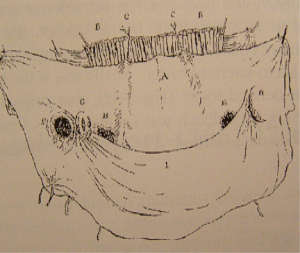
In the beginning, during the time of Anatomists, J. G. Cloquet [1790–1883] pictures for the first time a diagram of the posterior inguinal wall in “Research on anatomical hernia of the abdomen” (Figure 2) (14). It is essential to mention A. P. Cooper [1768–1841], who described for the first time the transversalis fascia (Figure 3) (15). But It was only after the development of the antisepsis leaded by Lister (16) that it was possible to operate in this space. In 1873, Annandale (Figure 4) (17) reported for the first time the concept of the posterior and preperitoneal approach concerning a patient with a combination of medial, lateral, and femoral hernias. In 1891, Lawson Tait from Birmingham said (18), “I have the impression that the radical cure of hernia, other than umbilical, will, by and by, be undertaken by abdominal section.” Sir G. Cheatle Lenthal from London [1820–1921] had treated inguinal and femoral hernias through a midline preperitoneal approach (Figures 5,6) (19). But the gold standard at that time was the anterior approach, following the works of Bassini [1844–1924] (Figure 7) (20), Shouldice [1890–1965] (Figure 8) (21) shows his famous technique, the gold standard in the 70’s and 80’s. For femoral hernias, McVay (Figure 9) described his famous technique in 1938 (22), but generally surgeons did not accept his original description. They omitted making the relaxation incision and results were not as good as those published by McVay.
Other authors then proposed to treat femoral hernias with plugs. This technique is a purveyor of drawbacks which are sometimes major chronic pain, infection, and/or recurrence. Even femoral vein compression (Figure 10). Quite often is it necessary to perform the operation a second time, after recurrence or chronic pain, and time to time to remove these plugs, often not incorporated (Figure 11). A few years before using these plugs, one of the masters of the modern era, Nyhus (Figure 12) (23,24), used the posterior approach, making a suture of the musculo-fascial plan. But after a high rate of recurrence, especially for large medial hernia, he used a synthetic mesh. At that time, many surgeons applied the principles published by Nyhus: Sheehan [1961], Mahorn and Goss [1962], Smith [1962], Huguier [1963], Estrin [1963], Andrews [1968] and Read [1968].
In 1956, Fruchaud (Figure 13), one of the masters of Rene Stoppa (Figure 14) and Jean Rives (Figure 15) in Alger, carried out an important study on anatomy, and published in French his famous book: “Anatomie chirurgicale des hernies de l’aine” (25), but unfortunately unknown, especially in English speaking countries. This book has been translated only recently by Robert Bendavid. It is interesting to note that Henry R. Fruchaud said in 1956: “Surgical treatment of inguinal or femoral hernias should not be the simple closure of the inguinal canal or femoral ring, but the deep abdominal wall reconstruction in the groin region.” This vision of Henry Fruchaud had particularly caught the attention of Jean Rives and René Stoppa. Jean Rives applied these ideas and started to use the polyester mesh in 1965, he published (26,27) his technique for the treatment of unilateral hernia, through a midline preperitoneal approach. Jean Rives communicated extensively with Rene Stoppa, and Stoppa made the cartography of the cleavable spaces with Odimba (28), he proposed his famous technique “The Giant Prosthesis for the Reinforcement of the Visceral Sac” (GPRVS) (29,30).
It is the first technique without tension and without suture. Stoppa used also a polyester prosthesis (31,32). George Wantz (Figure 16) (33,34) from New York, was a friend of Rene Stoppa, he applied this principle to treat unilateral hernias (Unilateral GPRVS). He also used the polyester prosthesis.
So, the preperitoneal technique initiated by Nyhus from Chicago return to New York. At that time (1974), Irving Lichtenstein described his plug technique and in 1986 his tension free technique with the introduction of the anterior prosthesis (35,36). Because of the simplicity and reproducibility of this technique, it quickly became very popular and has become the most widely used technique. But the positioning of the prosthesis is theoretically illogical regarding the pressure force, and the contact between the prosthesis and the sensory nerves in the region. As we have already mentioned above this anterior approach gave a higher rate of severe chronic pain.
In the 1980s, the laparoscopic revolution completely changed visceral surgery and influenced the parietal surgery.
Franklin in 1990, Arregui in 1991 described the TAPP technique (the laparoscopic treatment using the intra peritoneal and preperitoneal approach), published in 1992 in France by J. Leroy and G. Fromont (37). The same year in France; G. Begin (38), published the strictly extra peritoneal laparoscopic approach. For us, the laparoscopic approach is a minimal access surgery with extremely small incisions, but it is not a minimally invasive surgery because it requires general anesthesia with curarisation, with sophisticated and more expensive equipment. It is rarely but sometimes responsible for severe complications directly related to the surgical technique (visceral perforation, vascular injury).
The laparoscopic development goes against the idea of G. Wantz who wanted to develop a technique to treat complex hernias under ambulatory sitting, and under local anesthesia. The real challenge is to adapt the Nyhus, Fruchaud, Rives, Stoppa, Wantz techniques for a truly minimally invasive intervention. In this direction, Jean Henri Alexandre (Figure 17) published in 1984 an open preperitoneal technique (39), with a parietalization of the cord, and with the section of the inferior epigastric vessels, he putted a mesh in the preperitoneal space, this procedure is the first step in the direction of a minimal preperitoneal invasive technique.
The modern history of the minimal open technique
The real pioneer is Franz Ugahary (Figure 18) (40). He was in close contact with R. Stoppa, and G. Wantz (Figure19). He took up the Wantz challenge with the technique (grid iron) described in 1995, using a very short incision, located in the upper part of the groin area, approximately 2–3 cm above the deep inguinal ring (Figure 20).
This minimal access approach, mimicking the Mc Burney incision, enables the extensive dissection of cleavable area and retro peritoneal spaces to treat all types of inguinal or femoral hernias by putting in place a large prosthesis. The details of the technique are in (41,42).
The main steps of the original technique are:
- The 3 cm incision above the deep inguinal ring, as a Mc Burney incision without the incision of the peritoneum (Figure 20);
- The huge dissection in the preperitoneal space, typical from the Ugahary technique, with different size of atraumatic retractors, the dissection is so atraumatic that it is no necessary to use any electrosurgical devices;
- The reduction of a medial sac;
- The parietalization of the cord with a reduction of a lateral sac;
- The checking of the femoral and obturator areas;
- The use of a 15 by 10 regular flat mesh or a lightweight mesh unroll in the dissected space;
- No mesh fixation needed;
- No suture on the musculo fascial plane (transversalis fascia).
I used preferentially the original Ugahary technique between 2001 and 2011 for more than 1,000 hernias repair. With good results.
A prospective study on the first 300 operated hernias has been published (41) by the French National Academy of Surgery. It showed the good results of the technique in terms of recurrence and chronic pain:
- There were 12 (4%) seromas or hematomas, necessitating two very simple local procedures;
- No severe chronic pain was observed;
- The recurrence rate was 2.3% (seven cases).
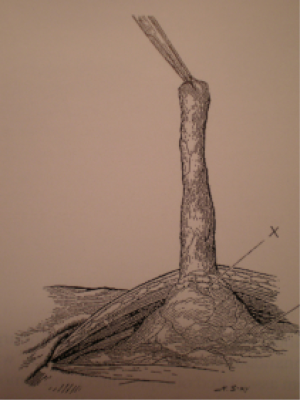
But unrolling the flat mesh (Figure 21) through the small incision according to the initial Ugahary technique appears difficult to reproduce for many colleagues.
In 2005, some surgeons in Europe use also the preperitoneal route with a similar technique, but with an incision in front of the deep inguinal ring, which is 3 cm under the Ugahary incision (Figure 22). The preperitoneal dissection is initiated through the deep inguinal ring or medially through the tranversalis fascia. The transinguinal preperitoneal (TIPP) technique was born. Using the Edouard Pelissier Ideas (43,44) and his new mesh with a rigid peripheral ring (Figure 23).
This technique was widely distributed in France by JF Gillion, and JM Chollet (5), in Belgium by F. Berrevoet, Maes L, Sommeling C, De Ghent (45,46) and many other.
The birth of the minimal open preperitoneal (MOPP) technique
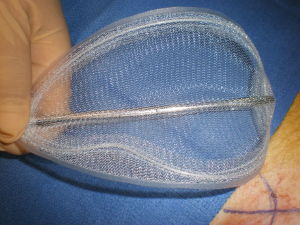
In 2011, I decided to adapt the TIPP technique using the so interesting F. Ugahary way of dissection, but with a new mesh, less rigid than the Pelissier one. I called this technique MOPP. The technique is described in “Inguinal Hernia Surgery” Giampiero Campanelli Editor (36). I use the same incision then the TIPP technique, but always going through the deep inguinal ring to penetrate in the preperitoneal space. I use the Franz Ugahary technique for the dissection. I have developed specific instruments to facilitate this dissection, it is a modification of the original retractors used by F. Ugahary (Figure 24). And I have also modified the original flat prosthesis to facilitate the placement in the preperitoneal space through the small incision (Figure 25). This mesh is also less rigid than the E. Pelissier one. This new mesh is a polypropylene ovoid prosthesis, it has a peripheral hem, with a peripheral not knit not woven reinforcement. Our results published in “Inguinal Hernia Surgery” (47) shows the good results and the very low chronic pain rate. Here is the technic.
Surgical technique (48)
The minimal open route between the skin and the deep inguinal ring
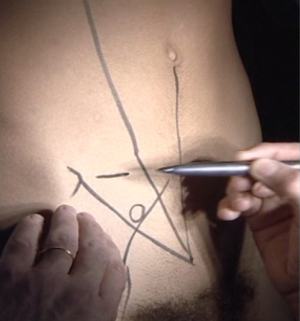
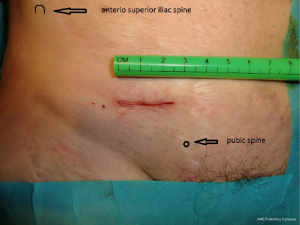
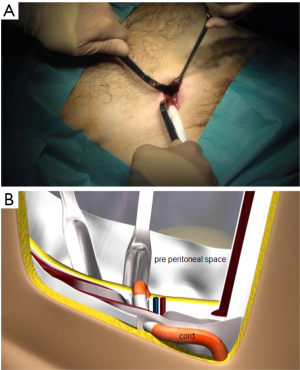
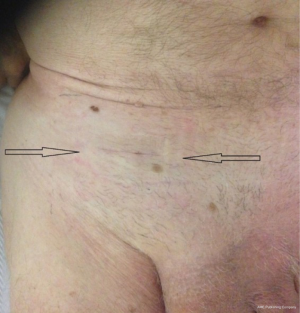
The skin incision (Figure 26) is deliberately reduced. With experience, it can be between 25 and 40 millimeters. It is immediately in front of the deep inguinal ring. Several landmarks can be materialized on the patient’s skin. It is easier to simply connect the superior anterior iliac spine to the pubic tubercle and draw the incision transversely to the union of the internal and middle third.
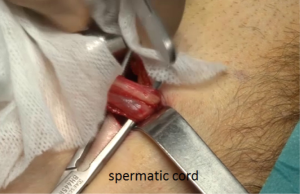
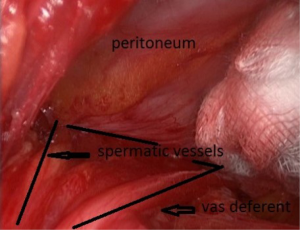
After skin and subcutaneous incision, the fascia of the external oblique muscle is incised in line with its fibers. The ilio inguinal nerve is generally identified and preserved. The spermatic cord is dissected (Figure 27), separating the funicular pedicle (the blue line) left behind. Time to time it is necessary to separate an old and fibrous medial sac from the spermatic cord. The cord is also separating from the ilio inguinal nerve. I never cut the cremaster fibers they are retracted medially. At this step, a lateral hernia sac is sought, locating a large and old bag is easy, sometimes you find a small sac in the most proximal part of the cord. The lateral sac is separated from the cord (Figure 28). Similarly, a lipoma of the cord will also be dissected and resected, the persistence of such lipoma may be responsible for postoperative pain Sometimes feel like a pseudo recurrence. The parietalization of the sac is initiated, pushing it through the deep inguinal orifice.
Cleavage of preperitoneal space
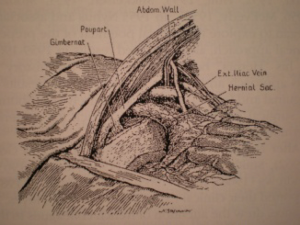
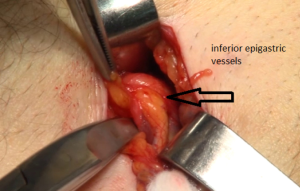
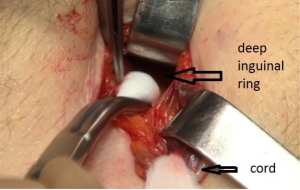
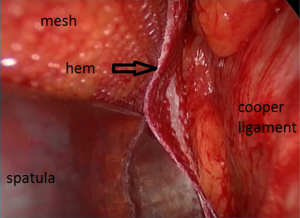
The penetration into the peritoneal space start through the deep inguinal ring, laterally to the epigastric vessels previously identified (Figure 29). The cleavage of the preperitoneal space is initiated (Figure 30), back to the tranvsersalis fascia, very fine at this location, pushing it medially and progressing back to the inferior epigastric vessels, the vessels are pressed against the anterior abdominal wall, they will be well protected during the entire duration of the intervention with a retractor. Using the retractors specifically dedicated to the procedure (Figure 31), the dissection extends into the avascular plane medially and laterally along the inferior epigastric vessels in the direction of the iliac vessels, quickly and easily, cleaving the Retzius and Bogros spaces. Cooper’s ligament is easily spotted, bladder pushed back, retro pubic space cleared (Figures 32,33). Dissection of the space dedicated to implanting the prosthesis continues with the increasing sizes of retractors, inwards and upwards. Facing the upper edge of the incision, the peritoneum may be more adherent to the superficial plane and must be time to time separated with scissors, it is imperative to widely open the plane at this level. The top and posterior dissection is easier to widely explore the psoas muscle.
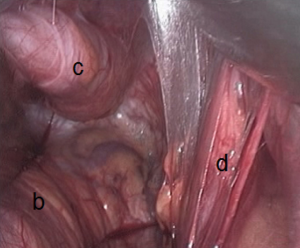
Parietalization of the spermatic cord
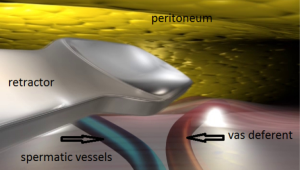
As G. Wantz said, the elements of the spermatic cord should be separated from the peritoneum, about 10 cm, compared to the deep inguinal ring, thus achieving the parietalization of the cord (Figure 34). During the dissection, the spermatic sheet described by R. Stoppa (7), uniting the vas deferens medially and the spermatic vessels laterally, must be carefully respected if possible. After the parietalization, this spermatic fascia can be interposed between the prosthesis and the external iliac vessels. After dissection of the cord, the “parietalization triangle” whose summit is the spermatic cord, the medial edge the vas deferens, and the lateral edge the spermatic vessels, is well exposed (Figure 35).
Placing the prosthesis
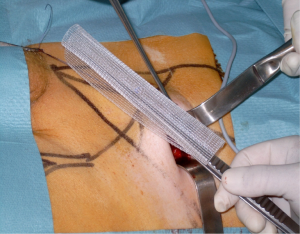
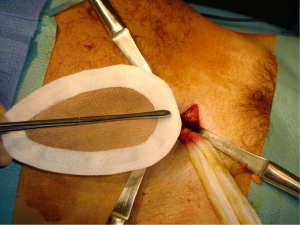
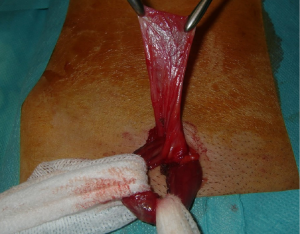
We use a mesh having a peripheral reinforcement with non-rigid hem (Figure 25). The dissected preperitoneal space is held open by three retractors. One of the retractor raises the anterior abdominal wall thereby protecting the epigastric vessels other two long and narrow retractors push back the visceral bag and the bladder. To prepare the introduction of the prosthesis, we use an atraumatic clamp (dressing forceps) that gauge the distance between the retro pubic region released and the incision. The prosthesis is grasped with this atraumatic forcep at the middle part of its lower and median edge, and introduced through the incision parallel to the inguinal ligament, up retro pubic region, taking into account the measurement previously performed (Figure 36). This same forcep grasps the upper and lateral part of the prosthesis and introduce it in the upper and lateral part of the preperitoneal dissection area. The lower end of the prosthesis is placed behind the pubis. The upper end of the prosthesis is placed in front of the psoas muscle. The prosthesis is thus partially deployed in the dissection space. The spreading of the prosthesis is completed using retractors, finger, and forceps. The correct position of the prosthesis can be controlled and improved by using a spatula instrument, which can go in the hem of the prosthesis and possibly remove a fold the edge of the prosthesis, thus optimizing good spread of its periphery (Figure 37). When the positioning of the prosthesis is satisfactory, spermatic cord is reintroduced under the external oblique muscle fascia. The prosthesis is never fixed.
The prosthesis in place, the operator sees the deep inguinal ring closes partially spontaneously, “as a sphincter”. It is not necessary to suture the musculo-fascial plane. During the closure of the external oblique aponeurosis, the ilio-inguinal nerve is carefully avoided. The subcutaneous plane is closed with two reversing stitches, adhesive strips are applied to the skin. The shower is permitted the day after. An adhesive bandage protects the adhesive strips, it is changed every day, without any special care until the final removal of the strips on the 10th day.
Indication
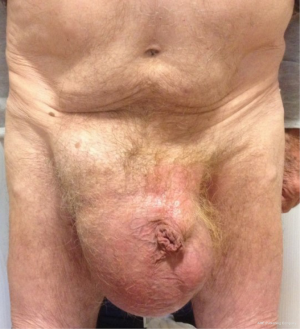
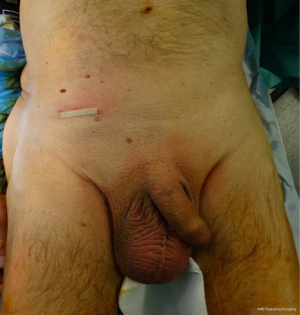
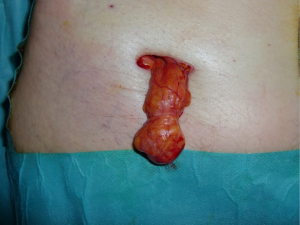
All primary, inguinal or femoral hernias, can be treated by this technique, in particular the large-scrotal inguinal hernias (Figures 38-40), or femoral hernias (Figure 41). In the presence of bilateral hernia, both sides are operated in the same operating session, the two prostheses are superimposed on the midline. Recurrent hernias without material previously established in the preperitoneal space are a very good indication. Recurrent hernias after Lichtenstein is also an excellent indication (Figure 42), with the possibility of setting up a new prosthesis, the preperitoneal space is often free to adhesion. Previous prosthesis is retained, sometimes a plug must be resected.

Special cases
Female hernias
The round ligament is always distally dissected and sectioned, it is largely repressed with a possible external oblique sac.
The femoral hernia
This is an excellent indication of the technique, it is easy to expand the femoral ring with the finger and repress back of fringes incarcerated fat. In its normal position, the prosthesis covers widely the femoral hole and the obturator foramen.
Scrotal hernia
It’s easy to dissect step by step a bulky inguinal scrotal sac, the distally part of the sac may be dropped in the scrotum.
Strangulated hernia
It is also possible to treat a strangulated hernia. An intestinal loop can be resected if necessary, through an enlarged transverse incision. Then it is possible to complete the operation by the same way with or without using a prosthetic material.
Contraindication
Previous radical prostatectomy, pelvic irradiation, or realization of a vascular bypass with dissection of the preperitoneal space can be a contraindication of the MOPP technique, also recurrent hernias with prosthesis implanted in the pre-peritoneal space. However, with experience, even in these situations it is often possible to start with the MOPP technique, and finally in case of failure to the preperitoneal dissection, it is possible to do the Lichtenstein technique. It is not a conversion.
Results
A total of 644 hernias (534 patients) have been operated between 2011 and 2015.
- Mean follow up: 711 days; day surgery: 598 (92.8%).
- Complications: bladder retention, 2; phlebitis, 1; superficial infection, 2; reoperation: 0.
- Post-operative pain—day 30, N=553; VAS: 0, 452 (81.73%); 1–3, 77 (13.92%); 4–6, 19 (3.43%); 7–8, 5 (0.9%);
- Chronic pain—at 3 months, N=97; VAS: 0, 77 (79.38%); 1–3, 9 (9.27%); 4–6, 10 (10.30%); 7–8, 1 (1.03%). No medication needed and no activity limitation.
- At 2 years, patient opinion: excellent result 212 (99.53%), medium result 1 (0.47%); no recurrence.
All these patients’ data are included in the “club hernie” data base for a long term prospective study.
MOPP and elderly patients
Another interest of the MOPP technique is to use it for elderly patients versus the endoscopic techniques (49).
In our “club hernie” data base, 14,254 groin hernias (12,089 patients between 18 and 101 years old) have been operated between September 2011 and 15 of April 2016, 1,504 patients were octogenarians [80–89] years of age, 289 were nonagenarians and more [90–101] years of age (38).
There was less laparoscopic procedure with the age: (I) (24.01%) in the nonagenarians group; (II) (38.80%) in the octogenarians group; (III) (52.02%) in the younger group, under 80 years old.
About the patients operated in emergency: (incarcerated hernia with or without intestinal obstruction), we can see also less laparoscopic procedure with the age: (I) (11.42%) in the nonagenarians group; (II) (19.14%) in the octogenarians group; (III) (38.84%) in the younger group, under 80 years old.
So, in France, the surgeons used preferentially the open procedure for the elderly patients in schedule or emergency surgery. In my personal practice, 140 patients up to 80 years old, have been operated with the MOPP technique, 111 patients (79.3%) in ambulatory setting. So, I recommend firmly this procedure for the elderly patients.
Conclusions
Nearly 150 years after the pioneers, the open preperitoneal route is more than ever one of the more attractive technique to operate the groin hernias. MOPP technique gave good results. The technique gave less chronic pain than the Lichtenstein technique. In the same spirit of G. Wantz, and F. Ugahary, we try to promote the really minimal invasive approach to treat bilateral big hernias routinely in an ambulatory setting. Our MOPP approach permit to treat nearly all type of groin hernias (primary or recurrent) under local (if necessary), or under "soft" general anesthesia with laryngeal mask without curarisation. These results are specifically interesting for the elderly patients. As we can see in our "club hernie" data base. The laparoscopic procedure is not currently adopted in France for the patient up to 80 years old, for these patients MOPP seems to be an appropriate technique, less invasive. For all the patients, our long term and prospective results showed the very low rate or recurrence and the very low rate of severe chronic pain after the MOPP technique.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Davide Lomanto and Anil Sharma) for the series “Inguinal Hernia Repair” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2017.06.08). The series “Inguinal Hernia Repair” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Soler M, Verhaeghe P, Stoppa R. Polyester (Dacron) Mesh. In: Bendavid R, Abrahamson J, Arregui ME, et al. editors. Hernias of the abdominal wall: principles and management, New York: Springer-Verlag, 2000:266-71.
- Soler M, Verhaeghe P, Stoppa R. Parietal reinforcement prostheses: an original intraperitoneal experimental study. Hernia 2000;4:1-66. [Crossref]
- Stoppa R, Soler M, Verhaeghe P. Treatment of groin hernias by giant preperitoneal prosthesis repair. In: Bendavid R. editor. Prostheses and abdominal wall hernias. Austin (TX): R.G. Landes, 1994;423-30.
- Soler M, Stoppa R, Verhaeghe P. Polyester (Dacron) mesh. In: Bendavid R. editor. Prosthesis and abdominal wall hernias. Austin (TX): R.G. Landes, 1994;268-77.
- Gillion JF, Chollet JM. 2013 Chronic pain and quality of life (QoL) after transinguinal preperitoneal (TIPP) inguinal hernia repair using a totally extraperitoneal, parietalized, Polysoft memory ring patch: a series of 622 hernia repairs in 525 patients. Hernia 2013;17:683-92. [Crossref] [PubMed]
- Koning GG, Keus F, Koeslag L, et al. Randomized clinical trial of chronic pain after the transinguinal preperitoneal technique compared with Lichtenstein's method for inguinal hernia repair. Br J Surg 2012;99:1365-73. [Crossref] [PubMed]
- Koc M, Aslar AK, Yoldas O, et al. Comparison of quality-of-life outcomes of Stoppa vs bilateral Lichtenstein procedure. Hernia 2004;8:53-5. [Crossref] [PubMed]
- Nienhuijs S, Staal E, Keemers-Gels M, et al. Pain after open preperitoneal repair versus Lichtenstein repair: a randomized trial. World J Surg 2007;31:1751-7. [Crossref] [PubMed]
- Berrevoet F, Maes L, Reyntjens K, et al. Transinguinal preperitoneal memory ring patch versus Lichtenstein repair for unilateral inguinal hernias. Langenbecks Arch Surg 2010;395:557-62. [Crossref] [PubMed]
- Eisenach JC. To our readers: an experiment in education. Anesthesiology 2010;112:1-2. [Crossref] [PubMed]
- Bay-Nielsen M, Nilsson E, Nordin P, et al. Chronic pain after open mesh and sutured repair of indirect inguinal hernia in youngmales. Br J Surg 2004;91:1372-6. [Crossref] [PubMed]
- Aasvang E, Kehlet H. Surgical management of chronic pain after inguinal hernia repair. Br J Surg 2005;92:795-801. [Crossref] [PubMed]
- Schmedt CG, Sauerland S, Bittner R. Comparison of endoscopic procedures vs Lichtenstein and other open mesh techniques for inguinal hernia repair: a meta-analysis of randomized controlled trials. Surg Endosc 2005;19:188-99. [Crossref] [PubMed]
- Cloquet J. The postérior inguinal wall as seen from the preperitoneal approach. In: Cloquet J. editor. Recherches anatomiques sur les hernies de l’abdomen. Paris: Mequigon-Marvis, 1817.
- Cooper AP. The anatomy and surgical treatment of Abdominal Hernia (2 vols). London: Longman, 1807.
- Lister BJ. The classic: On the antiseptic principle in the practice of surgery. 1867. Clin Orthop Relat Res 2010;468:2012-6. [Crossref] [PubMed]
- Annandale T. Case in which a reducible oblique & direct inguinal and femoral hernia existed on the same side and were successfully treated by operation. Edinburgh Med J 1876;27:1087.
- Seaton E. A discussion on treatment of hernia by median abdominal section. Br Med J 1891;2:285. [Crossref] [PubMed]
- Cheatle GL. An operation for the radical cure of inguinal and femoral hernia. Br Med J 1920;2:68. [Crossref] [PubMed]
- Bassini E. Sulla cura radicale dell’ ernia inguinale. Arch Soc Ital Chir 1887;4:30.
- Shouldice EE. Surgical treatment of hernia. Ontario Med Rev 1945;12:43.
- McVAY CB. ChAPP JD. Inguinal and femoral hernioplasty. Evaluation of a basic concept. Ann Surg 1958;148:499. [Crossref] [PubMed]
- Nyhus LM, Stevenson LM, Listerud MB, et al. Preperitoneal hernioraphy. A preliminary report on fifty patients. West J Surg Obstet Gynecol 1959;67:48-54. [PubMed]
- Duffy JJ, Condon RE, Harkins HN. Clinical experience with preperitoneal hernia repair for all types of hernia of the groin. Am J Surg 1960;100:434-8. [Crossref] [PubMed]
- Fruchaud H. Anatomie chirurgicale des hernies de l’aine. Paris: Doin Ed, 1957.
- Rives J, Lardennois B, Flament JB, et al. La pièce en tulle de dacron, traitement de choix des hernies de l’aine de l’adulte. A propos de 183 cas. Chirurgie 1973;99:564-75. [PubMed]
- Rives J, Stoppa R, Fortesa L, et al. Les pièces en dacron et leur place dans la chirurgie des hernies de l’aine. A propos de 65 cas recueillis sur une statistique intégrale de 274. Ann Chir 1968;22:159-71. [PubMed]
- Odimba BF, Stoppa R, Laude M, et al. Les espaces clivables sous-pariétaux de l'abdomen. Leur intérêt dans la chirurgie des hernies et des éventrations de la paroi abdominale antéro-latérale. J Chir 1980;117:621-7.
- Stoppa R, Petit J, Henry X. Plasties des hernies de l'aine par voie médiane sous-péritonéale. Actualités chirurgicales. Paris: Masson, 1972:448-52.
- Stoppa R, Petit J, Abourachid H. Procédé original de plastie des hernies de l'aine. L'interposition sans fixation d'une prothèse en tulle de dacron par voie médiane sous-péritonéale. Chirurgie 1973;99:119-23. [PubMed]
- Stoppa RE, Rives J, Warlaumont C, et al. The use of dacron in the repair of hernias of the groin. Surg Clin North Am 1984;64:269-85. [Crossref] [PubMed]
- Stoppa R, Warlaumont C, Verhaeghe P, et al. Tulle de Dacron et cure chirurgicale des hernies de l’aine. Chirurgie 1983;109:847-54. [PubMed]
- Wantz GE. Giant prosthetic reinforcement of the visceral sac. Surg Gynecol Obstet 1989;169:408-17. [PubMed]
- Wantz GE, Fischer E. Unilateral giant prosthetic reinforcement of visceral bag: Preperitoneal hernioplasties with Dacron. In: Bendavid R. editor. Abdominal wall hernias. Principles and management. New York: Springer-Verlag, 2001:396-400.
- Lichtenstein IL. Hernia repair without disability. St Louis: The C.V. Mosby Company, 1970.
- Lichtenstein IL, Shulman AG, Amid PK, et al. The tension free hernioplastie. Am J Surg 1989;157:188-93. [Crossref] [PubMed]
- Leroy J, Fromont G. Hernies de l’aine de l’adulte: prothèse sous péritonéale sous contrôle coelioscopique (à propos de 110 cas). J Coelio Chir 1992;1:22-5.
- Begin G. Cure coelioscopique des hernies de l’aine par voie propéritonéale. J Coelio Chir 1993;7:23-9.
- Alexandre JH, Dupin P, Levard H, et al. Cure des hernies de l’aine par prothèse non fendue de mersuture. Intérêt de la pariétalisation du cordon et de la ligature des vaisseaux épigastriques. Presse Med 1984;13:161-3. [PubMed]
- Ugahary F, Simmermacher RK. Groin hernia repair via a grid-iron incision year alternative technique for preperitoneal mesh incision. Hernia 1998;2:123-5. [Crossref]
- Beck M, Gillion JF, Soler M, et al. Traitement des hernies de l’aine par voie inguinale. EMC. Techniques chirurgicales-Appareil digestif 2017;12:1-21.
- Soler M, Ugahary F. Cure des hernies de l’aine par grande prothèse pré- péritonéale par voie sus inguinale latérale (technique de Ugahary). e-mémoires de l'Académie Nationale de Chirurgie 2004;3:28-33.
- Pélissier EP. Inguinal hernia: preperitoneal placement of a memory-ring patch by anterior approach. Preliminary experience. Hernia 2006;10:248-52. [Crossref] [PubMed]
- Pélissier EP, Monek O, Blum D, et al. The Polysoft patch: prospective evaluation of feasibility, postoperative pain and recovery. Hernia 2007;11:229-34. [Crossref] [PubMed]
- Berrevoet F, Maes L, Reyntjens K, et al. 2009 Transinguinal preperitoneal memory ring patch versus Lichtenstein repair for unilateral inguinal hernias. Langenbecks Arch Surg 2010;395:557-62. [Crossref] [PubMed]
- Berrevoet F, Sommeling C, De Gendt S, et al. The preperitoneal memory-ring patch for inguinal hernia: a prospective multicentric feasibility study. Hernia 2009;13:243-9. [Crossref] [PubMed]
- Soler M. Minimal Open Preperitoneal (MOPP) Technique. In: Campanelli G. editor. Inguinal Hernia Surgery. Milan: Springer, 2016:87-99.
- Soler M. Groin hernia repair with the MOPP technique. Available online: https://www.youtube.com/watch?v=F0iYbUenfbw
- Soler M. Long-term outcomes of groin hernia repair in octogenarians and nonagenarians: the "club hernie" data base results. Oral presentation. The 12th International Congress of the Asia Pacific Hernia Society (APHS). Tokyo, 27-28 October 2016.
Cite this article as: Soler M. The minimal open preperitoneal (MOPP) approach to treat the groin hernias, with the history of the preperitoneal approach. Ann Laparosc Endosc Surg 2017;2:133.